Rapid recovery (RP) in total knee arthroplasty may increase the functionality while reducing costs. The aim of this study is to prove the benefits of a rapid recovery programme compared to our classic protocol.
Patients and methodsWe performed a RCT (NCT03823573) in patients undergoing otal knee arthroplasty. Intervention group (RP protocol) received local infiltration of levo-bupivacaine in the periarticular tissue and supervized ambulation 4–6h after surgery. Control (C) group received a femoral nerve block with levo-bupivacaine, while a drain was used. Ambulation after its removal.
All the patients completed an Oxford Knee Score prior to surgery and 6 months after discharge. An ecodoppler to assess the presence of deep vein thrombosis was made 1 month after discharge. Minimum follow-up was of 6 months.
ResultsA total of 175 patients were included in the trial (92 patients in the control group, 83 patients in the RP group). There were no differences in sex, age, implanted prosthesis, haemoglobin drop, need for transfusion, range of motion on discharge (C: 82.6°, RP: 85°) and at the end of the follow-up (C: 105.1, RP: 106.6), Oxford knee score improvement (C: 17.5 points; RP: 19.3 points), patient satisfaction or re-admissions at the emergency department (C: 7.6%; RP: 10.8%).
Significancy was found on time of ischaemia (C: 81.29min; RP: 85.35min; p=.03), need for morphine shots (C: 19.7%; RP: 38.6%; p=.007), hospital stay (C: 3.84 days; RP: 2.54 days, p<.0001) and time until ambulation (C: 2.46 days; RP: 0.23 days; p<.0001).
ConclusionRapid recovery protocols can reduce hospital stay without increasing complications or need for re-admission.
Los programas de recuperación precoz (rapid recovery [RP]) en artroplastia total de rodilla pueden mejorar la funcionalidad a la vez que se reducen los costes. El objetivo del estudio es comparar los resultados de un programa de rehabilitación precoz con nuestro protocolo habitual.
Pacientes y métodosSe realizó un ensayo clínico aleatorizado (NCT03823573) en pacientes operados de artroplastia total de rodilla. El grupo intervención (RP) recibió infiltración periarticular con levobupivacaína e inició deambulación supervisada a las 4-6 h tras la intervención. El grupo control (C) empleó drenaje y recibió un bloqueo femoral e inició la deambulación al retirar el drenaje.
Los pacientes completaron un cuestionario Oxford Knee Score preoperatorio y a los 6 meses. La incidencia de trombosis venosa profunda asintomática se analizó mediante eco-doppler. El seguimiento mínimo fue de 6 meses.
ResultadosFueron incluidos 175 pacientes (92 pacientes en el grupo C y 83 en el RP). No hubo diferencias en sexo, edad, tipo de prótesis, descenso de hemoglobina, necesidad de transfusiones, balance articular activo al alta (C: 82,6°; RP: 85°) ni al finalizar el seguimiento (C: 105,1°; RP: 106,6°), mejoría del cuestionario (C: 17,5 puntos; RP: 19,3 puntos), satisfacción del paciente o retenciones hospitalarias (C: 7,6%; RP: 10,8%).
Se observó significación en el tiempo de isquemia (C: 81,29 min; RP: 85,35 min; p=0,03), necesidad de rescate con opioides (C: 19,7%; RP: 38,6%; p=0,007), estancia media (C: 3,84 días; RP: 2,54 días; p<0,0001) y demora en la deambulación (C: 2,46 días; RP: 0,23 días; p<0,0001).
ConclusiónEl protocolo RP puede reducir la estancia hospitalaria sin aumentar las complicaciones ni las retenciones.
As the demand for total knee arthroplasty increases,1 so does the interest in rapid recovery protocols. Although total knee arthroplasty is one of the most successful procedures in orthopaedic surgery,2 postoperative pain and the need for intensive rehabilitation protocols remain a problem. Clinical pathways for rapid recovery require a multidisciplinary approach and are primarily based on pain control and immediate mobilisation after surgery.
The reported benefits of these protocols include shorter hospital stays, reduced costs, and infection rates, and increased joint movement and patient satisfaction.3–9 We found no increased incidence of adverse events or need for readmission.5,6,8,9
Both general and neuraxial anaesthesia should be considered when implementing a rapid recovery protocol, although the latter has a lower incidence of complications and better outcomes.10,11 However, due to the need for early mobilisation, the use of hyperbaric bupivacaine is preferred, as it has earlier reversal of motor block.12 Regarding the prevention of postoperative nausea and vomiting, a combination of 8mg dexamethasone and 4mg ondansetron has been shown to be more effective than using either of them separately.13,14
Postoperative pain management can include the use of NSAIDs, opioids, 15 regional blocks, epidural analgesia, and intraoperative periarticular infiltrations. Multiple nerve blocks (femoral, sciatic and obturator) are more effective for pain control than epidural analgesia and periarticular infiltrations,16 but may affect limb strength, which is a clear disadvantage for early ambulation. Periarticular infiltrations are an alternative to femoral blocks17 while allowing earlier mobilisation than epidural analgesia and with a lower incidence of urinary retention.18 However, local infiltrations sometimes require more rescue analgesia. Long-acting local anaesthetics such as ropivacaine (6h) and levobupivacaine (10h) are useful for this purpose.
