Thiopurine immunomodulators are the most commonly used immunosuppressants in inflammatory bowel disease.
AimsTo evaluate the incidence of adverse events (AE) in patients with inflammatory bowel disease treated with azathioprine (AZA) or 6-mercaptopurine (MP) in our hospital, the features of these effects, the distribution of socio-demographic factors, and the possible predisposing factors.
MethodsWe included 377 patients with inflammatory bowel disease who were diagnosed through 2008 and who received AZA or MP during the course of their disease. We collected retrospective demographic, clinical, and laboratory data about their disease and detailed information on any AE.
ResultsFifty-one patients had some form of AE with AZA or MP (13.5%) and 11% discontinued therapy because of toxicity. Statistically significant association with Crohn's disease was found (P=.008). Myelotoxicity occurred in 18 patients (4.8%) with a mean time of laboratory abnormalities appearing after 16 months. Nine patients had hepatotoxicity secondary to these drugs (2.4%); one of them developed nodular regenerative hyperplasia and portal hypertension. Ten patients had acute pancreatitis (2.7%) with a mean time occurrence of 27 days and a statistically significant association with Crohn's disease (P=.03) and smoking (P=.01). Fifteen patients had gastrointestinal intolerance (4%) but 5 were able to continue with medication given in divided doses or switching to MP.
ConclusionsThiopurine immunomodulators have a significant percentage of AE (13.5%), which, although usually mild, forced us to follow up all cases and sometimes even suspend treatment.
Las tiopurinas son los inmunosupresores más utilizados para el tratamiento de la enfermedad inflamatoria intestinal.
ObjetivosEvaluar la incidencia de eventos adversos (EA) en pacientes con enfermedad inflamatoria intestinal tratados con azatioprina (AZA) o con 6-mercaptopurina (MP) en nuestro hospital, las características de dichos efectos, la distribución de los factores socio-demográficos y los posibles factores predisponentes.
MétodosSe incluyeron 377 pacientes con enfermedad inflamatoria intestinal que fueron diagnosticados hasta 2008 y que recibieron AZA o MP durante el curso de su enfermedad. Se recogieron retrospectivamente datos demográficos, clínicos y de laboratorio sobre su enfermedad e información detallada sobre cualquier EA.
ResultadosCincuenta y un pacientes tuvieron algún tipo de EA con AZA o MP (13,5%), y el 11% suspendieron el tratamiento por toxicidad. Se observó una asociación estadísticamente significativa con la enfermedad de Crohn (p=0,008). Hubo mielotoxicidad en 18 pacientes (4,8%) con un tiempo medio de aparición de las anomalías en los análisis de laboratorio de 16 meses. Nueve pacientes presentaron toxicidad hepática secundaria a estos fármacos (2,4%), uno de ellos desarrolló hiperplasia nodular regenerativa e hipertensión portal. Diez pacientes sufrieron pancreatitis aguda (2,7%) con un tiempo medio de aparición de 27 días y una asociación estadísticamente significativa con la enfermedad de Crohn (p=0,03) y tabaquismo (p=0,01). Quince pacientes presentaron intolerancia gastrointestinal (4%), pero cinco pudieron continuar con la medicación administrada en dosis fraccionadas o tras cambiar a MP.
ConclusionesLas tiopurinas presentan un porcentaje significativo de EA (13,5%), que si bien suelen ser leves, nos obligan a hacer un seguimiento de todos los casos y, en algunos incluso a suspender el tratamiento.
