The impact of bariatric surgery beyond its effect on weight loss has entailed a change in the way of regarding it. The term metabolic surgery has become more popular to designate those interventions that aim at resolving diseases that have been traditionally considered as of exclusive medical management, such as type 2 diabetes mellitus (T2D). Recommendations for metabolic surgery have been largely addressed and discussed in worldwide meetings, but no definitive consensus has been reached yet. Rates of diabetes remission after metabolic surgery have been one of the most debated hot topics, with heterogeneity being a current concern. This review aims to identify and clarify controversies regarding metabolic surgery, by focusing on a critical analysis of T2D remission rates achieved with different bariatric procedures, and using different criteria for its definition. Indications for metabolic surgery for patients with T2D who are not morbidly obese are also discussed.
El impacto de la cirugía bariátrica más allá de su efecto sobre la pérdida de peso ha supuesto un cambio en la visión que se tiene de ella. Así, se ha hecho más popular la denominación «cirugía metabólica» para referirse a las intervenciones encaminadas a resolver enfermedades que tradicionalmente se han considerado de tratamiento exclusivamente médico, como la diabetes mellitus tipo 2 (DM2). En numerosos foros internacionales se han abordado y debatido las indicaciones recomendadas de la cirugía metabólica, pero no se ha logrado aún un consenso definitivo. Las tasas de remisión de la diabetes tras la intervención han sido uno de los temas más candentes, y sus valores heterogéneos siguen siendo objeto de preocupación. El objetivo de esta revisión es identificar y aclarar las controversias en torno a la cirugía metabólica, centrándose en el análisis crítico de las tasas de remisión de la DM2 obtenidas con distintas técnicas bariátricas, y utilizando criterios diferentes para su definición. Se comentan también las indicaciones de la cirugía metabólica en los pacientes con DM2 sin obesidad mórbida.
Metabolic surgery has become the pathway for acceptance of surgical procedures for dealing with diseases traditionally considered as exclusively medically managed. If we look back in history, the classical example of metabolic surgery has been the Program on the Surgical Control of the Hyperlipidemias (POSCH),1,2 where it was observed that partial ileal bypass was an intervention capable of producing a marked reduction of low-density lipoprotein cholesterol (LDL-c), and was consequently associated with a statistically significant decline in cardiovascular events and increase in life expectancy. In this way, the idea that surgery could be regarded as a valid option for the treatment of metabolic diseases turned out to be true. Buchwald and Varco, in 1978, specified the conception of metabolic surgery by defining it as “the operative manipulation of a normal organ or organ system to achieve a biological result for a potential health gain”.3,4
Another example of metabolic surgery with current clinical relevance is bariatric surgery. This was initially conceived with the aim of achieving sustained and significant weight loss by using restrictive, malabsortive and mixed techniques. The rise in the number of interventions performed for people with morbid obesity during the 1990s, revealed that not only was this goal achieved, but, in fact, other associated comorbidities, including type 2 diabetes (T2D) were also improved.5 In 1995, the provocative title of Pories et al., “Who would have thought it? An operation proves to be the most effective therapy for adult-onset diabetes mellitus”,6 showed that, indeed, bariatric surgery was able to resolve T2D of the adult, and emphasized that there were mechanisms beyond those merely anatomical inherent to the surgical technique responsible for this effect.
Buchwald et al. published in 2004 a systematic review and metaanalysis7 of more than 22,000 patients who underwent bariatric surgery, and described improvement of diabetes in 86.6% of patients and complete resolution in 78.1%. Moreover, hyperlipemia was improved in 70% of cases, hypertension was resolved or improved in 78.5%, and obstructive sleep apnea was resolved in 85.7% of patients. Definitions of resolution and improvement differed across the studies included in this metaanalysis, but, given the results observed, it became evident that metabolic surgery was not used solely to designate procedures for the treatment of T2D, but also to refer to those interventions that aimed at reducing the impact of other cardio-metabolic aspects.
The impact of bariatric surgery beyond its effect on weight loss entailed a change in the way of thinking of scientific societies. In 2007, the executive boards of the so-called American Society for Bariatric Surgery (ASBS) and International Federation for the Surgery of Obesity agreed to include the term metabolic in their designations, advocating the idea that metabolic surgery truly operated on perfectly normal organs or organ systems to achieve a biological result for a potential health gain: the treatment of metabolic illnesses, and not just excess weight. Thus, these societies have become to be known as the American Society for Metabolic and Bariatric Surgery (ASMBS) and the International Federation for the Surgery of Obesity and Metabolic Disorders. This renovation has also been adopted by the homologous Spanish society, which is now named Sociedad Española de Cirugía de la Obesidad Mórbida y Enfermedades Metabólicas.8
Intensive lifestyle intervention, as reported in the LOOK-AHEAD study,9 achieves only modest T2D remission rates of 11.5% and 7.4% after one and four years of follow-up, respectively. These rates prove that an intensive lifestyle intervention is associated with a greater likelihood of partial remission of T2D in comparison with conventional support and educational management. However, intensive management still achieves results way lower than outcomes described after a surgical approach, where even the lowest rates reported were higher than with medical protocols.10 The opinion that surgical interventions may be an alternative option for chronic and progressive medical issues such as obesity and T2D has gained acceptance and popularity, and superiority has been subsequently described in several studies.10,11 Bariatric surgery has become, in this way, the prototype of metabolic surgery, to such extent, that both terms have often been considered synonymous.
But a recent study by Rubino et al.,12 which aimed at investigating the practical clinical consequences of offering two different surgery programs, identified differences in patients’ profiles depending on the denomination used: bariatric or metabolic. This may lead to considering distinctions between both denominations, based on what the foremost target is, i.e., weight reduction or treatment of metabolic illnesses. Nevertheless, once again, the debate may revive: can both problems be regarded on an individual basis, or should they be viewed as a whole?
This review aims to identify and clarify controversies regarding metabolic surgery for T2D and tries to guide the reader to an unbiased interpretation of published data.