Currently, the use of blood salvage agents is not recommended19 and has fallen in favour of local or intravenous administration of tranexamic acid, which has been shown to minimise blood loss.20,21 The use of drainage, although not formally contraindicated, is not supported by the American Academy of Orthopaedic Surgeons, as there is no difference in outcomes or complications.22
The aim of our study was to analyse the improvement in function and admission time after implementing a rapid recovery protocol in our department. This was undertaken by means of a randomised clinical trial compared to the usual or classic protocol used in our institution, a public university hospital under the National Health System, as there is currently no level I evidence in the published literature.
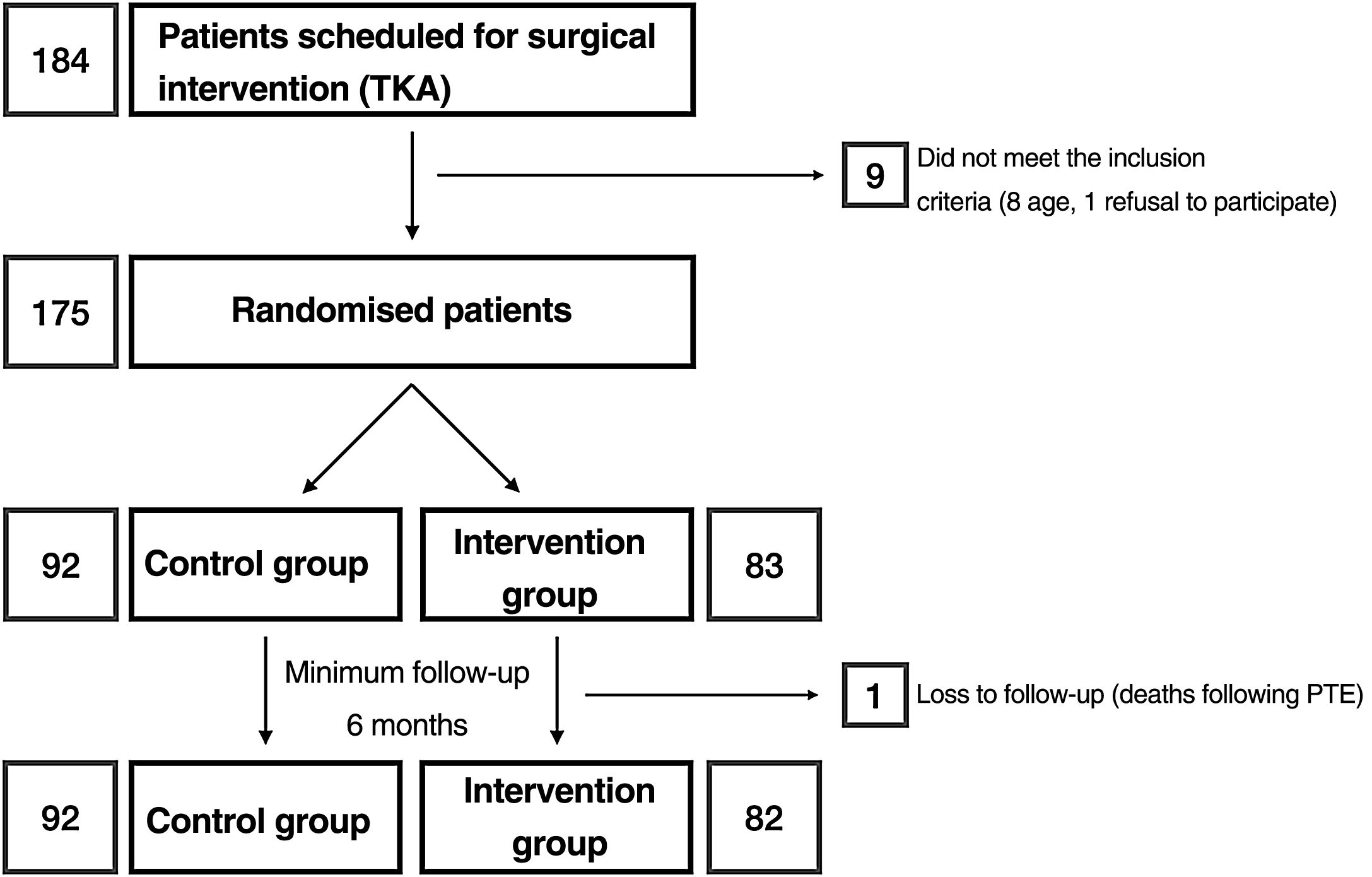
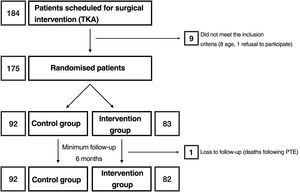
Materials and methodsAfter approval by the Drug Research Ethics Committee (reference number: P1102-16), we conducted a clinical trial (registered at clinicaltrials.gov with reference number: NCT03823573) in which, after obtaining informed consent, patients were randomised into 2 groups: group C and intervention group (RP) (Fig. 1). The sample size was estimated by the department's research support unit. Randomisation was performed using a Microsoft Excel spreadsheet for Mac® (v. 16, Microsoft, Redmond, WA, USA) using the function “=random.between(0;1)” using “0” for controls and “1” for the intervention group. Allocation was concealed using opaque, sealed envelopes with the results, which were opened after informing the patient of their participation in the study prior to scheduling the surgical intervention. Screening started in January 2019 and ended in March 2020. According to the inclusion criteria, all patients between 55 and 80 years of age,23 diagnosed with gonarthrosis and on the hospital waiting list for total knee arthroplasty were considered. This included as a requirement failure of appropriate conservative treatment used for at least 6 months. Patients outside this age range or refusing to participate in the study, those allergic to local anaesthetics, tranexamic acid or with a history of deep vein thrombosis, pulmonary thromboembolism or epilepsy were excluded.