Treatment of patients with inflammatory bowel disease (IBD) depends on several factors including disease location, severity, and complications. Therefore, treatment should be individualized and based on clinical response and tolerance to specific medical therapies.1
Azathioprine (AZA) and its metabolite 6-mercaptopurine (MP) are analogues of purines with immunosuppressive activity, which are used to treat various autoimmune diseases. AZA is a prodrug that is rapidly and completely converted into MP inside erythrocytes and other tissues by a non-enzymatic process. MP can be metabolized via methylation by thiopurine methyltransferase (TPMT), oxidized through xanthine oxidase to tiouric acid, or nucleotide catabolized to 6-thioguanine (6-TGN) by hypoxanthine-guanine-phosphoribosyl transferase.1–3 In sum, the effect of AZA and MP is the result of intracellular conversion to 6-TGN, which is fundamentally responsible for the activity of these drugs through its incorporation in DNA and RNA of leukocytes as fraudulent bases.1–4 Recent data suggest, however, that these nucleotides also have an immune effect through its blocking action of T lymphocytes co-stimulation and induction of its apoptosis.1,4
The dose of AZA and MP is generally adjusted based on patient weight in order to reach maximum therapeutic efficacy while reducing the incidence of adverse events (AE), although this is not always achieved. Various strategies have been suggested to monitor the dose individually and thus identify patients at increased risk of toxicity and those with subtherapeutic dose and insufficient immunosuppression. Among these strategies are the determination of changes in mean corpuscular volume, confirming the induction of a particular leukopenia, the quantification of 6-TGN, and monitoring of TPMT activity.2,3
Thiopurine immunomodulators are the most commonly used immunosuppressants in IBD. There is indication for their use in both Crohn's disease (CD) and ulcerative colitis (UC); they are mainly effective in maintaining remission. In clinical practice, AZA and MP are used equally with the only difference being dosing: 2.5mg/kg and 1.5mg/kg, respectively. Their main indications are steroid-dependence, fistulizing disease, prophylactic post-surgery use, and the treatment of extraintestinal manifestations. They reach efficacy after approximately 3 months of treatment and the slowness of action lessens their usefulness in severe acute patients.1,4
The major limitation for AZA/MP use comes from AE, both short and long-term, occurring in about 15% of cases. This toxicity will lead to suspension of treatment in about 10% of patients.1,5 The most important AE are myelosuppression, hepatotoxicity, acute pancreatitis, gastrointestinal intolerance, infections, and allergic and nonspecific reactions.1,4–7 They can be classified into 2 categories: idiosyncratic dose-independent reactions and those from non-allergic dose-dependent mechanism.1,7,8
The aim of this study was to evaluate the incidence of AE in IBD patients treated with AZA or MP in our hospital, the nature and seriousness of these effects, the distribution of socio-demographic factors in the patients who suffer these AE, and the possible predisposing factors.
There are few articles that specifically discuss thiopurine AE and most of these articles include a small number of patients. On the other hand, it is more common to find studies about one particular thiopurine adverse effect, but this work tries to compile all the different AE directly caused by the drug found in a large sample and for a long period of follow up.
MethodsWe included all patients diagnosed with IBD through 2008 who are taking or have taken AZA or MP during the course of their disease. Demographic, clinical, and laboratory data of these patients were collected retrospectively (by reviewing clinical documents and in some cases through personal interview), focusing on their disease and more in detail on the immunosuppressive drug AE (type of immunosuppressive and dose, time to onset of side effects, severity, evolution, withdrawal or reinstatement of the drug, concomitant therapy).
According to the behavior and the extent of their disease, the patients were stratified using the Montreal classification. UC was subdivided according to the extension (E) in proctitis (E1), left colitis (E2) and extensive colitis or pancolitis (E3). CD was divided by the age at diagnosis (A1 if <16 years, A2, 17 to 40 years and A3 if >40 years), depending on the location (L1: terminal ileum, L2: colon, L3: ileocolic and L4: upper gastrointestinal tract) and according to the clinical pattern in inflammatory (B1), stricturing (B2) or fistulizing (B3).9
We did not systematically assess the TPMT activity in all patients treated with thiopurines. Usually, at the begining of the treatment, the monitoring was performed at 2 weeks, at 4 weeks, at 8 weeks, and then each 3-6 months.
Definitions:
- —
Myelotoxicity: white blood cells <3,000/mm3 and/or platelets <100,000/mm3 and/or hemoglobin <10g/dl.1,4,5,7,10
- —
Neutropenia: neutrophil <1,500/mm3.11
- —
Hepatotoxicity: increase of over 2 times the upper limit of normal (N) in ALT or conjugate bilirubin or a combined increase in AST, total bilirubin, alkaline phosphatase, provided one of them is above 2N. The increase in AST, ALT, alkaline phosphatase or total bilirubin between N and 2N was considered abnormal liver profile but was not even considered hepatotoxicity.12,13
- —
Acute pancreatitis: abdominal pain usually accompanied by nausea and/or vomiting associated with increased amylase and/or lipase in blood.
- —
Trimodal distribution of TPMT activity: low if <5 U/ml, intermediate between 5 and 13.7 U/ml and high if >13.7 U/ml3.