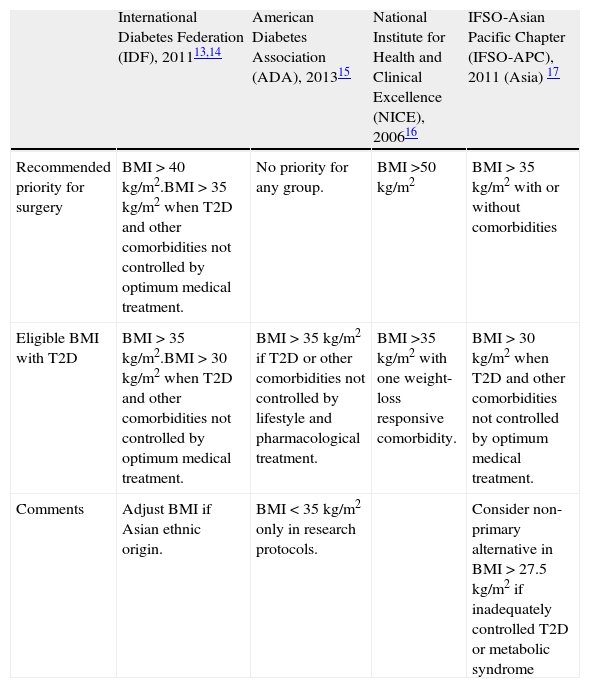
Metabolic surgery: for whom?There are several national and international guidelines and consensus statements that address the indications for bariatric surgery in patients with T2D.13–17 They all share basic terms and conditions, such as failure of previous weight-loss attempts, no specific contraindications to surgery and the patient's commitment to long-term follow-up and after care. These guidelines used to be based on specific body mass index (BMI) cut-off levels. However, this requisite has been questioned over the last recent years. Table 1 shows current guidelines established by the International Diabetes Federation (IDF),13,14 the American Diabetes Association (ADA),15 the National Institute for Health and Clinical Excellence (NICE)16 and the International Federation for the Surgery of Obesity and Metabolic Disorders – Asian Pacific Chapter (IFSO-APC).17 Only the IDF and the Obesity Work Group from the Sociedad Española de Endocrinología y Nutrición (SEEN)18 contemplate the possibility of bariatric surgery in cases with BMI<35kg/m2. Nevertheless, in spite of these general guidelines, consensus and position statements regarding the management of hyperglycemia in T2D have not included surgical intervention in their step-by-step algorithms.
Guidelines for eligibility for bariatric surgery in adults with T2D.
| International Diabetes Federation (IDF), 201113,14 | American Diabetes Association (ADA), 201315 | National Institute for Health and Clinical Excellence (NICE), 200616 | IFSO-Asian Pacific Chapter (IFSO-APC), 2011 (Asia) 17 | |
| Recommended priority for surgery | BMI>40kg/m2.BMI>35kg/m2 when T2D and other comorbidities not controlled by optimum medical treatment. | No priority for any group. | BMI>50kg/m2 | BMI>35kg/m2 with or without comorbidities |
| Eligible BMI with T2D | BMI>35kg/m2.BMI>30kg/m2 when T2D and other comorbidities not controlled by optimum medical treatment. | BMI>35kg/m2 if T2D or other comorbidities not controlled by lifestyle and pharmacological treatment. | BMI>35kg/m2 with one weight-loss responsive comorbidity. | BMI>30kg/m2 when T2D and other comorbidities not controlled by optimum medical treatment. |
| Comments | Adjust BMI if Asian ethnic origin. | BMI<35kg/m2 only in research protocols. | Consider non-primary alternative in BMI>27.5kg/m2 if inadequately controlled T2D or metabolic syndrome |
Distinction between successful treatment and cure may be difficult when talking about T2D, since diabetes is defined by hyperglycemia, which exists on a continuum, unlike other acute diseases. Cure is defined as restoration to good health, while remission is defined as reduction or disappearance of signs and symptoms of a disease.19 Due to the chronicity of T2D, it seems reasonable and more accurate to use the term remission, which will be the one used throughout this review.
The Cochrane Database System Review20 and Buchwald et al.’s review,21 both published in 2009, demonstrated the effectiveness of bariatric surgery for improvement and resolution of T2D. However, remission rates described across different studies have varied widely. In general, T2D remission has been defined as “the return to normal values of fasting glucose (FG) and glycosylated hemoglobin (HbA1c), in the absence of active pharmacologic treatment”. But cut-off levels used for these parameters have not always been homogeneous, and variations in the time of follow-up considered for evaluation have also occurred. Moreover, disparities amongst patients’ baseline characteristics have contributed to this heterogeneity.
In an attempt to overcome this controversy and establish standardized remission criteria, a consensus group comprised of experts in endocrinology, diabetes education, transplantation, metabolism, bariatric/metabolic surgery and hematology-oncology, proposed specific definitions for diabetes remission (Table 2).19 These new definitions rely on more stringent criteria for establishing glycemic control, and, consequently, remission rates have been communicated to be lower than previously reported in the literature.
