The implications of the Cryptococcus neoformans resistance to fluconazole on patient therapy have not been fully elucidated due to the discordant results found in published studies.
AimsTo establish the influence of C. neoformans resistance to fluconazole in the therapy of individuals with cryptococcosis and AIDS.
MethodsThis study retrospectively compared the clinical course of patients with cryptococcosis according to the level of fluconazole resistance of their C. neoformans isolates.
ResultsThis study included 71 episodes of cryptococcosis, defined as those isolates of C. neoformans obtained from patients with mycosis, of which 36 isolates were sensitive to fluconazole, 20 susceptible dose-dependent (SDD), and 15 were resistant. There were 5 treatment failures in the consolidation phase; two occurred in patients who had a susceptible strain, 2 in patients who had SDD strains, and one in a patient who had a resistant strain. During the maintenance treatment, relapses occurred in 4 of 33 patients (12%), seen during the follow-up period, none of which occurred in the group with resistant isolates. There were no significant differences in survival time free of treatment failure (p=0.65) or survival time free of failure or relapse (p=0.38). These results were not affected when tested in a Cox model that included age, CD4T lymphocyte counts, and use of antiretroviral therapy.
ConclusionsIn HIV patients with cryptococcosis, the resistance of C. neoformans appeared not to increase the risk of failure or relapse during treatment.
Las implicaciones de la resistencia de Cryptococcus neoformans al fluconazol en el tratamiento de pacientes infectados con esta levadura no han sido completamente definidas debido a hallazgos discordantes obtenidos previamente.
ObjetivosDilucidar la influencia de la resistencia de C. neoformans al fluconazol en el tratamiento de los pacientes con criptococosis y sida.
MétodosEn este estudio se compara retrospectivamente la evolución clínica de los pacientes con criptococosis según el grado de resistencia al fluconazol de los aislamientos de C. neoformans obtenidos de ellos.
ResultadosSe incluyeron 71 episodios de criptococosis definidos por el aislamiento de C. neoformans de pacientes con la micosis, que se distribuyeron de la siguiente manera: 36 aislamientos fueron sensibles, 20 sensibles dosis-dependiente y 15 resistentes. En la fase de consolidación, cinco fallos en el proceso de tratamiento tuvieron lugar: dos en pacientes con aislamientos sensibles, dos en pacientes con aislamientos dosis-dependiente y uno en un paciente con un aislamiento resistente. Durante la fase de mantenimiento se presentaron 4 recurrencias en los 33 pacientes que tuvieron seguimiento (12%), ninguna de las cuales ocurrió en el grupo con aislamientos resistentes. No se encontraron diferencias estadísticamente significativas en el tiempo de supervivencia de los casos sin fallo terapéutico (p=0.65) o en el tiempo de supervivencia de los casos sin fallo terapéutico o recaída (p=0.38). Estos resultados no se modificaron cuando fueron evaluados en un modelo de regresión de Cox en el que se incluyeron la edad, el conteo de linfocitos T CD4 y el uso de terapia antirretroviral.
ConclusionesEn pacientes con VIH y criptococosis la resistencia de C. neoformans a fluconazol parece no incrementar el riesgo de fallo terapéutico o recaída.
Cryptococcosis, a major opportunistic fungal infection mainly affecting patients living with human immunodeficiency virus (HIV) infection, remains a serious health problem with prevalence rates in HIV patients ranging from 4.2% in developed countries to more than 17% in African countries.2,9 In Colombia, where this mycosis reaches an incidence rate of 3.3 per 1.000 AIDS patients, cryptococcosis is an important cause of hospitalization.12
Current treatment of cryptococcosis is based on the initial administration of amphotericin B with flucytosine, followed by a consolidation phase with fluconazole. This therapeutic scheme has succeeded in decreasing the mortality from 14–25% to only 6%, and relapses from 17–24% to 2–4%.17 Many treatment alternatives are based on the use of fluconazole given as a single drug or as combination therapy.6,15–17 The success of these therapies, as well as of maintenance treatment with fluconazole, could be at risk by the emergence of resistant strains, whose prevalence is 20% in some regions of the world.22 However, the implications of this resistance have not been fully elucidated due to the discordant results found in published studies.1,5,7,23
This study was designed to establish the influence of C. neoformans resistance to fluconazole on the risk of failure during consolidation therapy and of relapse and failure of maintenance therapy in individuals with cryptococcosis and AIDS.
Patients and methodsStudy design and patientsThis retrospective cohort research focused on episodes of cryptococcosis that occurred in patients who had Western-blot confirmed HIV infection and who were treated in six hospitals in Medellín, Colombia. The C. neoformans isolates from these patients were classified as susceptible, susceptible dose-dependent (SDD) or resistant according to their susceptibility to fluconazole. We evaluated the impact of resistance to fluconazole on treatment failure during the consolidation phase, and on relapse of maintenance therapy.