Due to hospital needs, the patients were admitted to the ward on “day 0” (the afternoon before surgery). During that afternoon, the on-call resident or the principal investigator of the study provided the patients in the RP group with the appropriate preoperative information and assessed them according to the preoperative Oxford Knee Score (OKS). Surgery was performed on “day 1” early in the morning to allow for ambulation through the day. All surgeries were performed by a team of 22 specialist surgeons or by residents under their supervision. The use of spinal anaesthesia (0.5% bupivacaine hyperbaric solution) was preferred. General anaesthesia was used in cases where spinal block was not possible. After antibiotic (cefazolin 2g) and antiemetic (8mg dexamethasone and 40mg pantoprazole) prophylaxis, ischaemia was performed using an S-Mart type half-tourniquet (OHK Medical Devices, Newark, NJ, USA), which was removed after closure and bandaging of the limb. Careful haemostasis was performed in both groups. As anti-emetic prophylaxis 4mg ondansetron was administered prior to surgical wound closure.
A standard medial parapatellar approach was used. There are 2 prosthetic models available according to surgeon preference (with CR and PS options): Optetrak Logic® (Exactech, Gainesville, FL, USA) and Persona® (Zimmer Biomet, Warsaw, IN, USA). The NexGen® LPS-Flex prosthesis with Ti-Nidium® surface hardening (Zimmer Biomet, Warsaw, IN, USA) was chosen for patients with documented metal allergies. The tibial tray was cemented in all cases and the femoral component in the posterior-stabilised prostheses, according to the manufacturer's instructions.
Protocol of the intervention group (rapid recovery)Patients assigned to the intervention group underwent periarticular infiltration with a solution of 140mg levobupivacaine in 180mL physiological saline with a 90mm, 22 G spinal needle (Becton Dickinson, Franklin Lakes, NJ, USA) in 20mL syringes, according to the technique described by Quinn24: after testing the test components, 50mL were injected into the posterior capsule (20mL posterior to the medial condyle, 20mL posterior to the lateral condyle and 10mL into the intercondylar notch), 5mL into the medial collateral ligament, 5mL into the lateral collateral ligament and 30mL into the suprapatellar region. After capsular closure, 30mL of the solution was infiltrated into the arthrotomy margins and, after subcutaneous closure, 60mL was infiltrated into the surgical wound margins. Drains were not used in the RP group, as they would prevent early ambulation, although a small-diameter catheter was used to introduce 2g of intra-articular tranexamic acid after surgical wound closure, which was removed before the limb was dressed.
The patients were assessed by a rehabilitation specialist and the physiotherapist 4h after surgery, and started ambulation as tolerated with the aid of 2 crutches. In-hospital rehabilitation also included isometric quadriceps exercises, hip abduction and adduction, and knee flexion and extension.
Control group protocolPatients in group C followed the hospital's classic protocol until the start of the study. In this case, a Redon CH-16 drain was used, through which 2g of tranexamic acid was introduced after capsular and subcutaneous closure. The drain was kept closed for 10min after introducing the tranexamic acid to improve its efficacy. Patients in this group were not infiltrated with local anaesthetic during the operation; however, once in the post-anaesthesia resuscitation unit, the anaesthesiologist administered a femoral block with 20mL of 0.375% levobupivacaine under ultrasound or neurostimulator control, according to the anaesthesiologist's preference. Quadriceps strengthening exercises were started 24h after surgery, while ambulation was started when the drain was removed (per protocol, 24h after surgery, unless high debit) under the supervision of a physiotherapist. In this group, the start of ambulation was conditioned by the use of a drain.
After the surgery, patients in both groups received thromboprophylaxis with bemiparin (3500U every 24h for 30 days), and an elastic compression stocking at the time of the first dressing (24h after surgery). Basic analgesia included paracetamol at a dose of 1g every 8h alternating with 2g of metamizole (dipyrone) every 8h intravenously for the first 48h. After this time, 1g paracetamol every 8h was given orally, alternating with 575mg metamizole every 8h. The number of 4mg morphine chloride rescue boluses administered during the first 24h was recorded as a quantitative measure of perceived pain. Transfusion criteria included a haemoglobin drop below 8.5g/dL if associated with dizziness, headache, hypotension, or tachycardia (per the patient's usual levels). Discharge criteria included: well patient, ambulant, with dry dressing, and no need for intravenous analgesia.