Definitions of T2D remission according to Buse et al.19
| Partial remission | Subdiabetic hyperglycemia:- HbA1c not diagnostic for diabetes (<6.5%).- Fasting glucose 100–125mg/dL (5.6–6.9mmol/L).No active pharmacologic treatment or ongoing procedures.At least one year's duration. |
| Complete remission | Normal glycemic measures:- HbA1c in the normal range (<6%).- FG <100mg/dL (<5.6mmol/L).No active pharmacologic treatment or ongoing procedures.At least one year's duration. |
| Prolonged remission | Complete remission of at least five year's duration. |
For instance, in the review by Buchwald et al.,21 T2D remission was reported in 78% of patients after gastric bypass, but its definition was largely based on clinical reports, rather than in specific biochemical parameters, there were few good quality studies with a high level of evidence, and patients’ preoperative information and follow up of most cohorts was not described in detail. Nonetheless, these outstanding results reaffirmed surgeons’ optimism for the treatment of T2D. A low level of quality and evidence has been a common attribute of well-known studies, whose reports have been mostly based on case-series, with no methodological control.6,22–24 The studies by Sjostrom et al.,25 Dixon et al.,26 Schauer et al.10 and Mingrone et al.,11 on the other hand, have been considered of higher quality, given its controlled design. But remission rates still varied due to differences in criteria used and baseline patients’ features. Overall, data from large, adequately powered, long-term randomized controlled trials in bariatric surgery are still lacking, although a trend for an increasing number has been observed in recent years.27
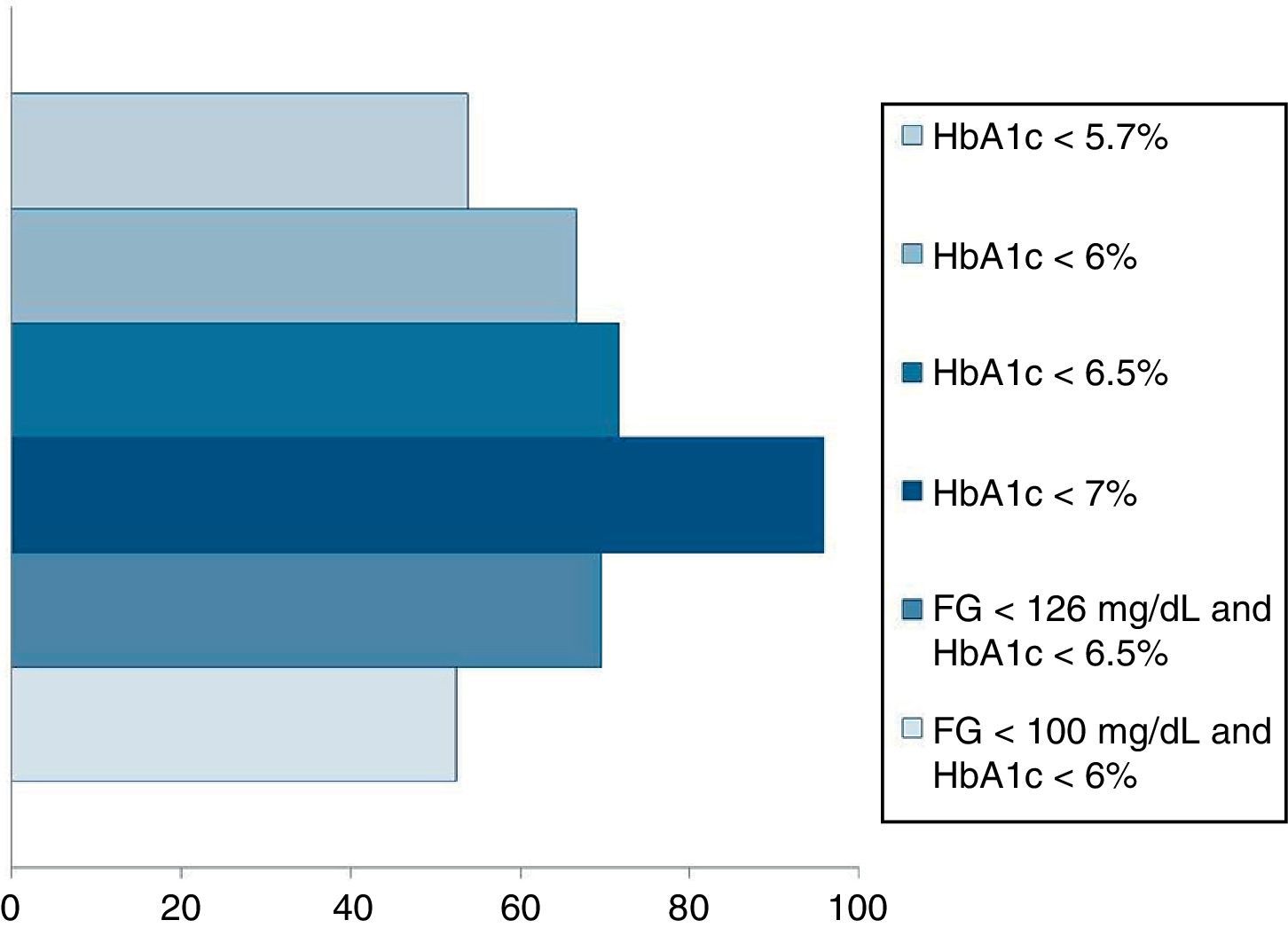
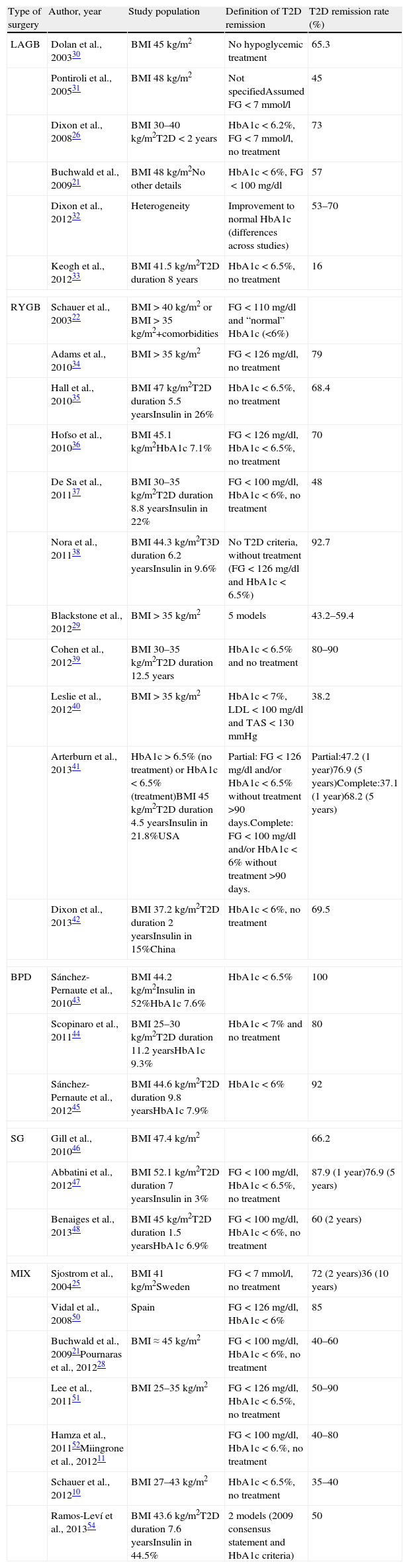
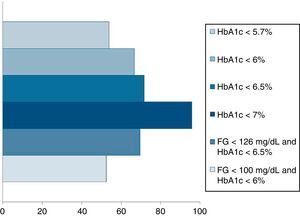
Pournaras et al.28 and Blackstone et al.29 compared remission rates using different models of remission criteria: those previously reported in literature, versus the ones proposed by Buse et al.,19 and observed a significant reduction in remission rates to around 50%. This may be a more realistic approximation of what can be expected after metabolic surgery, taking also into account existing differences between population's features. Table 3 highlights the main characteristics of the most relevant publications over the last recent years regarding T2D remission after bariatric surgery. Fig. 1 represents an estimate of the range of remission rates that would be obtained by using different criteria for the same population. It can be observed that frequencies vary from around 50%, when using the more stringent criteria, to near 90%, if only an adequate metabolic control is considered, defined by HbA1c levels below 7%. It must be noted, however, that these results are generally referred to the period of maximum weight-loss, that is, the first two years after surgery, and long-term evaluation may reveal lower remission rates.
