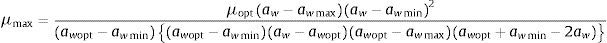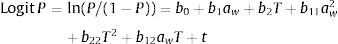Xerophilic fungi represent a serious problem due to their ability to grow at low water activities causing the spoiling of low and intermediate moisture foods, stored goods and animal feeds, with the consequent economic losses.
AimsThe combined effect of water activity and temperature of four Eurotium species isolated from animal feeds was investigated.
MethodsEurotium amstelodami, Eurotium chevalieri, Eurotium repens and Eurotium rubrum were grown at 5, 15, 25, 37 and 45°C on malt extract agar adjusted with glycerol in the range 0.710–0.993 of water activities.
ResultsThe cardinal model proposed by Rosso and Robinson (2001) was applied to fit growth data, with the variable water activity at fixed temperatures, obtaining three cardinal water activities (awmin, awmax, awopt) and the specific growth rate at the optimum aw (μopt). A probabilistic model was also applied to define the interface between growth and no-growth. The cardinal model provided an adequate estimation of the optimal aw to grow and the maximum growth rate. The probabilistic model showed a good performance to fit growth/no-growth cases in the predicted range.
ConclusionsThe results presented here could be applied to predict Eurotium species growth in animal feeds.
Los hongos xerófilos son un problema importante debido a su capacidad de crecer a bajas actividades del agua, lo que causa el deterioro de alimentos a humedades bajas e intermedias, de materias primas almacenadas y de piensos para animales, con las consecuentes pérdidas económicas.
ObjetivosSe llevó a cabo un estudio sobre el efecto de los factores ambientales (temperatura y actividad del agua) sobre el crecimiento de cuatro especies pertenecientes al género Eurotium aisladas de piensos para animales.
MétodosSe estudió el crecimiento de Eurotium amstelodami, Eurotium chevalieri, Eurotium repens y Eurotium rubrum a valores de actividad de agua en el rango 0,710-0,993 en el medio de cultivo agar extracto de malta modificado con glicerol, y valores de temperatura de 5, 15, 25, 37 y 45°C.
ResultadosEl modelo cardinal propuesto por Rosso y Robinson (2001) se aplicó para realizar el ajuste de datos con la actividad del agua como variable a una temperatura fija; se obtuvieron tres valores cardinales de actividad del agua (awmin, awmax, awopt) y la tasa de crecimiento específico en el valor óptimo de aw (μopt). También se aplicó un modelo probabilístico para definir la interfase entre crecimiento y no crecimiento. El modelo cardinal presentó una adecuada estimación del awopt y la máxima velocidad de crecimiento. El modelo probabilístico fue adecuado para el ajuste de los casos de crecimiento/falta de crecimiento en el rango previsto.
ConclusionesLos resultados presentados en este artículo pueden aplicarse para pronosticar el crecimiento de especies de Eurotium en piensos para animales.
Animal feeds are made from different ingredients such as corn, wheat, rice, soybean, sorghum, barley and other grain mill products, animal proteins, vitamins, minerals, etc. Appropriate amounts of all nutrients are required to maintain animals healthy and get faster at maximum weight. For feedstuff stability, maximum moisture contents are critical; below 0.6 water activity (aw) spoilage by microorganisms would not be expected.27 Feedstuffs are produced by commercial feed mills, as well as by home mixture, being low water activity a characteristic of these feeds (average 0.537). However, improper production or inadequate storage conditions allow the growth and proliferation of xerophilic fungi, resulting in a negative impact on nutritional and organoleptic properties of feeds and mycotoxin production.4,23 Thus, feedstuff should be discarded, with the subsequent economic losses.
According to the recent changes in nomenclature, the genus Eurotium was transferred to the anamorphic Aspergillus genus,31,41 although a proposal has been recently submitted to Taxon by Pitt and Taylor36 in order to keep the name Eurotium, advocating the recognition of the diversity in the morphological and physiological phenotypes among these fungi. The genus belongs to the phylum Ascomycota, characterized by the production of ascospores resistant to high temperatures inside bright yellow cleistothecia.