After discharge, all the patients underwent the same rehabilitation programme, in which, after evaluation in the outpatient department, the suitability of a home exercise programme supervised as an outpatient or a rehabilitation programme supervised by the physiotherapist in the hospital facilities was decided (in general, those who did not achieve 90° of flexion in the outpatient department).
One month after discharge, all patients underwent Doppler ultrasound in the radiology department to study the incidence of asymptomatic deep vein thrombosis (DVT). At the end of follow-up (6 months after surgery), a personal interview was conducted to assess postoperative OKS25 and the overall satisfaction of each patient. The patients were also asked about their overall satisfaction with the procedure, which they rated from 0 to 10.26
Study variables and statistical analysisPatient demographics, existence of preoperative varus-valgus, type of prosthesis used, need for blood product transfusion, duration of surgery (or surgical time, defined as ischaemia time), haemoglobin decrease at 24h, and estimated blood loss (according to the formula described by Good27,28), delay in ambulation (not having started ambulation within 24h after removal of the drain or the procedure), delay in ambulation (not having started ambulation within 24h after removal of the drain or the procedure, if no drain), hospital stay, delayed hospital discharge (more than 48h in the RP group and more than 72h in group C after surgery), complications (including incidence of deep vein thrombosis), need for rehabilitation in hospital facilities after discharge, active joint movement at baseline and discharge from the rehabilitation programme, OKS before surgery and at 6 months after discharge, and need for readmission were recorded as the study variables. Data were recorded in a Microsoft Excel for Mac® table (v. 16, Microsoft, Redmond, WA, USA) and statistical analysis of the study (by intention-to-treat) was performed using SPSS Statistics for Mac® (v. 25, IBM, Armonk, NY, USA). Parametric (Student's t-test, χ2, Fisher's exact) and non-parametric (Mann–Whitney U) tests were used as appropriate. Results were considered statistically significant at p<.05.
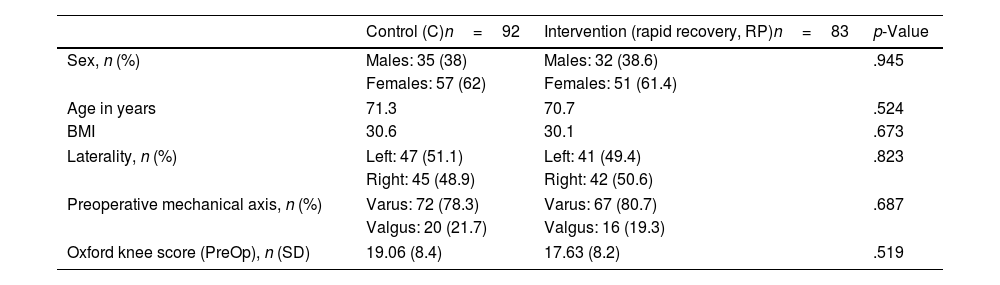
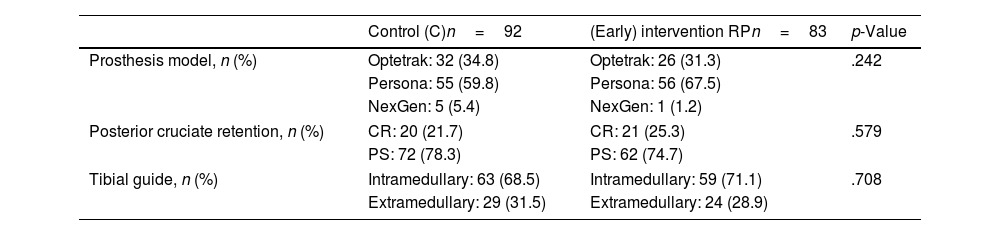
ResultsA total of 175 patients were included in the study and were distributed into group C, 92 patients, and intervention group (RP), 83 patients. The main demographic characteristics are shown in Table 1. No preoperative differences were observed between the groups. No patellar prosthesis was performed in any case. The mean operative time was 83.80min (range: 60–175min; SD: 13.37). For statistical purposes, one patient in group C whose surgery duration was 175min was not included in the analysis due to intraoperative complications: RP (85.35min; 60–115min; 12.26) and C (81.29min; 65–116min; 12.99) with p=.03. Therefore, we estimate the mean time taken to infiltrate local anaesthetic at 4.06min. All patients in the RP group received periarticular infiltration with levobupivacaine. However, only 71.7% of patients in group C (66 patients) had femoral anaesthetic block administered by the anaesthesiologist. Other surgery-dependent variables are included in Table 2.