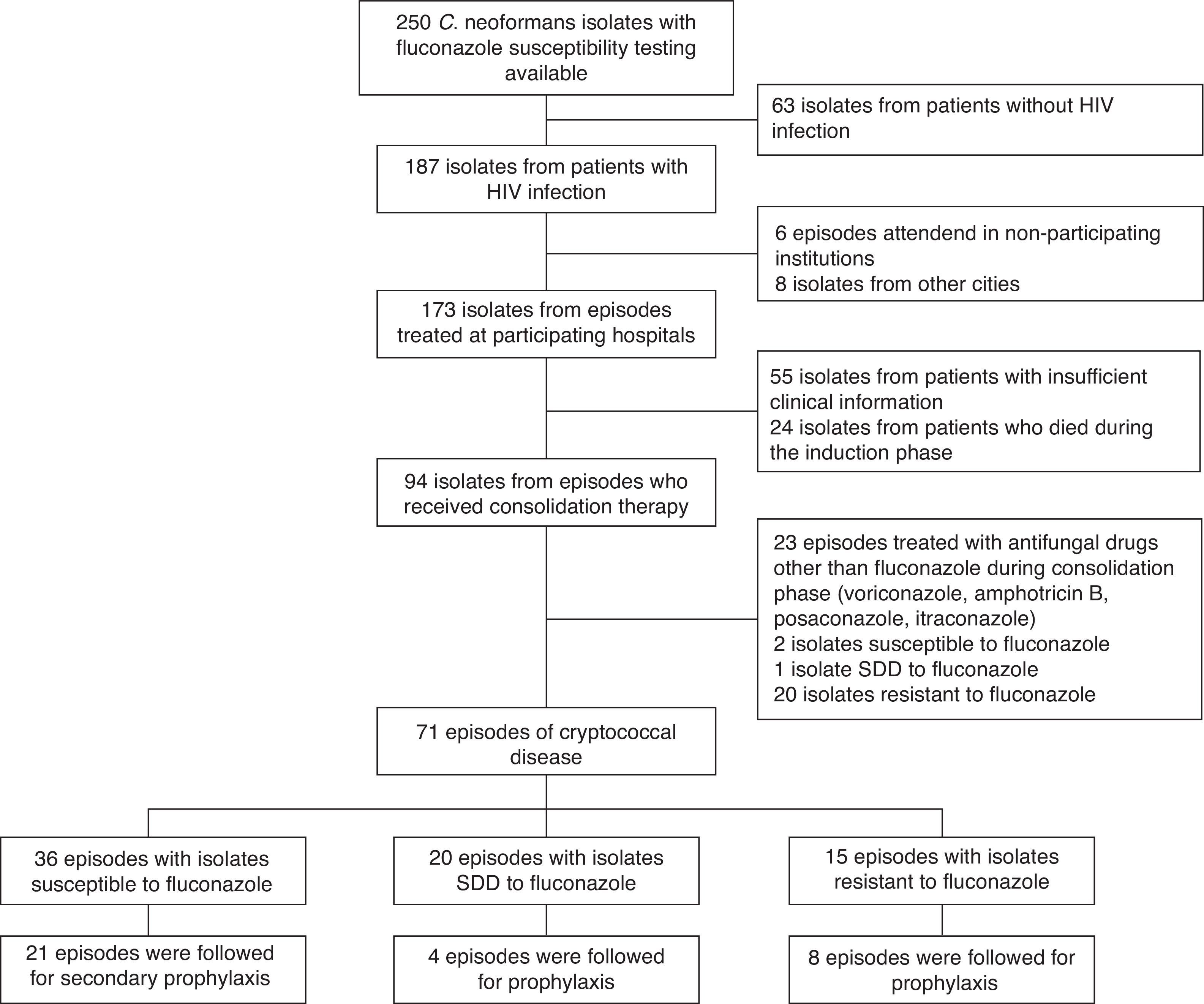
Episodes of cryptococcosis were identified from the database of the Medical Mycology and Experimental Unit of the Corporation for Biological Research (CIB). This unit performed fluconazole susceptibility tests on 250 C. neoformans isolates collected during the period of January 2000–December 2008, 187 (74.8%) of which came from AIDS patients. All of the isolates were identified as C. neoformans var. grubii. Episodes of cryptococcosis were included in the study if the disease had been confirmed by the isolation of C. neoformans from cerebrospinal fluid, blood, lung, skin or lymph nodes, with samples being obtained for microbiological and pathological analyses for each clinically affected organ if there was information on fluconazole susceptibility reported as minimal inhibitory concentration (MIC), and if the information available in the clinical records was complete enough to determine the outcome of treatment.
For purposes of inclusion in the cohort, patients had to have a positive culture for C. neoformans and the presence of at least one sign or symptom compatible with the disease. The patients included presented with their initial episode or with recurrence of a previously diagnosed episode.
Treatment followed mostly the recommendations that appeared in Practice Guidelines for the Management of Cryptococcal Disease published in 2000 by the Infectious Diseases Society of America.21 However, no patient received flucytosine because this drug was not available in Colombia at the time of the study. Briefly, patients with cryptococcal disease received induction therapy with amphotericin B deoxycholate, 0.7–1mg/kg/day, for two weeks, followed by consolidation therapy with fluconazole, 400–800mg/day, for 10–12 weeks. Some patients did not receive induction therapy with amphotericin B deoxycholate, but were managed with fluconazole alone at 400–800mg/day for 10–12 weeks. At the end of the consolidation phase, maintenance treatment consisted of fluconazole 200–400mg/day, until achievement of CD4T cell counts of at least 200cells/mm3 on two measurements made at least 3 months apart. No patient had intracranial pressure measurement or underwent a new lumbar puncture for pressure management.
A therapeutic failure was considered to have occurred when an event was observed during the 12 weeks of the consolidation phase of treatment. Relapse was taken to be an event occurring after initiation of maintenance treatment and up to 36 weeks on treatment. Failure and relapse were considered proven when recurrence or exacerbation of signs and/or symptoms of disease were accompanied by microbiological (isolation on culture) demonstration of C. neoformans. Failure and relapse were considered probable when mycological tests did not demonstrate the organism, but the patient showed recurrence or exacerbation of signs and/or symptoms compatible with cryptococcosis, had no other disease documented, and had response to antifungal therapy.
The outcomes evaluated were those corresponding to survival time free of failure (to evaluate the consolidation phase) and survival time free of failure or relapse (a combined outcome to evaluate the consolidation and maintenance phases).