Statistical analysis: For quantitative variables, the mean and standard deviation were calculated, (the variables that did not follow a normal distribution by the Kolmogorov-Smirnov were defined with median, minimum, maximum and interquartile range), and qualitative variables were expressed as percentages with 95% confidence intervals. A P value <.05 was considered statistically significant. In univariate analysis, categorical variables were compared using the chi-square test and quantitative variables using the appropriate test (Student t test, Wilcoxon, etc, depending on whether or not their values followed a normal distribution). A model of unconditional logistic regression was created for the study of some variables. The dependent variable was the presence or absence of AE and then set each of them separately, and the independent variables were age, sex, smoking habit and type of IBD.
ResultsThe number of patients who received AZA/MP was 377. One hundred and seventy-seven were men (47%) and 199 women (53%), with an overall mean age of 43±15 years (range 14 to 89 years). The most common type of IBD was CD with 55%, UC with 42% and finally indeterminative colitis in 3% of the patients.
Referring to the classification of extension in UC we meet 25% proctitis, 41% of left colitis and 34% extensive colitis. The distribution of CD in our sample according to the Montreal classification is detailed in Table 1.
348 patients were treated with AZA and 44 with MP (8 of them had MP in second intention). In the majority of patients the recommended doses of these drugs were used, that are 2.5mg/kg in the case of AZA and 1.5mg/kg for MP.
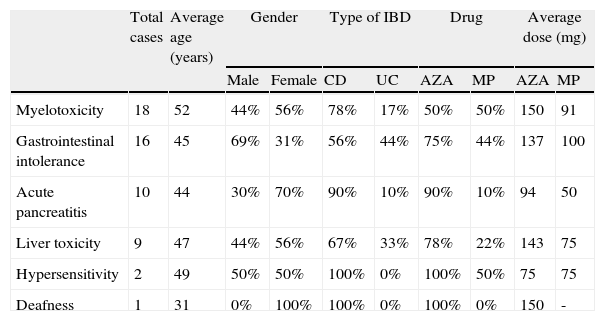
Of the 377 subjects, 51 had some form of AE with AZA or MP (13.5% of the total). We noted myelotoxicity in 18 patients (4.8%), 15 patients had gastrointestinal intolerance (4%), 10 patients had acute pancreatitis (2.7%) and 9 patients had hepatotoxicity secondary to these drugs (2.4%). Three more patients had rare AE and they all pointed to the withdrawal of treatment: One had a hypersensitivity reaction with skin rash, another suffered severe asthenia with AZA and then polyarthralgia and fever when trying to switch to MP, and the last patient presented with bilateral deafness. We detailed the characteristics of all these subjects in Table 2. No neoplasm or isolated infections were registered.
Sociodemographic data and distribution of patients with adverse events.
| Total cases | Average age (years) | Gender | Type of IBD | Drug | Average dose (mg) | |||||
| Male | Female | CD | UC | AZA | MP | AZA | MP | |||
| Myelotoxicity | 18 | 52 | 44% | 56% | 78% | 17% | 50% | 50% | 150 | 91 |
| Gastrointestinal intolerance | 16 | 45 | 69% | 31% | 56% | 44% | 75% | 44% | 137 | 100 |
| Acute pancreatitis | 10 | 44 | 30% | 70% | 90% | 10% | 90% | 10% | 94 | 50 |
| Liver toxicity | 9 | 47 | 44% | 56% | 67% | 33% | 78% | 22% | 143 | 75 |
| Hypersensitivity | 2 | 49 | 50% | 50% | 100% | 0% | 100% | 50% | 75 | 75 |
| Deafness | 1 | 31 | 0% | 100% | 100% | 0% | 100% | 0% | 150 | - |
AZA: azathioprine; CD: Crohn¿s disease; IBD: inflammatory bowel disease; MP: 6-mercaptopurine; UC: ulcerative colitis.
Of the 51 patients with AE, 84% discontinued the therapy because of toxicity (11% of total) and only 16% continued with AZA or MP.
The average age of the patients who developed AE was higher than the age of those who tolerated AZA/MP (48 vs. 43 years; P<.05).
In the univariate analysis there were no significant differences for the occurrence of AE according to the sex (P=0.98) but there was significant difference for the fact of having CD (P=.008).