Demographic and preoperative variables.
| Control (C)n=92 | Intervention (rapid recovery, RP)n=83 | p-Value | |
|---|---|---|---|
| Sex, n (%) | Males: 35 (38) | Males: 32 (38.6) | .945 |
| Females: 57 (62) | Females: 51 (61.4) | ||
| Age in years | 71.3 | 70.7 | .524 |
| BMI | 30.6 | 30.1 | .673 |
| Laterality, n (%) | Left: 47 (51.1) | Left: 41 (49.4) | .823 |
| Right: 45 (48.9) | Right: 42 (50.6) | ||
| Preoperative mechanical axis, n (%) | Varus: 72 (78.3) | Varus: 67 (80.7) | .687 |
| Valgus: 20 (21.7) | Valgus: 16 (19.3) | ||
| Oxford knee score (PreOp), n (SD) | 19.06 (8.4) | 17.63 (8.2) | .519 |
BMI: body mass index; PreOp: preoperative; RP: rapid recovery; SD: standard deviation.
Surgery-related variables.
| Control (C)n=92 | (Early) intervention RPn=83 | p-Value | |
|---|---|---|---|
| Prosthesis model, n (%) | Optetrak: 32 (34.8) | Optetrak: 26 (31.3) | .242 |
| Persona: 55 (59.8) | Persona: 56 (67.5) | ||
| NexGen: 5 (5.4) | NexGen: 1 (1.2) | ||
| Posterior cruciate retention, n (%) | CR: 20 (21.7) | CR: 21 (25.3) | .579 |
| PS: 72 (78.3) | PS: 62 (74.7) | ||
| Tibial guide, n (%) | Intramedullary: 63 (68.5) | Intramedullary: 59 (71.1) | .708 |
| Extramedullary: 29 (31.5) | Extramedullary: 24 (28.9) | ||
CR: cruciate retaining; PS: posterior stabilised; RP: rapid recovery.
The mean haemoglobin drop 24h after surgery was 2.50g/dL for RP and 2.58g/dL for C (p=.685). Estimated blood loss was 109.3mL for RP and 112.2mL for C (p=.514). One patient (1.1%) in C required a blood product transfusion. No transfusion was required in the RP group.
Regarding the need for opioid rescue medication, 38.6% of patients in the RP group required at least one dose of morphine chloride, while only 27.2% of patients in group C received it (p=.079). If we only consider the patients in C who were correctly analogised by the anaesthesiologists by femoral block according to protocol (per protocol analysis), only 19.7% of patients required opioid administration (p=.007).