Mycological proceduresThe mycological procedures were performed at the CIB's Medical Mycology Laboratory. The isolates were identified by assimilation of urea, production of melanin, and assimilation studies done by means of the Api Aux 20C kit (bioMérieux, Marcy. l’Étoile, France). Species were determined by growth in the canavanine-glycine-bromothymol blue (CGB) medium, while the serotype were defined by means of a commercial kit with specific polyclonal antibodies (Crypto-Check, Iatron, Japan).
Susceptibility tests were performed using the M 44A disk diffusion method, using 25μg fluconazole disks (Becton Dickinson, Sparks, MD), according to CLSI.13 Reading was done with the video camera BIOMIC®, which measures inhibition in millimeters, converts them into MICs by a regression curve, and stores the data electronically (Giles Scientific, 1999, Santa Barbara, CA). Quality control strains tested were ATCC C. albicans 90028 and C. parapsilosis 22019. Given that there is no standard cutoff points for resistance specific for C. neoformans and fluconazole, we used the values published by the CLSI and by Barry et al. for Candida spp., which consider susceptible isolates to be those with a MIC≤8μg/ml (≥19mm inhibition), SDD between 16–32μg/ml (15–18mm inhibition) and resistant MIC≥64μg/ml (≤14mm inhibition).4,13,14
Cryptococcal episodes were classified into three groups according to the fluconazole susceptibility pattern exhibited by the particular C. neoformans isolate, as follows: susceptible, MIC≤8μg/ml; SDD, MIC ranging from 16 to 32μg/ml, and resistant, MIC≥64μg/ml. A second classification was based on a MIC cutoff of 16μg/ml, as described by Aller et al.,1 who grouped the isolates as susceptible if the MIC was <16μg/ml and resistant if they had a MIC≥16μg/ml (epidemiological cut points).1
Data management and statistical analysisThe mycological data, obtained from the records of the Medical and Experimental Mycology Unit of the CIB, and the clinical data extracted from medical records in the participating hospitals, were recorded in a format designed for the purpose and entered into a database created in Microsoft Excel® (Microsoft, Redmond, WA).
The software SPSS 16.0 (SPSS Inc., Chicago, IL) and EPI DAT 3.1 (Consejería de Sanidad, Junta de Galicia and OPS/OMS) were used for statistical analysis. For descriptive analysis, measures of absolute frequency and percentage for qualitative variables were calculated, while for quantitative variables the median and the inter-quartile range (IQR) were used. The baseline characteristics of the three groups (susceptible, SDD and resistant) were compared in a univariate analysis using the Pearson Chi2 test or Fisher's exact test when applied to compare qualitative variables and the Kruskal–Wallis test for comparison of quantitative variables, accepting a two-tailed p-value ≤0.05 as statistically significant. The Shapiro–Wilk test showed that all quantitative variables did not adopt a normal distribution curve (p≤0.05 for at least one group in each variable). Survival analysis was done using the Kaplan–Meier method to estimate the survival function for each of the outcomes and the log-rank test to compare groups. To identify other possible variables associated with the risk of therapeutic failure or relapse, a Cox's proportional hazards model with the Enter method was obtained, including variables with p-values ≤0.25 in the univariate analysis.
Ethical approvalThis research was approved by the Ethics Committee of Universidad Pontificia Bolivariana and the Research Committees of the participant Hospitals: Hospital La María, Hospital Pablo Tobón Uribe, Clínica Universitaria Bolivariana and Hospital General de Medellín. The study was subjected to the principles of Declaration of Helsinski. No informed consent was prepared due to the retrospective nature of the study and, also, due to the difficulties for contacting those patients seen in consultation years ago.
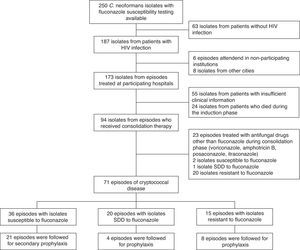
ResultsPatients’ characteristicsSeventy-one episodes of cryptococcosis experienced by 68 patients met the study's inclusion criteria (Fig. 1). Male patients (n=59) accounted for 81% of the episodes. The median age of the study population was 35 years (IQR 29–40), and the median duration of the HIV infection was 121 days (3–159). In 25 (35%) episodes, the patients were receiving antiretroviral therapy at the time they developed cryptococcosis. The median viral load was 236,000copies/ml (93,200–733,000copies/ml), and the median CD4 count was 22.5cells/mm3 (10–51cells/mm3). The use of fluconazole prior to the development of cryptococcosis was reported in 22 (31%) episodes.
For 60 (85%) episodes of cryptococcosis it was the first event, and for 11 (15%) it was a relapse; for the latter, the initial episode of cryptococcosis had occurred a mean of 5.2 months before relapse. Only 3 of these 11 patients had received fluconazole for maintenance treatment.
Meningeal involvement was present in 66 (93%) episodes; in the remaining 5 episodes involvement of a different site (fungemia, pulmonary, cutaneous, and/or lymph node) other than central nervous system was documented. In 12 (17%) of the episodes, simultaneous isolation of C. neoformans from more than one site was recorded; sixteen episodes had documented fungemia. In 7 of these episodes, susceptibility tests were run for isolates obtained from cerebrospinal fluids, in another 3 from lung samples, and from an equal number from blood cultures. In a single case, the isolate subjected to susceptibility tests came from a lymph node, and in two patients more these studies were carried out using isolates coming from two different involved sites. Documented concurrent opportunistic infections were primarily tuberculosis and pneumocystosis, which were demonstrated in 20 (28%) episodes.