Main characteristics of the most relevant reports concerning T2D remission after metabolic surgery.
| Type of surgery | Author, year | Study population | Definition of T2D remission | T2D remission rate (%) |
| LAGB | Dolan et al., 200330 | BMI 45kg/m2 | No hypoglycemic treatment | 65.3 |
| Pontiroli et al., 200531 | BMI 48kg/m2 | Not specifiedAssumed FG<7mmol/l | 45 | |
| Dixon et al., 200826 | BMI 30–40kg/m2T2D<2 years | HbA1c<6.2%, FG<7mmol/l, no treatment | 73 | |
| Buchwald et al., 200921 | BMI 48kg/m2No other details | HbA1c<6%, FG<100mg/dl | 57 | |
| Dixon et al., 201232 | Heterogeneity | Improvement to normal HbA1c (differences across studies) | 53–70 | |
| Keogh et al., 201233 | BMI 41.5kg/m2T2D duration 8 years | HbA1c<6.5%, no treatment | 16 | |
| RYGB | Schauer et al., 200322 | BMI>40kg/m2 or BMI>35kg/m2+comorbidities | FG<110mg/dl and “normal” HbA1c (<6%) | |
| Adams et al., 201034 | BMI>35kg/m2 | FG<126mg/dl, no treatment | 79 | |
| Hall et al., 201035 | BMI 47kg/m2T2D duration 5.5 yearsInsulin in 26% | HbA1c<6.5%, no treatment | 68.4 | |
| Hofso et al., 201036 | BMI 45.1kg/m2HbA1c 7.1% | FG<126mg/dl, HbA1c<6.5%, no treatment | 70 | |
| De Sa et al., 201137 | BMI 30–35kg/m2T2D duration 8.8 yearsInsulin in 22% | FG<100mg/dl, HbA1c<6%, no treatment | 48 | |
| Nora et al., 201138 | BMI 44.3kg/m2T3D duration 6.2 yearsInsulin in 9.6% | No T2D criteria, without treatment (FG<126mg/dl and HbA1c<6.5%) | 92.7 | |
| Blackstone et al., 201229 | BMI>35kg/m2 | 5 models | 43.2–59.4 | |
| Cohen et al., 201239 | BMI 30–35kg/m2T2D duration 12.5 years | HbA1c<6.5% and no treatment | 80–90 | |
| Leslie et al., 201240 | BMI>35kg/m2 | HbA1c<7%, LDL<100mg/dl and TAS<130mmHg | 38.2 | |
| Arterburn et al., 201341 | HbA1c>6.5% (no treatment) or HbA1c<6.5% (treatment)BMI 45kg/m2T2D duration 4.5 yearsInsulin in 21.8%USA | Partial: FG<126mg/dl and/or HbA1c<6.5% without treatment >90 days.Complete: FG<100mg/dl and/or HbA1c<6% without treatment >90 days. | Partial:47.2 (1 year)76.9 (5 years)Complete:37.1 (1 year)68.2 (5 years) | |
| Dixon et al., 201342 | BMI 37.2kg/m2T2D duration 2 yearsInsulin in 15%China | HbA1c<6%, no treatment | 69.5 | |
| BPD | Sánchez-Pernaute et al., 201043 | BMI 44.2kg/m2Insulin in 52%HbA1c 7.6% | HbA1c<6.5% | 100 |
| Scopinaro et al., 201144 | BMI 25–30kg/m2T2D duration 11.2 yearsHbA1c 9.3% | HbA1c<7% and no treatment | 80 | |
| Sánchez-Pernaute et al., 201245 | BMI 44.6kg/m2T2D duration 9.8 yearsHbA1c 7.9% | HbA1c<6% | 92 | |
| SG | Gill et al., 201046 | BMI 47.4kg/m2 | 66.2 | |
| Abbatini et al., 201247 | BMI 52.1kg/m2T2D duration 7 yearsInsulin in 3% | FG<100mg/dl, HbA1c<6.5%, no treatment | 87.9 (1 year)76.9 (5 years) | |
| Benaiges et al., 201348 | BMI 45kg/m2T2D duration 1.5 yearsHbA1c 6.9% | FG<100mg/dl, HbA1c<6%, no treatment | 60 (2 years) | |
| MIX | Sjostrom et al., 200425 | BMI 41kg/m2Sweden | FG<7mmol/l, no treatment | 72 (2 years)36 (10 years) |
| Vidal et al., 200850 | Spain | FG<126mg/dl, HbA1c<6% | 85 | |
| Buchwald et al., 200921Pournaras et al., 201228 | BMI≈45kg/m2 | FG<100mg/dl, HbA1c<6%, no treatment | 40–60 | |
| Lee et al., 201151 | BMI 25–35kg/m2 | FG<126mg/dl, HbA1c<6.5%, no treatment | 50–90 | |
| Hamza et al., 201152Miingrone et al., 201211 | FG<100mg/dl, HbA1c<6.%, no treatment | 40–80 | ||
| Schauer et al., 201210 | BMI 27–43kg/m2 | HbA1c<6.5%, no treatment | 35–40 | |
| Ramos-Leví et al., 201354 | BMI 43.6kg/m2T2D duration 7.6 yearsInsulin in 44.5% | 2 models (2009 consensus statement and HbA1c criteria) | 50 | |
LAGB=laparoscopic adjustable gastric band; RYGB=Roux-en Y gastric bypass; BPD=biliopancreatic diversion; SG=Sleeve gastrectomy; MIX=mixed techniques.