The time until ambulation was .23 days in RP and 2.46 days in C (p=.003). Of the patients in the RP group, 81.9% started ambulation on the day of the intervention. The main reasons for delayed ambulation were nausea or vomiting, dizziness, pain, and weakness due to persistent motor block. The mean postoperative length of stay was 2.54 days in RP and 3.84 in C (p<.0001). The main causes for delayed discharge were, according to the number of cases: pain (9), holiday (8), delayed treatment by the rehabilitation service (5), dizziness, nausea, and vomiting (5), bleeding from the surgical wound (5), social problems (4), refusal of the patient (3), and other medical disorders (3).
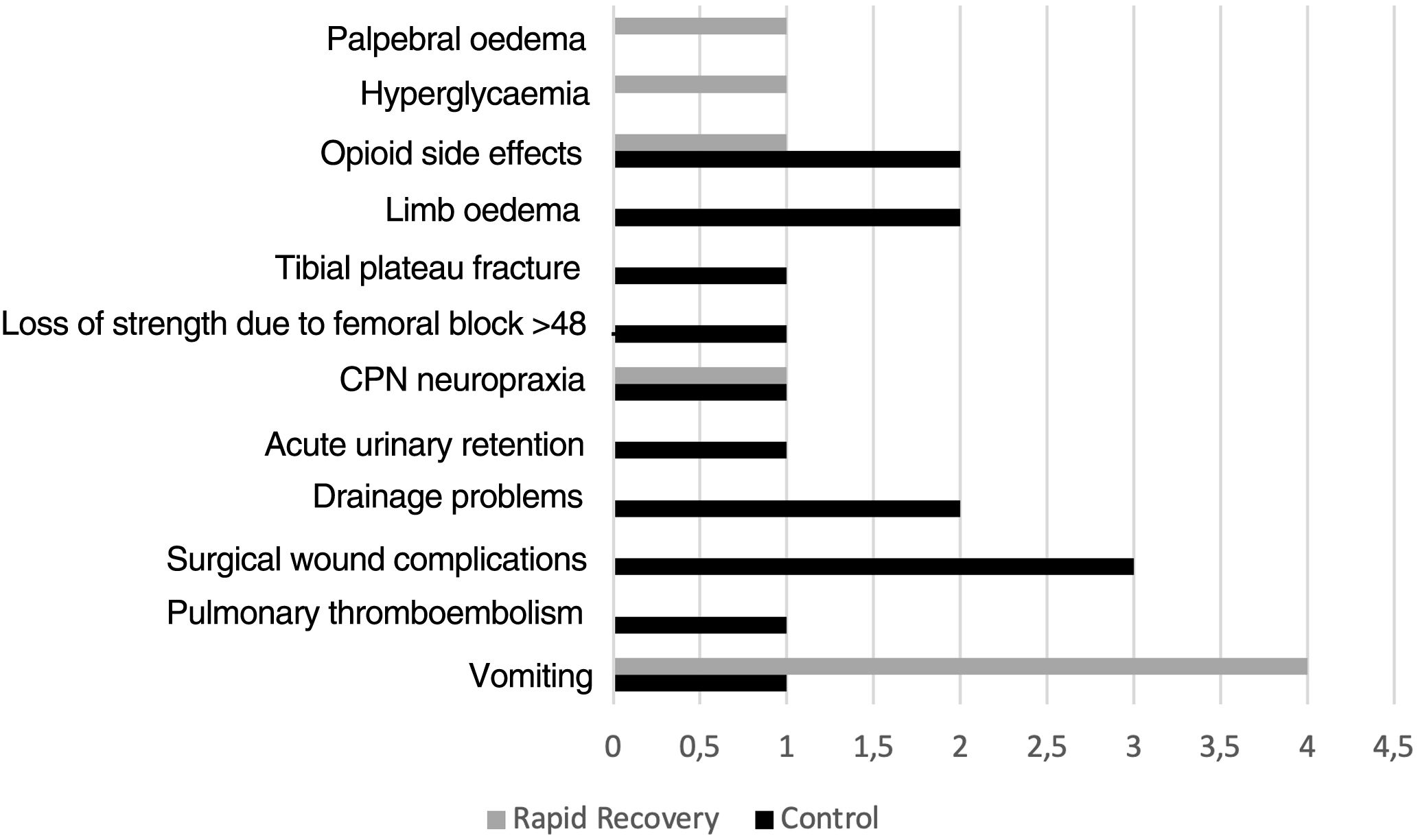
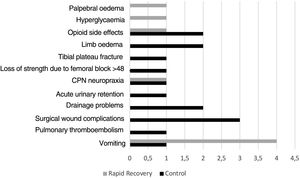
Thirteen patients in group C had complications during admission C (14.1%), whereas there were 8 (9.6%) in group RP (p=.361) (Fig. 2).
After discharge and during follow-up (9.27 months; 6–15.3 months), 7 patients in group C (7.6%) required hospital readmission due to pain (2), swelling/oedema (1), suspected deep vein thrombosis (2, only one confirmed by Doppler ultrasound), acute infection (1), acute stroke (1). In the RP group, 9 patients (10.8%) required rehabilitation: pain (3), haemarthrosis (1), fever (1), dressing allergy (1), delayed surgical wound healing (1), and SARS-CoV-2 infection (1) (p=.458).
Rehabilitation in hospital facilities was required by 40 patients (43.5%) in group C and 30 patients (36.1%) in group RP (p=.502). Those in the RP group required shorter follow-up by the rehabilitation service, although this was not statistically significant (C: 63.3 days; RP: 55.5 days; p=.298). The active joint movement and OKS questionnaires were assessed during follow-up, the results of which are shown in Table 3. When asked about their overall satisfaction, patients in the C group gave a mean score of 8.26, while those in the RP group rated it at 8.20 (p=.856).
Main postoperative variables.
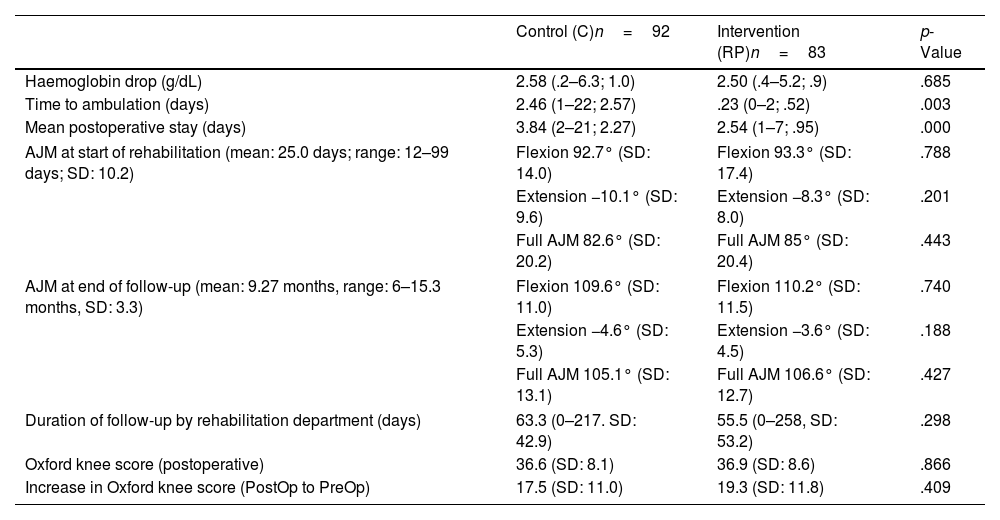
| Control (C)n=92 | Intervention (RP)n=83 | p-Value | |
|---|---|---|---|
| Haemoglobin drop (g/dL) | 2.58 (.2–6.3; 1.0) | 2.50 (.4–5.2; .9) | .685 |
| Time to ambulation (days) | 2.46 (1–22; 2.57) | .23 (0–2; .52) | .003 |
| Mean postoperative stay (days) | 3.84 (2–21; 2.27) | 2.54 (1–7; .95) | .000 |
| AJM at start of rehabilitation (mean: 25.0 days; range: 12–99 days; SD: 10.2) | Flexion 92.7° (SD: 14.0) | Flexion 93.3° (SD: 17.4) | .788 |