The multivariate analysis showed that the occurrence of AE was not related to sex or smoking behavior, but it was with age (odds ratio [OR] 1.03; 95% confidence interval (CI): 1.01-1.05; P=.003) and CD (OR 3.2; 95% CI: 1.6 – 6.4; P=.001).
The data found regarding the principal AE will be detailed separately.
MyelotoxicityBone marrow toxicity was secondary to AZA in 9 cases (with an average dose of 150mg daily) and in 9 more patients, it was secondary to MP (with an average dose of the drug of 90mg daily). The time when laboratory abnormalities appeared from the start of treatment with AZA/MP ranged from 15 days to 10 years, with a mean time of 16 months.
Sixty-five percent of patients remained asymptomatic and 71% did not require any treatment, 2 required transfusions of packed red blood cells to treat anemia, 2 other patients needed granulocyte colony stimulating factor and 3 required antibiotics. Six patients required hospital admission (33% of those with myelotoxicity), with a mean stay of 18±14 days. Two of them in an intensive care unit, i.e. 11%, without fatal complications in any case. The intensive care unit admission of these patients was 30 and 26 days respectively; one case had bilateral interstitial pneumonia associated with severe respiratory failure, leukopenia and neutropenia while the other one consisted of cytomegalovirus pneumonia and significant pancytopenia (which required intravenous therapy with vancomycin, imipenem, ganciclovir and granulocyte colony stimulating factor).
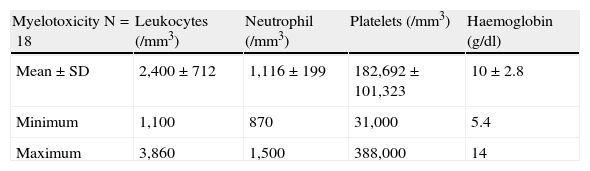
All patients had leukopenia and/or neutropenia. Thrombocytopenia was associated in the 23% of patients and anemia in the 69%. The most representative analytical figures are detailed in Table 3.
In all cases of myelotoxicity secondary to AZA the medication was discontinued, while 2 of 9 patients treated with MP the toxicity was solved by reducing the drug dose. The average time for analytic normalization ranged from 3 days to 8 months, with a median of 1 month.
Fourteen of 18 patients were determined to have TPMT activity, with a mean of 20±6 U/ml and a minimum figure of 10. None had low activity, only 14% had intermediate and 86% of patients had high activity of the enzyme.
Regarding other concomitant treatments among the 18 subjects, 6 were taking aminosalicylates, 9 were taking oral corticosteroids, and 2 ciprofloxacin.
Multivariate analysis demonstrated a statistically significant association between myelotoxicity and age (OR 1.05; 95% CI: 1.02-1.09; P=.002) and CD (OR 5.1; 95% CI: 1.5-17; P=.009). There were no significant differences in AZA or MP doses among patients with CD and UC.
Liver injuryNine patients had liver toxicity and none of them referred alcohol consumption. Seven cases were secondary to AZA and 2 cases were secondary to MP. The mean time for the appearance of laboratory abnormalities in their liver profile was 11±27 months (range 3 days to 82 months).
Medication was discontinued in 6 patients, while in 2 cases their was a dose reduction to resolve toxicity (one case was solved analytically and was able to continue treatment, while the other discontinued medication). Another patient's liver profile normalized spontaneously within 15 days without dose modification and treatment with AZA was continued without further AE.
Sixty-seven percent of patients with hepatotoxicity remained asymptomatic. Two patients experienced nausea and vomiting, which resolved upon discontinuation of medication. One patient had an episode of jaundice, choluria and acholia and was the only one who required hospitalization; serology for hepatitis C virus was positive, but did not require specific treatment. Aside from the previous patient, all others were serologically negative for hepatitis B and C virus.
In all patients, hepatotoxicity was finally resolved, except in one patient who had alterations in liver profile after discontinuing AZA. Therefore, he was evaluated using abdominal magnetic resonance imaging and an upper endoscopy, which revealed signs of portal hypertension. His serologies and autoimmune markers were negative and finally underwent a liver biopsy that was compatible with the diagnosis of nodular regenerative hyperplasia (NRH). All patients had a prothrombin activity within the normal range.