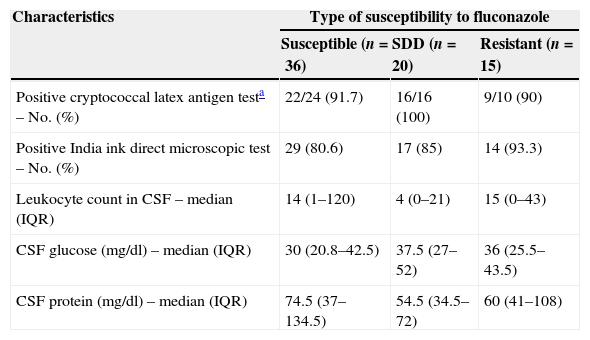
For those patients who had meningitis, lower glucose concentration was the most relevant finding in cerebrospinal fluid (CSF); the increase in protein concentration and leukocytes was mild in all groups. The qualitative cryptococcal antigen test on CSF was positive in 47 (94%) of the 50 episodes in which this test was performed. The India ink test was positive in 60 (85%) of the 71 episodes.
Induction treatment consisted of amphotericin B deoxycholate in 64 (90%) of the episodes; in the remaining 7 episodes, only fluconazole treatment was given from the time of diagnosis. The total mean dose of amphotericin B administered was 737 (581–1000) mg. In all episodes, but two, the patients received 400mg daily of fluconazole during the consolidation phase; these two patients were given 600 and 800mg daily.
Fluconazole susceptibility testingFluconazole susceptibility testing of the 71 isolates showed that 36 (51%) were susceptible, twenty (28%) were SDD, and the remaining 15 (21%) isolates resistant (MIC≥64μg/ml).
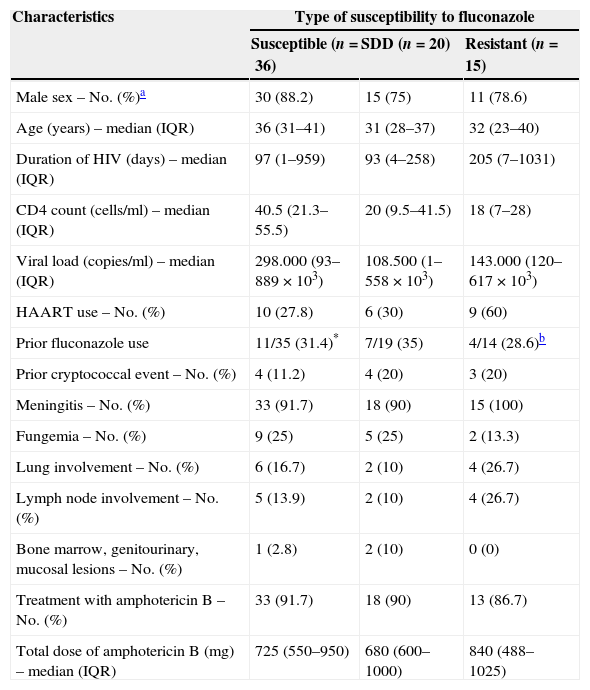
Correlation of fluconazole susceptibility with patient characteristics and their outcomeWhen comparing baseline and clinical characteristics from the patients whose isolates fell into each of the 3 groups defined by the fluconazole susceptibility, no statistically significant differences were observed (Tables 1 and 2). From the 71 episodes evaluated during the consolidation phase, 66 completed such a phase while the remaining 5 (7%) experienced treatment failures. From the 33 episodes followed during the maintenance stage, 4 (12%) had cryptoccocal relapses.
Baseline characteristics of 71 episodes of cryptococcosis according to fluconazole susceptibility patterns of Cryptococcus neoformans isolates.
| Characteristics | Type of susceptibility to fluconazole | ||
|---|---|---|---|
| Susceptible (n=36) | SDD (n=20) | Resistant (n=15) | |
| Male sex – No. (%)a | 30 (88.2) | 15 (75) | 11 (78.6) |
| Age (years) – median (IQR) | 36 (31–41) | 31 (28–37) | 32 (23–40) |
| Duration of HIV (days) – median (IQR) | 97 (1–959) | 93 (4–258) | 205 (7–1031) |
| CD4 count (cells/ml) – median (IQR) | 40.5 (21.3–55.5) | 20 (9.5–41.5) | 18 (7–28) |
| Viral load (copies/ml) – median (IQR) | 298.000 (93–889×103) | 108.500 (1–558×103) | 143.000 (120–617×103) |
| HAART use – No. (%) | 10 (27.8) | 6 (30) | 9 (60) |
| Prior fluconazole use | 11/35 (31.4)* | 7/19 (35) | 4/14 (28.6)b |
| Prior cryptococcal event – No. (%) | 4 (11.2) | 4 (20) | 3 (20) |
| Meningitis – No. (%) | 33 (91.7) | 18 (90) | 15 (100) |
| Fungemia – No. (%) | 9 (25) | 5 (25) | 2 (13.3) |
| Lung involvement – No. (%) | 6 (16.7) | 2 (10) | 4 (26.7) |
| Lymph node involvement – No. (%) | 5 (13.9) | 2 (10) | 4 (26.7) |
| Bone marrow, genitourinary, mucosal lesions – No. (%) | 1 (2.8) | 2 (10) | 0 (0) |
| Treatment with amphotericin B – No. (%) | 33 (91.7) | 18 (90) | 13 (86.7) |
| Total dose of amphotericin B (mg) – median (IQR) | 725 (550–950) | 680 (600–1000) | 840 (488–1025) |
SDD: susceptible dose-dependent; IQR: inter-quartile range; HAART: highly active antiretroviral therapy.
Cerebrospinal fluid findings in 71 episodes of cryptococcosis according to fluconazole susceptibility patterns of their Cryptococcus neoformans isolates.
| Characteristics | Type of susceptibility to fluconazole | ||
|---|---|---|---|
| Susceptible (n=36) | SDD (n=20) | Resistant (n=15) | |
| Positive cryptococcal latex antigen testa – No. (%) | 22/24 (91.7) | 16/16 (100) | 9/10 (90) |
| Positive India ink direct microscopic test – No. (%) | 29 (80.6) | 17 (85) | 14 (93.3) |
| Leukocyte count in CSF – median (IQR) | 14 (1–120) | 4 (0–21) | 15 (0–43) |
| CSF glucose (mg/dl) – median (IQR) | 30 (20.8–42.5) | 37.5 (27–52) | 36 (25.5–43.5) |
| CSF protein (mg/dl) – median (IQR) | 74.5 (37–134.5) | 54.5 (34.5–72) | 60 (41–108) |
SDD: susceptible dose-dependent; IQR: inter-quartile range; CSF: cerebrospinal fluid.