Given the difficulty of establishing remission of a disease whose definition is based on a continuous variable, we suggest that, for example, ADA's HbA1c cut-off levels for diagnosis of diabetes53 could perhaps be helpful, more straightforward, and clinically practical for doing so (Table 4). A comparison of this model of criteria with the one proposed by the 2009 consensus group was recently published,54 and no significant differences were found.
Proposed simplified criteria for T2D remission, according to ADA's definition of diabetes.53,54
| Remission | HbA1c<5.7%No active hypoglycemic treatment |
| Improvement | HbA1c 5.7–6.4%No active hypoglycemic treatment |
| No remission | HbA1c>6.5%Active hypoglycemic treatment |
| Optimal control | HbA1c<7%With or without active hypoglycemic treatment |
However, scientific societies have not yet formally accepted any specific criteria and there has been no definitive positioning regarding this matter, so heterogeneity across subsequent publications persists.
What factors may affect type 2 diabetes remission after metabolic surgery?Published reports have described a set of predictors of T2D remission:
- 1.
Previous duration of diabetes. Duration of diabetes has been taken as an estimate of disease severity. The longer this period, the lower remission rates. It is one of the most frequently identified predictors of remission across numerous reports.35,42,52,54,55 Some have proposed different thresholds, ranging between 5 and 10 years, for which the probability of achieving diabetes remission decreases, and it has been proposed that T2D duration longer than 15 years would imply no remission.
- 2.
Previous insulin treatment. The use of insulin therapy prior to surgery has been associated to lower rates of remission, in comparison to those patients who were treated with oral agents exclusively: 13.5% vs. 53.8% in Blackstone et al.’s report.29 The explanation lies on the assumption that insulin treatment could also be regarded as an estimation of disease severity, duration of diabetes and worse control, presumably due to deterioration of pancreatic beta-cells.
- 3.
Weight loss. The complex association between obesity and T2D justifies the fact that percentage of weight loss (%WL) and percentage of excess weight loss (%EWL) influence diabetes remission. Insufficient %WL seems to be one of the main reasons for inadequate T2D control, and this may sometimes be linked to a lack of patient's commitment to long-term follow-up, or even to failure of the surgical technique.56 A %EWL of at least 50% (or 30% of %WL) is required to allow a significant improvement or even remission of T2D.57 Nevertheless, improvement of glycemic control after surgery can be observed as early as in the immediate postoperative period, when a significant weight loss has not yet been achieved,58,59 and, consequently, surgeons have promoted the use of metabolic surgery in patients with only mild obesity, or even with mere overweight.39,44,60,61 Several weight-independent mechanisms are involved in the short and mid-term amelioration of glucose metabolism, but, there are no studies that evaluate their efficacy in the long-term, in the absence of an associated fat reduction. The association between T2D and excess adiposity is complex; in fact, it has been observed that up to 80% of individuals classified as overweight (BMI 25.0–29.9kg/m2) and up to 29% of lean subjects (BMI 20.0–24.9kg/m2) have a body fat percentage that could be judged as within the obesity range, when determined by air displacement plethysmography.62 Excess body fat, especially abdominal visceral fat, may explain the development of metabolic disorders such as T2D and atherogenic dyslipemia in patients with BMI<30kg/m2. This circumstance can be remarked in the particular case of Asian populations, in whom and elevated risk of T2D, hypertension and hyperlipemia has been observed at a relatively low level of BMI. As a result, surgical indications have been deliberated and BMI criteria have been adapted for this specific group of patients, as it was described earlier.17 Revaluation of T2D in patients with lower BMI with other alternative assessment tools deems, therefore, necessary to manage these individuals, for which well-designed randomized trials should be carried out.
- 4.
Preoperative baseline C-peptide. Baseline C-peptide is a surrogate of pancreatic beta-cell mass and insulin secretion. High levels have been identified in obese patients, and significant reductions have been observed after bariatric procedures. Some reports have proposed high preoperative C-peptide levels (around >3ng/mL) as predictors of remission,42,55,63,64 although there is no established agreement on preoperative cut-off values and its clinical relevance has not been thoroughly analyzed, especially because levels may be influenced by hypoglycemic treatment. Stimulated C-peptide levels and evaluation of incretin effects require further investigation.
- 5.
Age. Older age has been associated to no remission, presumably due to deterioration of pancreatic beta-cells and T2D progression.55 However, not all studies have found differences in age between remitters and non-remitters. Furthermore, the majority of studies include patients under the age of 60, making the distinction between the effect of age itself and T2D duration confusing.
- 6.
Sex. Some studies suggest male sex as a predictor of remission, although this has not been consistent across all reports.29 However it is worth remarking the fact that, in general, men tend to lose more weight than women, but T2D remission rates adjusted for gender have not been found to be significantly different.
- 7.