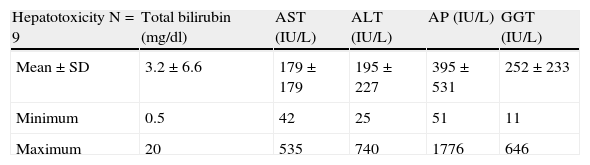
The analytical figures reached in the liver profile in these subjects are summarized in Table 4.
Detailed analytical figures of cases of liver toxicity.
| Hepatotoxicity N=9 | Total bilirubin (mg/dl) | AST (IU/L) | ALT (IU/L) | AP (IU/L) | GGT (IU/L) |
| Mean±SD | 3.2±6.6 | 179±179 | 195±227 | 395±531 | 252±233 |
| Minimum | 0.5 | 42 | 25 | 51 | 11 |
| Maximum | 20 | 535 | 740 | 1776 | 646 |
ALT: alanine aminotransferase; AP: alkaline phosphatase; AST: aspartate aminotransferase; GGT: γ-glutamyl transferase; SD: standard deviation.
Thirty-three percent of the patients were taking concomitant aminosalicylates to the thiopurine immunomodulator, one of them received metronidazole and 44% were taking oral corticosteroids.
Acute pancreatitisOf the 10 patients with acute pancreatitis secondary to AZA or MP, 80% were smokers and none had alcohol consumption or gallstones.
The univariate analysis showed a statistically significant association between the occurrence of acute pancreatitis and the diagnosis of CD (P=.03). The multivariate analysis found no relationship between the onset of pancreatitis and sex, age, or the CD (OR 3.5; 95% CI: 0.4-31; P=.2), but an association was found with smoking habit (OR 8.4; 95% CI: 1.6-42; P=.01).
Nine patients with acute pancreatitis were taking AZA and only one took MP. The time of occurrence of the adverse event since the patient began taking the medication was mostly less than one month, with a mean time of 27±18 days. Eight of these patients required hospitalization and all exhibited typical symptoms of pancreatitis, such as nausea and vomiting accompanied by abdominal pain. None of these patients developed severe pancreatitis or complications. All patients stopped the immunomodulating medication, resolving the event, with a mean hospital stay of 8±5 days.
Five of 10 patients took oral corticosteroids as concomitant treatment and 4 of them received mesalazine. None were taking sulfasalazine.
Gastrointestinal intoleranceFifteen patients had symptoms of gastrointestinal intolerance, primarily in the form of nausea and nonspecific abdominal symptoms. None of them required hospitalization.
Eighty percent of these patients had gastrointestinal intolerance to AZA and the 47% had this adverse event secondary to the intake of MP (therefore, 27% had an intolerance to both drugs). The mean time to onset of symptoms was less than a month, from 24±21 days with a range from 4 days to 2 months.
In 5 patients an attempt to control the symptoms was done by dividing the dose, in 2 of these 5 patients there was a reduction in the drug dose. From this small group of patients, only 2 were able to continue treatment and resolved the adverse event, the rest had to discontinue the medication. In 3 other cases, an attempt to solve the toxicity was done by decreasing the dose of the immunomodulator; we achieved this in one case.
In the 5 patients with gastrointestinal intolerance to AZA we attempted to switch to MP. Three of them had the same symptoms with the second drug, and finally had to stop both medications, while the other 2 tolerated MP and continued.
Regarding other concomitant treatments when the adverse event occurred, 87% of these patients took oral corticosteroids and 20% were receiving aminosalicylates.
Other adverse effectsOther less common AE occurred in 3 patients. Two of them presented with hypersensitivity reactions after about a month of treatment in the form of skin rash, fatigue or arthralgia, and the last patient had an episode of bilateral deafness at 6 months after starting treatment with AZA daily. In all cases, medication was discontinued and the symptoms resolved (the patient with bilateral deafness took 2 years to fully restore hearing).
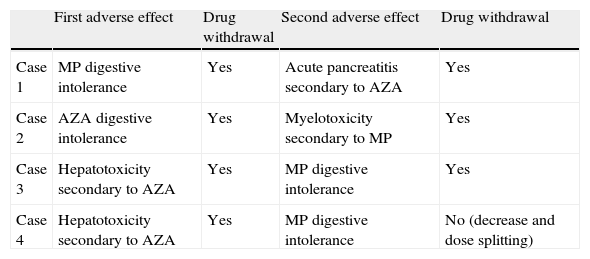
In addition, 4 patients with digestive intolerance had a different AE when trying to change the AZA to MP or vice versa. In the Table 5 is detailed what occurred to these patients.