Concurrent opportunistic infections were present in 12 (33%) of the 36 episodes with susceptible isolates, in 6 (30%) of the episodes with SDD isolates, and 2 (13%) of the episodes with resistant isolates. In the group with susceptible isolates the concurrent opportunistic infections were tuberculosis (9) and Pneumocystis jirovecii pneumonia (3). In the SDD group, such concurrent infections were P. jirovecii pneumonia (3), Salmonella spp. bacteremia (1), disseminated Mycobacterium avium complex (1) and cytomegalovirus (1). In the group with resistant isolates, Salmonella spp. bacteremia and Kaposi's sarcoma occurred in one patient each. No relapse or death events occurred in patients receiving simultaneous anti-tuberculosis treatment.
There were 5 (7%) treatment failures, all mycologically confirmed; two were in the group of episodes whose isolates were susceptible to fluconazole, two in the SDD group, and one was in the group of patients whose isolates were fluconazole resistant. The latter patient died of cryptococcosis at week 4 after diagnosis. Two patients in the group whose isolates were susceptible to fluconazole died within one week of switching from amphotericin B to fluconazole therapy, and their deaths were considered to be attributable to cryptococcosis. Two patients in the SDD group died in the consolidation phase from causes unrelated to cryptococcosis. All negative outcomes occurred in patients with meningitis; one of these patients had, additionally, fungemia and skin involvement.
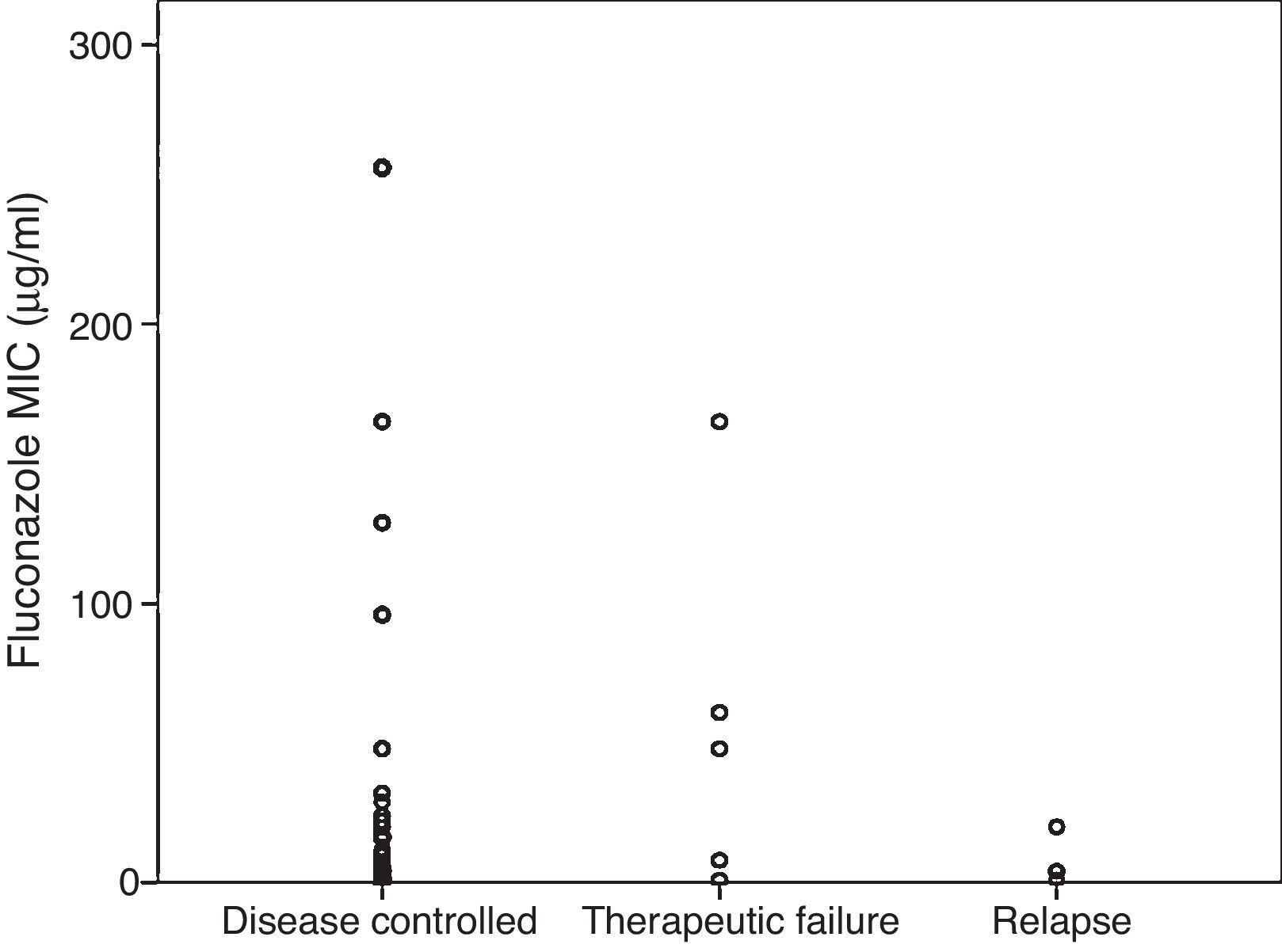
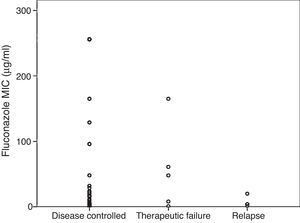
Fig. 2 shows MICs grouped by results of therapy with fluconazole. For episodes in which the cryptococcal infection was controlled, the mean MIC was 14 (2–48)μg/ml, compared with a mean of 48 (4.4–113)μg/ml in the group that failed during the consolidation phase, and a mean of 4 (1.8–16)μg/ml in the group that relapsed during maintenance treatment (p=0.51).