Eurotium species are universally known as spoiling fungi, and are responsible for substantial economic losses of food commodities, leather goods and textiles. Most of its species are of particular interest to food and feed mycology because of their xerophilic physiology; many isolates are able to grow at water activities below 0.75 and some have been reported to grow at values as low as 0.64 aw.10 Twenty Eurotium species are known approximately; among the most common ones, Eurotium amstelodami, Eurotium chevalieri, Eurotium repens, and Eurotium rubrum are usually associated with stored goods.35 The growth and development of these fungi cause changes in the organoleptic characteristics and the nutritional quality of the raw materials and commodities, affecting the shelf life of processed products. Furthermore, these species have been reported to produce secondary metabolites.1,10,24 Some of these compounds, such as echinulin, physcion and flavoglaucin, have shown toxicity to animals.24 Although most of the studies on feedstuffs mycobiota have been focused on the presence of the micotoxigenic genera Aspergillus, Fusarium and Penicillium,11,12,18,22,29,32,34,38Eurotium species have also been reported as frequent contaminants.7,43 In Argentina, Eurotium species have been determined as major components of the mycobiota in poultry,18,23 chinchilla, rabbit and rainbow trout feeds.22,25 The interaction between environmental factors and food characteristics might provide the required conditions for the growth and development of filamentous fungi, followed by potential toxic metabolite production. In order to predict and avoid undesirable fungal growth it is necessary to determine the influence of those environmental factors, such as water activity and temperature.
The aim of the present work was to evaluate the effect of aw and temperature on the germination time and growth of four xerophilic Eurotium species isolated from animal feeds. Mathematical modelling tools were tested to describe and characterize the ecophysiology of the xerophilic fungi on synthetic media.
Materials and methodsFungal isolatesFour species belonging to the genus Eurotium were used in the present study: E. amstelodami, isolated from rabbit feed, and E. chevalieri, E. repens and E. rubrum, isolated from chinchilla feeds. The identity of the four isolates had been previously confirmed by scanning electron microscopy of ascospores and DNA sequencing of two independent DNA loci (ITS and beta-tubulin).24
MediaThe basal medium used was malt extract agar (MEA), consisting of 2% malt extract (Oxoid, LP0039, UK), 0.1% peptone (Britania, Buenos Aires, Argentina), 2% glucose (Oxoid, LP0071) and 2% agar (Oxoid, LP0011), at nine different water activities (aw). Media were adjusted by substituting equal parts of water by glycerol (w/w). Different water contents were prepared in the range 0.710–0.993 (0.710, 0.750, 0.790, 0.830, 0.870, 0.914, 0.952, 0.989 and 0.993). Glycerol concentrations were calculated according to Chirife et al.13 and Ross.38 All media were sterilized by steam treatment at 121°C for 15min. The aw was measured with an AquaLab CX-2 water activity metre (Decagon Devices, Inc., USA).
Preparation of the inoculumFungi were grown on Czapek yeast extract agar with 20% sucrose (CY20S) for seven days at 25°C to obtain heavily sporulating cultures. After incubation, conidia from CY20S plates were removed and suspended in 5ml of sterile water/glycerol solutions with their aw previously modified to the required value, and containing 0.05% of Tween 80 (Anedra, Research AG, Buenos Aires, Argentina). The number of conidia from this stock suspension was determined using a Neubauer chamber, and then the final concentration was adjusted to 105conidia/ml.
Inoculation and incubationPetri plates (55mm diameter) with approximately 10ml of medium were centrally inoculated with a calibrated loop containing 1μl of the conidia suspension (average 100 conidia). Petri plates with the same aw values were enclosed in polyethylene bags and incubated at 5, 15, 25, 37 and 45°C for a maximum of 90 days. To minimize the transfer of water to or from the media, plates with glycerol solutions adjusted to the corresponding aw, were placed inside the plastic bags and changed weekly. Non-inoculated control plates were included inside each bag and measured at the end of the experiment to detect any significant deviation of the initial aw, and no variation higher than 0.001 was detected. Each treatment (awT) was carried out in four replicates for each Eurotium species, either for germination and growth rate measurements. To obtain enough growth/no-growth data for the probabilistic models, four additional plates were inoculated and incubated under each set of conditions, and the combined data of 8 replicates were used for each species at each awT treatment.
Germination measurementTo determine the germination, Petri plates were examined twice a day using a stereomicroscope (×40). The criterion to decide that germination had occurred was the production of a germination tube with a similar length to the diameter of the conidia in at least 50% of the conidia.26 Germination times were recorded in hours and reported in days as the mean of four replicates for each species under each awT treatment. Plates were examined until 90 days.