Inadequate diagnosis of T2D. Late autoimmune diabetes of the adult (LADA), type 1 diabetes and some forms of maturity-onset diabetes of the young (MODY) prove lower rates of resolution after bariatric surgery, apparently due to other concomitant pathogenic factors involved. Inclusion of these patients in bariatric series may, therefore, influence remission reports.
Bariatric surgery offers an effective treatment option for glycemic control in patients with T2D and morbid obesity, and numerous papers have reported profound weight loss and amelioration of all obesity-related comorbidities in patients with BMI>35kg/m2.7,21,25 These encouraging results, together with the identification of weight-independent mechanisms responsible for resolution of diabetes, have prompted consideration of bariatric surgery for less obese individuals.
Recent reports have described that bariatric surgery was also effective for diabetes resolution in cases with BMI<35kg/m2,39 a finding with important clinical relevance, since patients with a BMI between 30 and 35kg/m2 constitute the most numerous class of obese people. In this setting, metabolic surgery purely aims at resolving T2D, not so much obesity. However, as it was previously outlined, individuals with BMI 30–35kg/m2 still have an elevated body fat percentage, with visceral preponderance, making reduction of this adipose tissue a perdurable main priority for the amelioration of metabolic disorders. It is, therefore, important that patients with T2D and only “mild” obesity receive correct information regarding the main objectives and what to expect of the surgical procedure. Moreover, reduction of micro and macrovascular complications, as well as of long-term cardiovascular morbidity and mortality, should also be aimed, not only an improved BMI, but data in literature is still scarce.65–67
Additionally, several questions have been set to debate in this context. Firstly, the appropriate time to recommend surgery in these cases is not well established, and there are no agreements in consensus statements, since there is insufficient evidence-based data from large randomized controlled trials in this range of BMI. Also, deliberation regarding risks and benefits of surgery should be contemplated. Furthermore, previous diabetes status should be taken into account, since patients would obtain benefit from surgery mainly due to weight-independent mechanisms, and if pancreatic reserve is downgraded, effectiveness would be diminished.
Due to the above-mentioned issues, metabolic surgery for patients with T2D and BMI<35kg/m2 is contemplated by the IDF13,14 and the Obesity Work Group from the Sociedad Española de Endocrinología y Nutrición (SEEN),18 for patients with poor glycemic control and associated cardio-metabolic risk factors, in whom other alternative treatment strategies have failed. Additionally, the American Association of Clinical Endocrinologists, The Obesity Society, and the American Society for Metabolic and Bariatric Surgery (AACE/TOS/ASMBS) in their recently updated guidelines,81 consider that patients with BMI of 30–34.9kg/m2 with diabetes or metabolic syndrome may also be offered a bariatric procedure. Nevertheless, they acknowledge that current evidence is limited by the number of subjects studied and lack of long-term data demonstrating net benefit, and they also remark that there is insufficient evidence for recommending bariatric surgical procedures specifically for glycemic control alone, lipid lowering alone, or cardiovascular disease risk reduction alone, independent of BMI criteria. The ADA,15 on the other hand, considers that there is not enough safety and efficacy data to be able to recommend metabolic surgery in this group of patients outside clinical trials and research protocols. But the Food and Drug Administration (FDA) has recently approved the use of gastric banding and sleeve gastrectomy also for patients with BMI of 30–34.9kg/m2. Further high-quality long-term studies seem necessary.
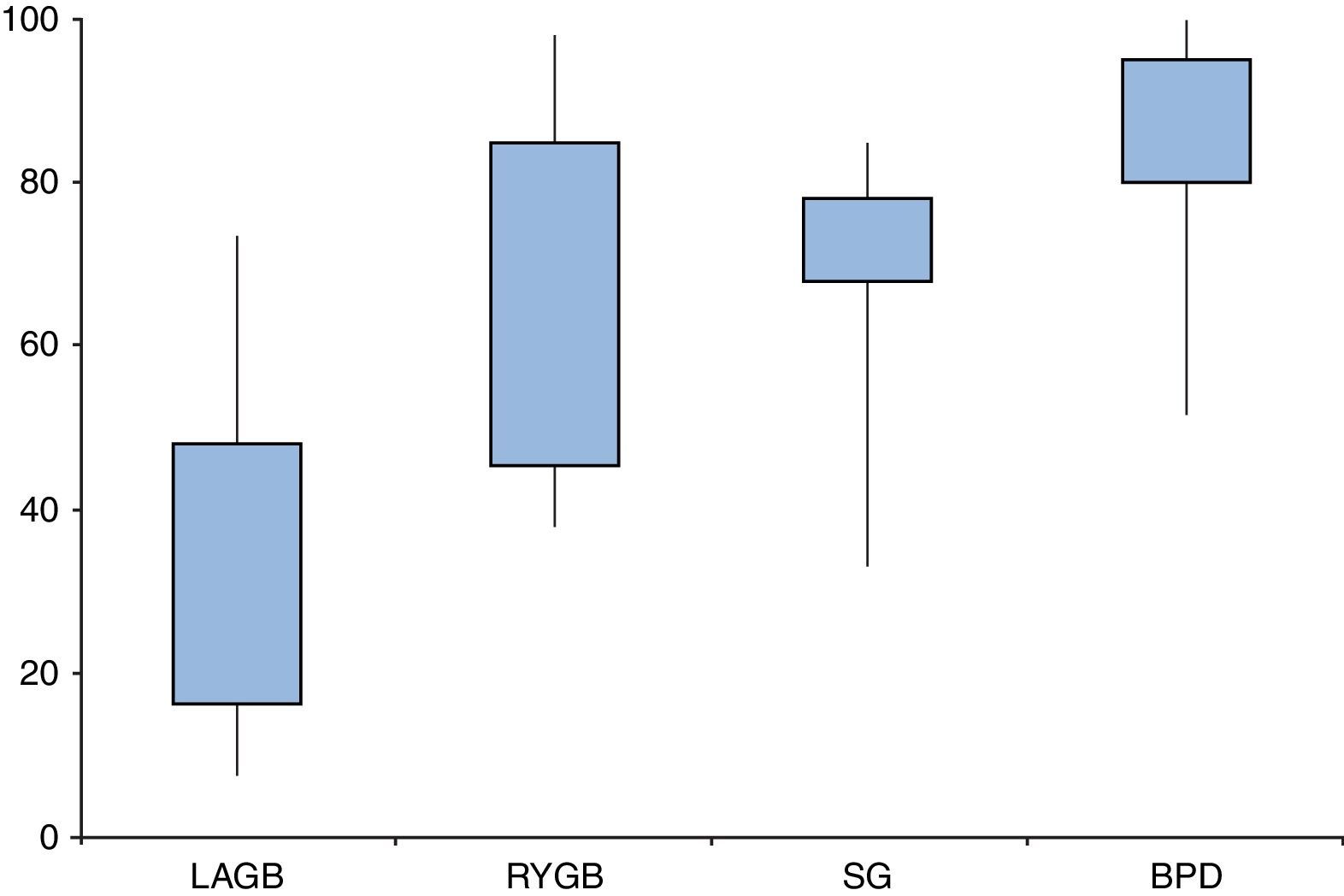
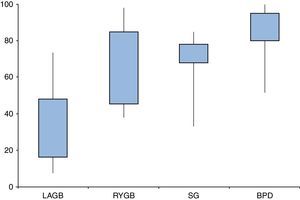
Are all types of surgeries equally effective?Resolution of diabetes following bariatric surgery occurs because of both weight-dependent and independent mechanisms.58,59,68 Therefore, the type of surgical procedure will influence rates of remission. Several studies and reviews have addressed the different approaches and their overall results, reporting T2D remission rates ranging from 7–70% for laparoscopic adjustable gastric banding (LAGB), 38–98% for Roux-en-Y gastric bypass (RYGB), 33–85% for sleeve gastrectomy (SG), and 52–100% for biliopancreatic diversions (BPD) (Fig. 2).