Patients with more than one type of adverse event.
| First adverse effect | Drug withdrawal | Second adverse effect | Drug withdrawal | |
| Case 1 | MP digestive intolerance | Yes | Acute pancreatitis secondary to AZA | Yes |
| Case 2 | AZA digestive intolerance | Yes | Myelotoxicity secondary to MP | Yes |
| Case 3 | Hepatotoxicity secondary to AZA | Yes | MP digestive intolerance | Yes |
| Case 4 | Hepatotoxicity secondary to AZA | Yes | MP digestive intolerance | No (decrease and dose splitting) |
AZA: azathioprine; MP: 6-mercaptopurine.
Despite the effectiveness of thiopurine immunomodulators its use is limited by its toxicity. Present et al. in their article regarding MP toxicity, found 7.6% of AE induced directly by the drug from 396 patients6; however, in other series this percentage reaches 30% in adults7 and 46% in children.14 These numbers vary depending on the ranges used by each author to define each adverse event (for example, number of leukocytes) and, of course, on how many of these are included.5,6,8,15,16
Three hundred and seventy-seven patients were included in our study, of which 13.5% had AE and most of them (11%) had to discontinue medication for this reason. The percentage of medication withdrawal is a very important factor for the clinical practice and varies from 8.9% to 18% in the different series.1,4,5,14,15
Subjects with some toxicity had a significantly higher age than the ones who showed no side effects to AZA/MP (48 vs. 43 years old). The multivariate analysis also noticed the occurrence of toxicity related to age, but with an OR close to 1. This relationship with age is not reported in other studies and there even exists high rates of AE in pediatric and teenage patients, thus the clinical relevance of this association is uncertain. The relationship found in our study with CD, with an OR of 3.2, seemed to be most relevant, although in previous literature there is some controversy. Present et al.6 found a higher incidence of AE in CD as in our work, Mart’inez et al.7 found a significant association with UC in a small series of 92 cases, and Saibeni et al.4 found no differences between both types of IBD.
Myelotoxicity occurred in 4.8% of patients in our sample. In other studies this figure varied between 1.7% and 11%5–8,10,11,14,17 depending on the definition of leukopenia (below 4,000, 3,500 or 3,000 leukocytes/mm3), since this was in all studies the most common form of presentation.
Treatment time until appearance of myelotoxicity was highly variable, from 15 days to 10 years. This supports the nonallergic mechanism of its appearance, as described by Connell et al.10 in his extensive sample of 760 patients with IBD, in which bone marrow toxicity also occurred with a mean of 16 months and a range from 0.01 to 184 months. This marked variability in the time of myelotoxicity appearance emphasizes the requirement for analytical monitoring of the full blood count in all cases throughout the treatment, independently of the time the patient is taking the thiopurine drug.1,5,10,11 We recommend the first visit 15 days after the initiation of the treatment, a second visit at one month, the third at 2 months, and then a visit each 3-6 months with clinical and analytical monitoring.
In our sample, there was no relationship found between myelotoxicity or its severity and TPMT, as none of the patients with bone marrow toxicity had a low activity of this enzyme and only 14% had intermediate activity (not finding among them the patients that required hospital admission or intensive care unit stay). The lower activity of this enzyme may be responsible for myelosuppression induced by AZA/MP in some patients (through metabolic shunt that leads to excessive production of 6-TGN), but in many other patients different factors seem to be involved.2
In 89% of myelotoxicity cases, immunosuppressive medication was discontinued (2 cases improved by reducing the dose of MP) and the condition was resolved in all patients in a mean time of 1 month. These results are similar to those reflected in other articles, with the time of normalization of laboratory parameters varying from 1 to 16 weeks.6,8,10,11,14
In the multivariate analysis performed for myelotoxicity, a statistically significant association with CD was observed, with an OR of 5.1, indicating that it is 5 times more likely to suffer myelotoxicity in CD than in UC. This relationship is not reported in other studies and the explanation for this finding is unknown, but we found no differences in AZA/MP doses between patients with CD and UC.