Growth measurementThe radial mycelial growth rate was determined by periodical measurement of two perpendicular diameters of the colonies. Growing colonies were measured daily until the end of the experiment (90 days) or until the colony reached the edge of the Petri plate.
Statistical methodsGrowth rates (mm/day) for each aw and temperature combination were calculated as the linear regression from the linear phase of the growth curve. Secondary models were used to describe the influence of water activity and temperature on fungal growth. The Rosso cardinal model39,40 at a fixed temperature was applied to describe the effect of water activity on the radial growth rate. As the Eurotium strains isolated from animal feeds used in the present study did not grow at many of the temperatures tested, a secondary model was applied at fixed temperatures of 15 and 25°C, at which enough growth data were available.
The model is described by the following equation:
The equation was fitted using the Statistica v 10.0 (StatSoft Inc.) non-linear estimation procedure. The goodness of fit of the model was evaluated by the root mean square error (RMSE). Homogeneity of the variance of the dependent variable was tested by Levene's test using Statistica v 10.0.
To treat the data of each of the four fungal species, a linear logistic regression analysis was applied to determine the growth/no-growth boundaries under the different aw and temperature values evaluated. Data from 8 replicates per species were expressed as values of 1 or 0 corresponding to growth or no-growth, respectively. The resulting data were fitted to a logistic regression equation37 with a full second order logistic regression model8 that includes the linear term for time:
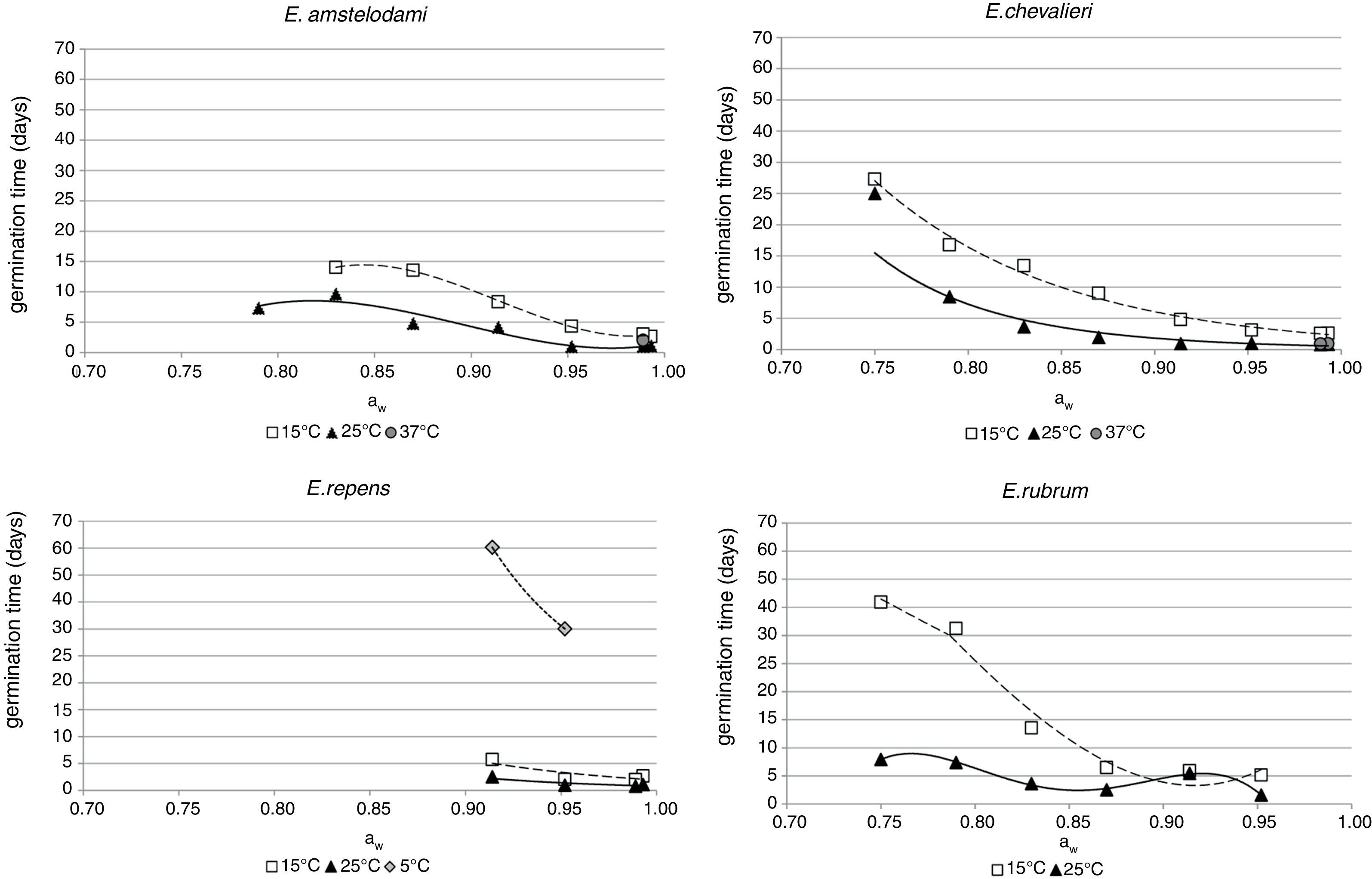
where P is the probability of growth (range 0–1), bi are the coefficients to be estimated, t (days) is the incubation time, aw is the water activity of the medium, and T is the incubation temperature in °C. The equation was fitted using Statistica 10.0 linear logistic regression procedure. The predicted growth/no-growth interfaces for P=0.1, 0.5 and 0.9 were calculated