| Extension −10.1° (SD: 9.6) | Extension −8.3° (SD: 8.0) | .201 | |
| Full AJM 82.6° (SD: 20.2) | Full AJM 85° (SD: 20.4) | .443 | |
| AJM at end of follow-up (mean: 9.27 months, range: 6–15.3 months, SD: 3.3) | Flexion 109.6° (SD: 11.0) | Flexion 110.2° (SD: 11.5) | .740 |
| Extension −4.6° (SD: 5.3) | Extension −3.6° (SD: 4.5) | .188 | |
| Full AJM 105.1° (SD: 13.1) | Full AJM 106.6° (SD: 12.7) | .427 | |
| Duration of follow-up by rehabilitation department (days) | 63.3 (0–217. SD: 42.9) | 55.5 (0–258, SD: 53.2) | .298 |
| Oxford knee score (postoperative) | 36.6 (SD: 8.1) | 36.9 (SD: 8.6) | .866 |
| Increase in Oxford knee score (PostOp to PreOp) | 17.5 (SD: 11.0) | 19.3 (SD: 11.8) | .409 |
AJM: active joint movement; RP: rapid recovery; SD: standard deviation.
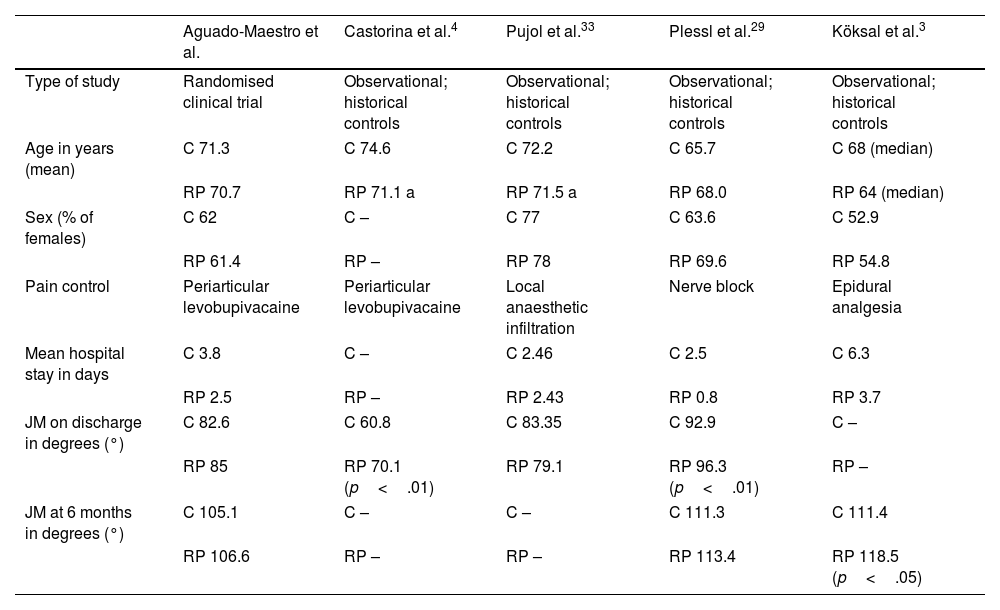
Our study included a sample of 175 patients, who were randomised into 2 groups by concealed allocation. We demonstrated that, after removal of the drain and initiation of a rapid recovery programme, the mean length of stay decreased significantly from 3.84 to 2.54 days. Most authors agree on the decrease in length of stay after implementing the protocols. However, new protocols that include outpatient prosthetic surgery show significantly shorter mean lengths of stay,29–32 although the focus of our study was not to analyse this type of intervention. The most frequent cause for delayed hospital discharge was related to postoperative pain, followed by decreased staff availability in the department during the weekend or holidays. These variables could be the target for future action to further shorten mean lengths of stay. The overall patient demographics were like those published by Castorina et al.4 and Pujol et al.,33 although they were older compared to the samples of Plessl et al.29 and Köksal et al.3 We decided not to include patients over 80 years of age due to their greater need for non-surgical hospital readmissions after discharge.23
Table 4 shows the main study variables. The studies employed used historical cohorts that already had a protocol for analysis. They all rely on early rehabilitation on the day of surgery.