Type 2 diabetes remission rates according to different bariatric procedures: laparoscopic gastric banding (LAGB), Roux-en-Y gastric bypass (RYGB), sleeve gastrectomy (SG) and biliopancreatic diversion (BPD). Percentages taken and adapted from results from different studies, which include different remission criteria.
Nevertheless, it is essential to bear in mind that there are few studies that directly compare two different techniques10,11,48–51,69; therefore, it is difficult to reach definitive conclusions about the relative effectiveness of various surgical techniques beyond mere and prudent speculations concerning each individual report. And moreover, once again, the use of arbitrary remission criteria and heterogeneous study populations, mainly regarding differences in BMI and previous insulin use, may bias results and confound our interpretations.
Laparoscopic adjustable gastric bandingLaparoscopic adjustable gastric banding (LAGB).7,26,32,58,70 Overall, remission rates after LAGB have been described to be lower than with other types of interventions, presumably because the core mechanism involved is weight loss. Buchwald et al.7 described remission rates below 50%. Conversely, in the unblinded randomized controlled trial by Dixon et al.,26 which included patients with BMI between 30 and 40kg/m2 and a recently diagnosed T2D (<2 years) undergoing LAGB, 73% of patients achieved remission, defined as FG<126mg/dL and HbA1c<6.2%, without diabetes medications. The systematic review by Dixon et al.32 included studies from 2000 to 2011 and reported that remission or improvement in diabetes ranged from 53% to 70%, with consistent results in patients with sustained weight loss, but there were few high-quality and long-term studies.
Roux-en-Y-Gastric BypassRoux-en-Y Gastric Bypass (RYGB), with different lengths of the alimentary and biliopancreatic limbs, has been one of the most reported interventions for the surgical resolution of T2D, and its safety and long-term efficacy has been well established. Two large case-series studies by Pories et al.6 and Schauer et al.22, described return to “normal” HbA1c levels without hypoglycemic medications, in 91% and 83% of patients, respectively, yet these series included patients with prediabetes. The multicenter Swedish Obese Subjects (SOS) study,71 which compared three types of surgery versus medical treatment, reported the highest weight loss in patients with RYGB and a 2-year recovery rate from diabetes significantly higher (73%), although it decreased at 10-year follow-up to 36%.25
Variations in intestinal limb lengths have often made comparison and assessment of operation outcomes difficult. Two recent reviews72,73 aimed to compare the effect of different limb lengths on weight loss: Orci et al.72 observed a trend to support that a longer Roux-limb would be more efficient in superobese patients. Stefanidis et al.73 described that a Roux-limb of at least 150cm was associated with a modest weight loss advantage in the short-term in superobese patients, but no significant impact was seen in patients with BMI<50kg/m2; they focused on the common channel as the way to achieve a significant weight loss due to malabsorption, promoting a length of at least 100cm.
However, debate exists on whether or not the length of intestinal limbs may affect T2D remission rates, to such an extent, that some surgeons have empirically, and not evidence-based, attempted to use different measures and distinguish between bariatric and metabolic surgery.
Sleeve gastrectomySleeve gastrectomy (SG) was initially conceived as a merely restrictive procedure, but has gained popularity over recent years. Ongoing observations have described its efficacy for T2D improvement, mainly because, apart from its effect on reduced food intake, several physiologic mechanisms develop, such as reduction of ghrelin secretion due to resection of the gastric fundus, and early incretin stimulation due to rapid gastric emptying.46–48 Additionally, the easiness of its laparoscopic performance and its low rate of complications has prompted its use as a first-step intervention in extremely obese patients, whose surgical risk is high and T2D control is poor. Thus, data is becoming more available.