and plotted using Microsoft Excel 2003 Solver and MatLab (MathWorks, Version 7.0.1R14SP1).ResultsGermination dataInfluence of aw on the germination time for each species is shown in Fig. 1. For E. amstelodami, germination occurred in the range 0.830–0.993 aw at 15°C and 0.790–0.993 aw at 25°C. Germination at 37°C was only observed at 0.989 aw (2 days). No germination was registered at any of the tested aw values at 5 and 45°C. The lowest germination time was observed at 0.952 aw and 25°C (0.96 days) and the highest was at 14 days, occurring at 15°C and 0.830 aw.
Germination of E. chevalieri occurred in a wider range of aw (0.750–0.993), both at 15 and 25°C. Moreover, at 37°C and 0.989 aw or 0.993 aw, germination followed by mycelial growth was also registered after 0.88 and 0.92 days, respectively. No germination was observed at the other aw values at 37°C or at any of the aw values tested between 5 and 45°C. The shortest time required for germination was 0.79 days, registered at 25°C and 0.989 aw, while the longest for this species (27.3 days) was observed at 15°C and 0.750 aw.
E. repens presented a quite different behaviour from the abovementioned species. Germination only occurred at the highest aw values (range 0.914–0.993), both at 15 and 25°C. In the range 0.914–0.952 aw, germination was also observed at 5°C, being this species the only one capable of growing at this temperature. No germination was registered at any of the tested aw values at 37 and 45°C. The fastest germination for E. repens occurred at 25°C and 0.989 aw (0.79 days), while it took 60.4 days to germinate at 5°C and 0.914 aw; this was the longest germination time observed during the whole incubation period at all the evaluated conditions.
Unlike the other Eurotium species, E. rubrum presented an upper limit of aw, since it did not germinate above 0.952 aw at any of the tested temperatures. The lower limit for germination was 0.750 aw, at 15 and 25°C. No germination was observed at any other assayed temperature (5, 37, and 45°C). The shortest germination time was 1.57 days at 0.952 aw and 25°C, and the longest was 41.8 days at 0.750 aw and 15°C.