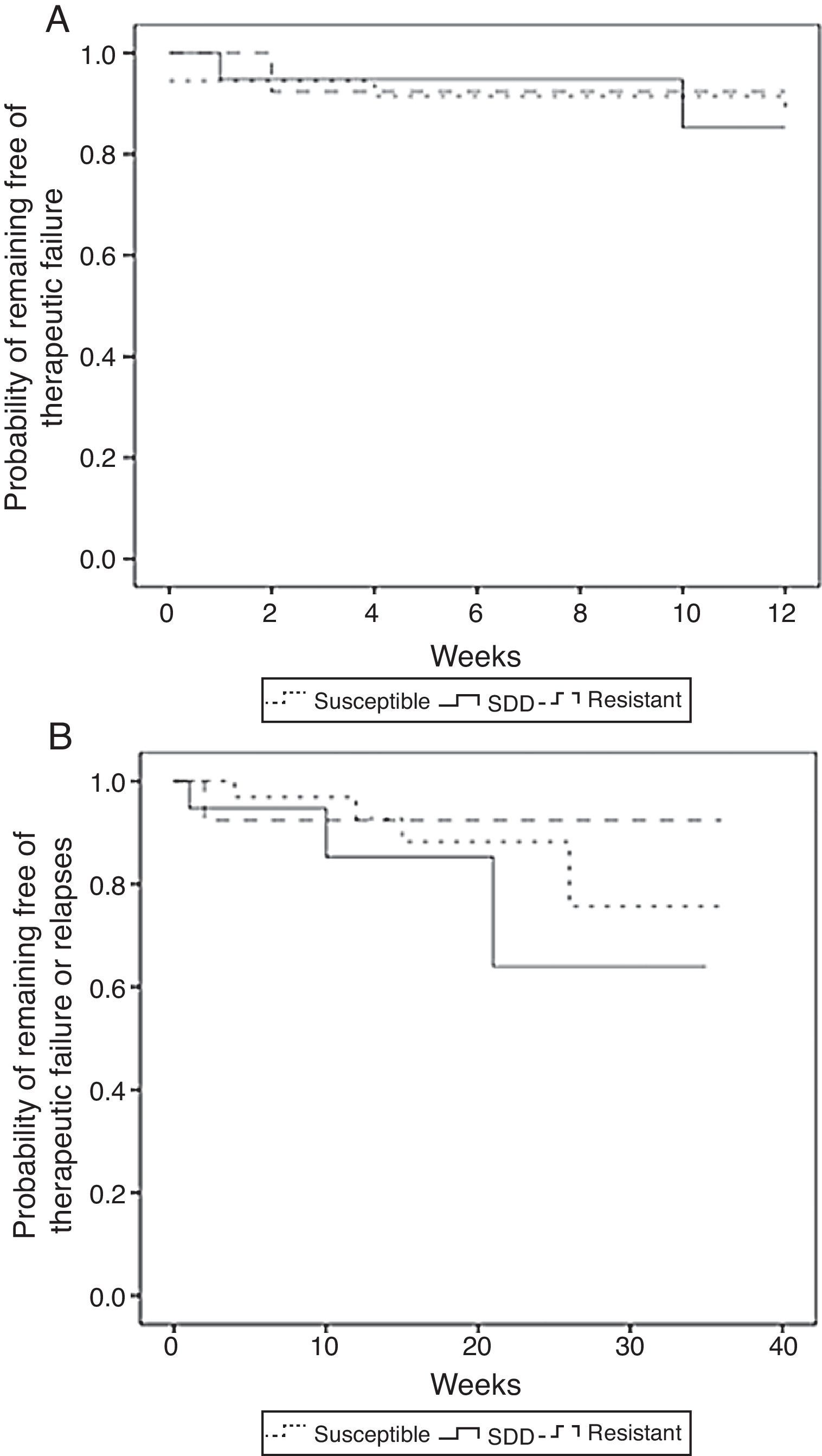
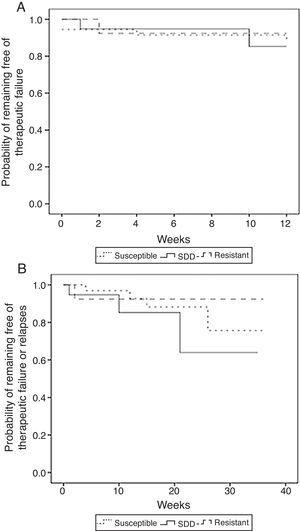
The unadjusted comparison of time of survival free of therapeutic failure and time of survival free of failure or relapse showed no statistically significant differences among the three groups when using the log-rank test (p=0.65 and p=0.38, respectively) (Fig. 3).
Kaplan–Meier estimates of the probability of (A) remaining free of therapeutic failure according to the level of resistance to fluconazole of (B) remaining free of therapeutic failure or relapse according to the level of resistance to fluconazole (susceptible, SDD and resistant) (N=71).
For 33 of the 71 episodes (46%), follow-up was recorded during the maintenance treatment phase. A total of 4 (12%) relapses occurred, 3 in the group of susceptible isolates and 1 in the SDD group. Of these 4 relapse episodes, 3 were confirmed and one was probable. No differences were found between the 3 groups in regard to relapses (p=0.42).