Hepatotoxicity occurred in 2.4% of patients and in other articles this rate varied between 0.3% and 14%.5,6,8,12–14,17,18 Most cases were secondary to AZA and the fundamental indication was the steroid dependency, so that almost half of the cases received corticosteroids concomitantly at the time of the side effect occurrence. This could be related to the association observed by Bastida et al. in his work between the appearance of thiopurine-induced hepatotoxicity and concomitant use of corticosteroids.18
The onset of the liver toxicity was highly variable, as in the myelotoxicity, with a mean time of 11 months, and ranged from 3 days to 7 years. In their 2007 review, Gisbert et al. described that most liver toxicity cases occur in the first months of treatment (from 1.5 to 5 months), although in some cases they may appear later.13
Regarding the types of hepatotoxicity, 5 of our patients had laboratory abnormalities in the first weeks of treatment, which might be due to a hypersensitivity syndrome, while in 2 cases there was marked elevation of cholestasis enzymes compared with those of cytolysis, suggesting a idiosyncratic cholestatic reaction. Only one patient developed dose-related hepatotoxicity with fibrosis and NRH in the histological study, starting after 9 months of treatment with 200mg (2.5mg/kg) of AZA daily. Several publications have linked the use of thiopurine immunomodulators in IBD with the appearance of NRH, which is a type of dose-dependent toxicity characterized by the damage of the endothelial cells, the sinusoids, and the hepatic venules. NRH usually occurs between 3 months and 3 years of thiopurine treatment and results in a non-thrombotic occlusion of vessels and the development of fibrosis and portal hypertension. The exact pathogenesis of NRH is unknown but seems to be related to the depletion of glutathione and several studies show an association with the use of AZA, MP and especially with 6-TGN.13,18–25Sixty-seven percent of patients with hepatotoxicity remained completely asymptomatic and only one (who was positive for hepatitis C virus serology) required hospitalization for severe jaundice with total bilirubin levels up to 20mg/dl. All AE were resolved in all patients except in the patient who developed portal hypertension secondary to NRH. These findings indicate that in most patients the liver damage is mild and reversible, although special care must be taken in patients with previous chronic liver disease, and liver biopsy should be considered in patients with persistent liver profile alteration despite the withdrawal of the immunomodulatory drug.
In our study, 2.7% of the patients treated with AZA/MP had a typical clinical presentation of acute pancreatitis with abdominal pain, nausea, and elevated amylase, which is similar to previous publications, where this percentage varies from 1 to 5%.5–8,17,26,27 We found no statistically significant association between the occurrence of pancreatitis and gender or age; however, we found association with smoking habit (OR of 8). With respect to the type of IBD, the diagnosis of CD did not reach statistical significance in multivariate analysis, although an OR of 3.5 was found. This may be due to the small sample size and the consequent low statistical power (note that only one patient with UC presented acute pancreatitis). The association of this adverse event with CD is described in previous articles26,27 and although the pathogenic mechanisms underlying are not clear, it is suggested that there may be a delayed allergic reaction in relation with the production of antibodies, in addition to genetic individual predisposition.27
Drug doses used in the patients who developed pancreatitis were lower than in the rest of toxicities (less than 1.5mg/kg of AZA and less than 1mg/kg of MP) and the time of appearance of the symptoms was less than 1 month in most of the cases, which seems to be related to allergic or idiosyncratic nature, coinciding with previously published articles.5,6,8,14,26–28 Finally, gastrointestinal intolerance was observed in 4% of the patients and although they had no clinical severity, it resulted in a significant percentage of drug suspension. In these cases, however, various strategies are described to prevent this adverse event without directly rejecting the thiopurine option. The easiest thing to do is divide the doses and the most recently studied is the change to MP in cases of intolerance to AZA, as referred to by Bowen et al.29 and Domènech et al.,30 both with very good results in tolerance to the second drug (over 50%). In our study, these strategies worked in the 40% of the patients. It seems that both actions are valid and successful, so they should be considered before discontinuing a drug that can provide major benefits for IBD.
In conclusion, the thiopurine immunomodulators are agents of particular usefulness in IBD, but with a significant percentage of AE, which, although usually mild, force us to follow up in all cases and sometimes even suspend the treatment.
FundingCIBEREHD is funded by the Instituto de Salud Carlos III.
Conflict of interestThe autors declare not to have any conflict of interest.