Vidal et al.50 reported resolution of T2D (defined as fasting plasma glucose <126mg/dl and HbA1c in the normal range, in the absence of hypoglycemic treatment) at 12 months in 85% of the 39 patients whose mean BMI and HbA1c was 52kg/m2 and 7.4%, respectively, without significant differences compared to gastric bypass. Benaiges et al.,48 also found equivalent results between SG and RYGB, with T2D remission rates around 60%. On the other hand, in the randomized controlled trial by Lee et al.,51 only 14 of 30 patients (47%) achieved remission after SG, yet their mean preoperative BMI was 30kg/m2 and their mean HbA1c level was 10%. Recently, Abbatini et al.47 evaluated long-term remission of T2D after SG in 33 patients with mean BMI of 52kg/m2 and mean HbA1c 7.3%. They reported discontinuation of diabetes medications in 89% of patients at 3 months, 85% at 36 months, and 77% at 60 months, remarking the benefit of SG for the treatment of T2D. There is insufficient evidence to recommend SG over RYGB or BPD, and, again, patients’ preoperative features, including their T2D conditions, will influence surgical outcome.
Biliopancreatic diversionBiliopancreatic diversion and other variations, such as duodenal switch and single-anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S), have been reported to be the most effective procedures for resolution of T2D,7,21,24,43–45 with rates reaching more than 90%. Buchwald et al., in their metaanalysis of 20047 and 2009,21 reported remission rates in 98% and 95% of patients, respectively, and these rates have been observed to be maintained for several years.24,74 However, attention must be drawn to the fact that these malabsorptive procedures may lead to undesirable adverse effects such as vitamin deficiency and risk of malnutrition.
Novel techniquesNovel techniques have been recently attempted, conceived as primarily antidiabetic, such as duodeno-jejunal bypass, ileal interposition and endoluminal duodeno-jejunal sleeve, with promising results in the short-term evaluation.58
What can we expect on the long-term follow-up?The promising results for the short and mid-term follow up may be jeopardized when patients are evaluated in the long-term. In fact, different case series have reported a rate of T2D recurrence of 50–90% 5 years or more after metabolic surgery.25,33,41,75–78 T2D relapse may be explained by unfavorable preoperative factors, inadequate weight loss and weight regain, lack of patient's commitment to treatment and follow-up, failure of the surgical technique, and inadequate incretin stimulation following surgery.56 It is probable that a publication bias exists, with studies that obtain disappointing results not being reported.
Management of T2D relapse after metabolic surgery may be challenging and has not been thoroughly evaluated. Anatomic modifications determine pathophysiological changes that may alter the effects of oral hypoglycemic medications and increase insulin sensitivity.79 The presence of weight regain may be helpful to rule out pancreatic beta-cell exhaustion, and may guide us into the best treatment alternatives. However, there are no evidence-based data to make clinical recommendations. Increase in the number of metabolic procedures performed and long-term follow-up will provide additional data regarding the best approach in these cases.
What happens with those who do not achieve diabetes remission?As it has been previously outlined, stringent criteria have lowered diabetes remission rates. Furthermore, observational studies with mid and long-term follow-up have shown an increased number of patients who do not strictly fulfill these criteria, or in whom T2D reappears after an initial amelioration. This was observed, for example, in the SOS study, where T2D remission rates went from around 70% at 2 years, to around 30% at 10 years, basically due to weight regain.
However, defining an individual as “non-remitter” of diabetes according to biochemical criteria should not underestimate the role that metabolic surgery plays in cases with poorly controlled and long-standing T2D. Indeed, these patients benefit from significant reductions in FG and HbA1c levels, as well as form decreasing insulin requirements and use of hypoglycemic medications. Likewise, the number of patients who reach an optimum combined metabolic control, as defined by ADA's target recommendations15 (HbA1c<7%, LDLc<100mg/dL, tryglicerides<150mg/dL and HDLc>40mg/dL in male or >50mg/dL in female), significantly increases, with barely any difference between “remitters” and “non-remitters”82. Furthermore, since hypertension is also improved or even resolved after metabolic surgery, ADA's target blood pressure levels15 (<140/80mmHg) may be more easily achieved, yet amelioration of hypertension is less obvious, due to other pathogenic mechanisms involved. And also, preliminary analysis point out that bariatric surgery appears to be cost-effective, although long-term data are still needed.80
For these reasons, even though a patient could be initially considered as a poor candidate for T2D resolution after bariatric surgery owing to, for example, his long diabetes duration, high insulin requirements and previous deficient control, this possibility should not be disregarded. In fact, quite the opposite: indication for surgical procedures should be taken into account, since overall metabolic improvement, which is the foremost goal of bariatric surgery, may be attained, with little surgical risk.21,25,34
Final considerationsThe impact of bariatric surgery for the treatment of diabetes and other obesity-associated comorbidities has led to widespread of the term metabolic surgery to describe interventions that aim at improving these diseases. T2D remission rates have varied over a wide range across published studies, mainly due to heterogeneity in criteria used for definition, patients’ baseline characteristics, and the type of procedure performed. A valid estimate would be to establish remission of diabetes in around 50% of patients, yet these observations can only apply in the short-term evaluation (up to 2 years of follow-up).
Even in “non-remitters”, surgery allows and overall benefit regarding combined metabolic control. Thus, T2D remission should not be aimed as the foremost goal, to the extent of developing new types of interventions that would risk nutritional health status. Correct metabolic control using the least number of medications as possible should be thought of as enough, and therefore regarded as the overall core objective if patient's quality of life improves.
In this way, metabolic surgery should not be applied only to those with established T2D diagnosis or a specific BMI cut-off level, but also to patients with further signs of metabolic syndrome, such as insulin resistance, arterial hypertension, hyperlipemia, hyperuricemia, liver steatosis, polycystic ovarian syndrome and endothelial disease, amongst others. These patients benefit as well from the impact of surgery on cardio-metabolic comorbidities.
It would be desirable that scientific societies dealing with T2D and metabolic surgery elaborated a consensus document in which guidelines for eligibility for surgical procedures were addressed for patients with T2D and different BMI categories, and universal criteria for evaluation, both in the short and long-term, were also established.
Conflict of interest disclosure statementThe authors declare no conflict of interest.
We acknowledge grant support from the Fundación Mutua Madrileña de Investigación BiomédicaAP 89592011.