A Cox's model, including as covariates age, use of antiretroviral treatment, and CD4 counts, was built for each outcome as these variables had a p-value ≤0.25 in the bivariate analysis. The time of survival free of failure was not significantly affected by age (hazards ratio (HR) per year of change in age, 1.13; CI 95% 0.97–1.32; p=0.11), CD4 count (HR per change in cell/mm3, 1.00; CI 95% 0.97–1.03; p=0.88), or the use of antiretroviral drugs (HR 2.66; CI 95% 0.22–31.82; p=0.44). The time of survival free of failure and relapse was not affected by age (HR per year of change in age, 1.05; CI 95% 0.95–1.17; p=0.35), CD4 count (HR per change in cell/mm3, 0.99; CI 95% 0.97–1.02; p=0.60), or by the use of antiretroviral drugs (HR 2.55; CI 95% 0.39–16.59; p=0.33).
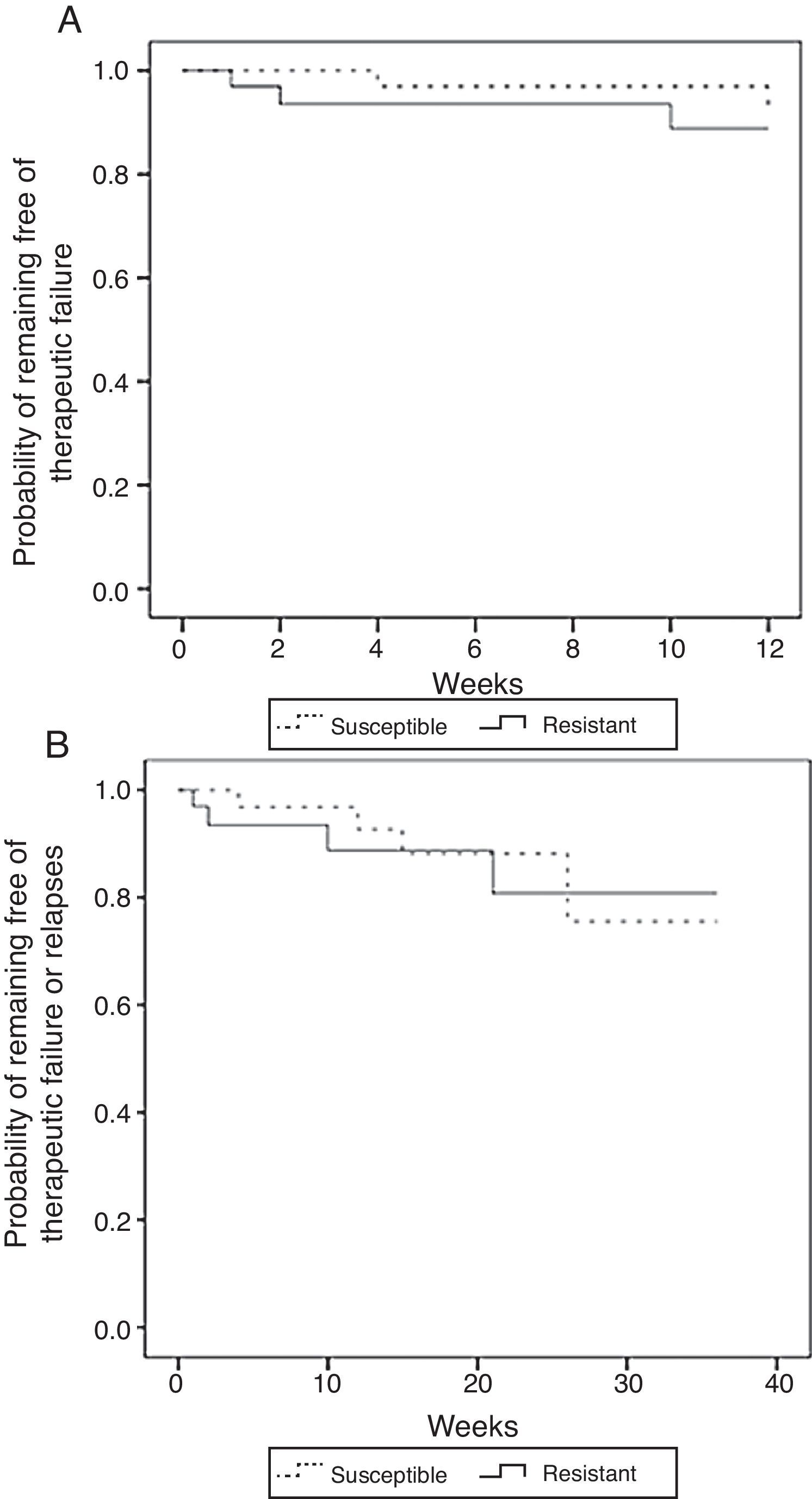
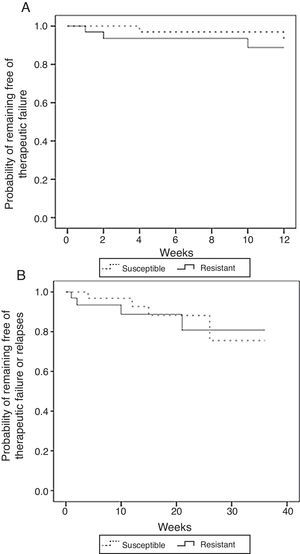
A comparison between isolates with MIC <16μg/ml (susceptible) versus those with MIC≥16μg/ml (resistant) yielded similar non-significant results. The unadjusted comparison of time of survival free of therapeutic failure and time of survival free of failure or relapse showed no statistically significant differences among the two groups when using the log-rank test (p=0.49 and p=0.86, respectively) (Fig. 4).