Discussion. Comparison of results with other references.
| Aguado-Maestro et al. | Castorina et al.4 | Pujol et al.33 | Plessl et al.29 | Köksal et al.3 | |
|---|---|---|---|---|---|
| Type of study | Randomised clinical trial | Observational; historical controls | Observational; historical controls | Observational; historical controls | Observational; historical controls |
| Age in years (mean) | C 71.3 | C 74.6 | C 72.2 | C 65.7 | C 68 (median) |
| RP 70.7 | RP 71.1 a | RP 71.5 a | RP 68.0 | RP 64 (median) | |
| Sex (% of females) | C 62 | C – | C 77 | C 63.6 | C 52.9 |
| RP 61.4 | RP – | RP 78 | RP 69.6 | RP 54.8 | |
| Pain control | Periarticular levobupivacaine | Periarticular levobupivacaine | Local anaesthetic infiltration | Nerve block | Epidural analgesia |
| Mean hospital stay in days | C 3.8 | C – | C 2.46 | C 2.5 | C 6.3 |
| RP 2.5 | RP – | RP 2.43 | RP 0.8 | RP 3.7 | |
| JM on discharge in degrees (°) | C 82.6 | C 60.8 | C 83.35 | C 92.9 | C – |
| RP 85 | RP 70.1 (p<.01) | RP 79.1 | RP 96.3 (p<.01) | RP – | |
| JM at 6 months in degrees (°) | C 105.1 | C – | C – | C 111.3 | C 111.4 |
| RP 106.6 | RP – | RP – | RP 113.4 | RP 118.5 (p<.05) | |
BA: joint movement (active in our series); C: control group; RP: intervention group (rapid recovery).
In relation to blood loss, we understand that the estimation method proposed by Nadler and Good is influenced by other variables that affect haemoconcentration. The formula was used for comparative purposes with other publications in our department. We were unable to demonstrate statistically significant differences in the increased transfusion requirements of group C (using drainage) due to the minimal incidence of this event.
We observed that post-operative pain was lower (measured as decreased need for morphine chloride salvage) in patients in whom the anaesthesiologist performed a femoral block. However, we were unable to demonstrate less postoperative pain in group C because only 71.7% of the patients in that group received the block according to the protocol. This fact could speak in favour of finding surgeon-dependent analgesic alternatives, such as local infiltration analgesia34 which, although it has been shown to be less effective,16 is not influenced by other variables such as assistance pressure of the anaesthesiologist in charge of the post-anaesthesia resuscitation unit.
A few studies3,29 agree that early rehabilitation protocols may improve patients’ active joint movement during early follow-up, although only Köksal et al.3 reported that these differences were also observed 6 months later. Our study did not show any significant difference in the short term (2 weeks) or in the medium term (6 months). Our joint movement was lower than that reported by Köksal and Plessl in their publications; however, we could not establish in their articles whether the measurement corresponded to active or passive joint movement, whereas we evaluated active joint movement in our trial.
Regarding repeat hospital attention and readmissions during follow-up, 7.6% of the patients in group C and 10.8% of the intervention group were re-admitted. These results are in line with those described by Petersen et al. in data extracted from the Danish National Register, who reported readmission of 8% during the first 90 days after fast track hip and knee arthroplasty.32 Finally, although not observed in our series, Jenny et al. published an increased incidence of reoperation in patients operated under rapid recovery protocols in 10 centres in France (2%) in the first 90 days after discharge.7 We could not confirm this theory, since only one patient in our study, in group C, required reoperation due to acute prosthetic infection.
The incidence of deep vein thrombosis in our series was 0.5%, lower than expected even though all the patients underwent Doppler ultrasound one month after surgery.35–37 This difference could be related to the use of bemiparin 6h after surgery and the use of elastic compression stockings.
Our sample rated the total knee arthroplasty procedure with scores of 8.26 and 8.2 out of 10. These results are lower than those published by Jansson et al.,26 with a mean score of 9 points. However, as they point out, their results should be viewed with caution due to the small sample size and the inclusion in the study of total knee and hip arthroplasty.
To our knowledge this is the first randomised clinical trial comparing the efficacy of implementing an early rehabilitation protocol with periarticular anaesthesia infiltration and without using drains with classical protocols. Although its main weakness is the smaller sample size (a sample size of 200 patients was planned, which had to be reduced due to the COVID-19 pandemic) and the impossibility of masking, there are, however, other limitations that should be highlighted, as they could condition the results. We compared the classic or usual protocol in the department with the implementation of a new protocol for rapid recovery. The use of drain and anaesthetic block was standard practice and could clearly condition the start of ambulation. The same was true for preoperative education, which was not routine in the department until the rapid recovery protocol was implemented, and may have been influenced by the resident physician in charge of providing it. Some of the actions within the anaesthesiology department could not be fully monitored. It was not possible to standardise the type of anaesthesia used in group C, and therefore some of these patients (less than 10%) received spinal anaesthesia with isobar levobupivacaine. On the other hand, up to 29% of patients in group C did not receive anaesthetic block, which in our opinion reflects the need for surgeon-dependent analgesic techniques.
ConclusionsRapid recovery protocols may decrease hospital stay and costs without increasing complications or the need for hospital re-admission.
Level of evidenceLevel of evidence II.
Conflict of interestsThe authors have no conflict of interests to declare.
FundingThe authors declare that they have received no funding for the conduct of the present research, the preparation of the article, or its publication. However, the corresponding author would like to state that he received a grant from Zimmer Biomet during the period when he was doing this work, which was not a conflict of interest.