Kaplan–Meier estimates of the probability of (A) remaining free of therapeutic failure according to the level of resistance to fluconazole or (B) remaining free of therapeutic failure or relapse according to the level of resistance to fluconazole (MIC<16μg/ml vs MIC≥16μg/ml) (N=71).
The Cox's model for a breakpoint of 16μg/ml also included as covariates age, CD4 T-cells and the use of antiretroviral drugs. The time of survival free of therapeutic failure was not significantly influenced by age (HR per year of change in age, 1.13; CI 95% 0.97–1.32; p=0.11), CD4 T-cells count (HR per change in cell/mm3, 1.0; CI 95% 0.97–1.03; p=0.95) or by the use of antiretroviral drugs (HR 2.4, CI 95% 0.23–24.62; p=0.46). The time of survival free of failure or relapse was not significantly influenced by age (HR per year of change in age, 1.06; CI 95% 0.95–1.18; p=0.30), CD4 T-cells count (HR per change in cell/mm3, 0.99; CI 95% 0.97–1.02; p=0.57), or by the use of antiretroviral drugs (HR 0.97; CI 95% 0.39–10.70; p=0.40).
DiscussionThe role of fluconazole in the treatment of AIDS-associated cryptococcosis has been readdressed by recent studies that evaluated fluconazole as induction treatment.6,15,16 The increased resistance to this azolic compound, among C. neoformans isolates studied in the ARTEMIS program,18 indicated the need for determining the impact of resistance to the treatment of patients with cryptococcosis. Despite numerous studies, this issue has not been resolved.1,3,5,7,11
The present retrospective study evaluated the impact of fluconazole resistance in a cohort of AIDS patients with C. neoformans disease, mostly with meningeal involvement. We assessed the relationship between fluconazole resistance and failure of consolidation therapy and relapses during maintenance treatment. There were 5 (7%) failures during consolidation therapy and 4 (12%) relapses during maintenance treatment. These numbers are lower than the 23% reported in a previous study that compared the effectiveness of maintenance therapy with fluconazole versus itraconazole.20 The mycological demonstration of C. neoformans in patients experiencing poor outcomes does not favor an immune reconstitution syndrome as the cause of the patient's clinical deterioration. Nonetheless, it is probable that the presence of concurrent opportunistic infections recorded in 28% of the patients could have affected the final results considering that the mortality was attributable not only to the latter infections but also to the interactions with the medications prescribed for such concurrent infections, as well as to the adverse effects and the impact, among others, of multiple medications on patients’ adherence to medical recommendations.
Our study found no impact of fluconazole resistance on the outcome of the therapy with this compound. Only one failure occurring during the consolidation phase was demonstrated in patients with resistance to this drug. On the other hand, 14 patients with isolates with MIC≥64μg/ml failed to show adverse results during consolidation or maintenance phases. An analysis using a cutoff of 16μg/ml, which was related to the outcome of therapy in another study,1 also showed no statistically significant differences in the therapeutic response between resistant and susceptible cases.
These findings are similar to those reported by Dannaoui et al.7 who evaluated the impact of the susceptibility to fluconazole, measured by three different techniques, on the early outcome of cryptococcosis treatment. In this study, the susceptibility to fluconazole did not predict the outcome of the therapy although the power was low due to the small number of patients who were receiving fluconazole.7 In a larger population, Arechavala et al.3 only found one strain of C. neoformans resistant to fluconazole in 11 patients who experienced relapse.
On the other hand, several studies have found a correlation between the results of susceptibility testing and the outcome of therapy. Larsen et al.11 showed a correlation between C. neoformans in vitro susceptibility to fluconazole and treatment response in a murine model. Aller et al. found a relation between MIC <16μg/ml and a better response to fluconazole compared with patients who had isolates with MIC≥16μg/ml,1 and Bicanic found decreased susceptibility to fluconazole in 66% of relapses occurring in HIV patients with cryptococcal meningitis.5
The lack of consensus about the cutoff point for C. neoformans susceptibility testing to fluconazole,8,19 associated with the poor correlation between different techniques reported in certain studies,10,23 has hindered clinical studies aiming at defining the impact of resistance to fluconazole on the treatment of cryptococcosis. The current research supports the thesis that there is a lack of correlation between susceptibility to fluconazole and the outcome of therapy for C. neoformans in AIDS patients. However, its retrospective design prevented the evaluation of variables, such as fungal burden, time to culture negativity in CSF, fluconazole levels in CSF, drug interactions, and adherence to treatment, which are known to influence the outcome of treatment. Proper evaluation of the effect of these factors would require a prospective study. It is possible that the inclusion of the episodes corresponding to relapses introduced bias in the results. However, it would be expected that those who relapsed would be more likely to have fluconazole-resistant isolates, but this was not the case.
In conclusion, this work found no effect of in vitro resistance to fluconazole on the outcome of consolidation therapy or maintenance treatment in AIDS patients with C. neoformans disease. Prospective studies comparing different techniques to determine the susceptibility of C. neoformans to fluconazole and to assess patient's adherence to treatment, to measure drug levels in cerebrospinal fluid, as well as determine fungal burden, should be undertaken to define the role of fluconazole susceptibility testing in the management of cryptococcosis.
Funding sourceThis research was supported by Universidad Pontificia Bolivariana and Corporación para Investigaciones Biológicas. The study sponsors did not have any role in the study design or in the collection, analysis and interpretation of data, in the writing of the manuscript, and in the decision to submit the manuscript for publication.
Conflict of interestNone.
The authors specially thank Dr. Carol A. Kauffman for her invaluable help in review of the manuscript. Our thanks goes to the Medical and Experimental Mycology Unit personnel of the Corporación para Investigaciones Biológicas and also to the Hospitals participating in this study.