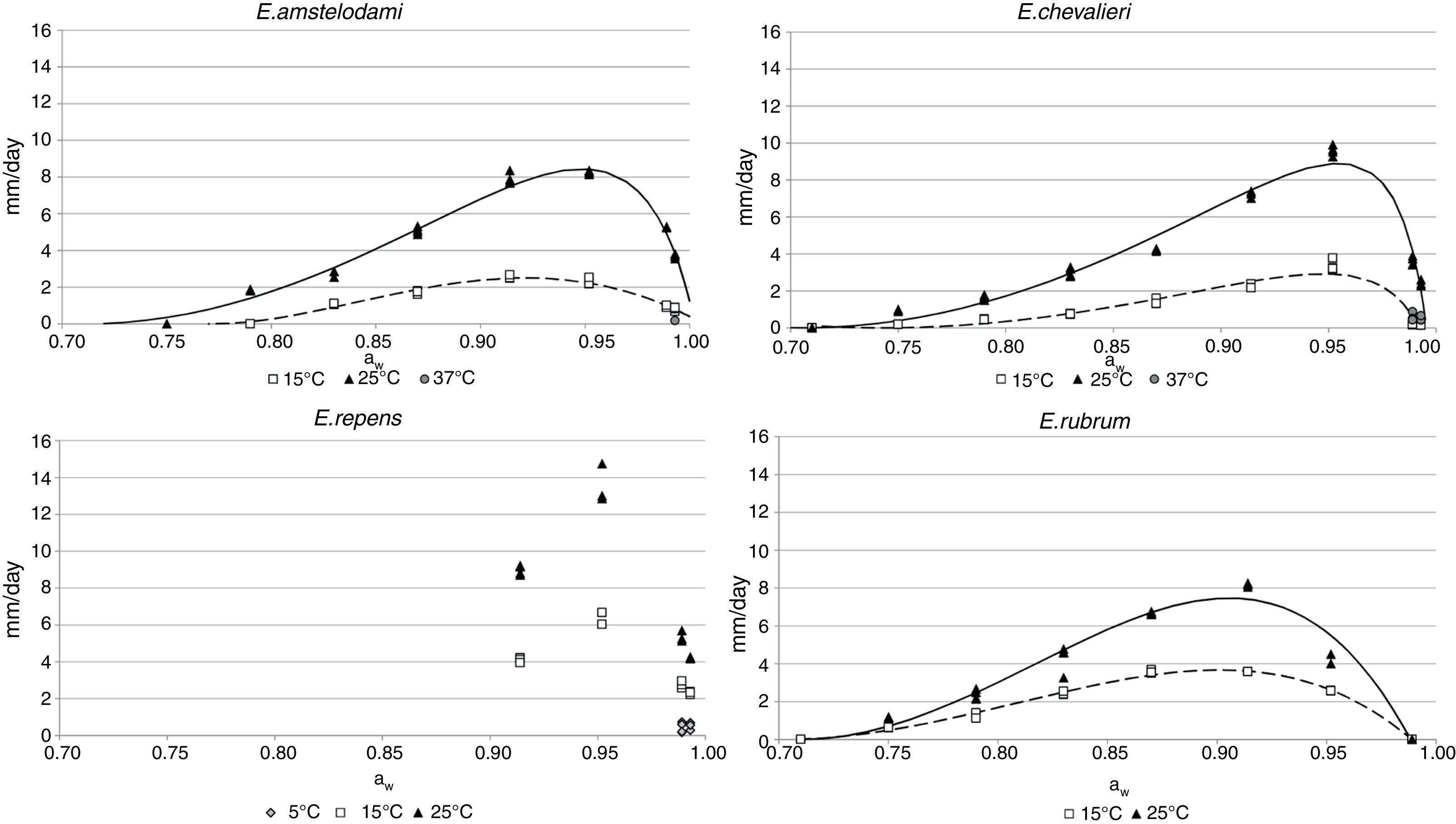
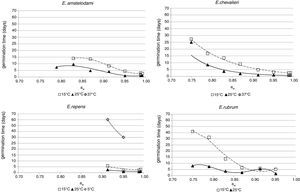
Growth dataGrowth of E. amstelodami was observed in the whole range of conditions in which germination occurred (0.790–0.993 aw at 25°C, 0.830–0.993 aw at 15°C). The minimum growth rate was registered at 15°C and 0.830 aw (1.1mm/day). Nevertheless, it grew slowly at 37°C and 0.989 aw (0.18mm/day). The optimal aw levels for growing were in the range 0.914–0.952 aw at 15 and 25°C. The maximum growth rate was 8.3mm/day at 25°C and 0.952 aw (Fig. 2).
E. chevalieri growth also occurred at all the conditions which allowed germination (0.750–0.993, both at 15 and 25°C). It was also capable of growing at 37°C and 0.989 and 0.993 aw, but with low growth rates (0.6 and 0.5mm/day, respectively). The optimal conditions for growing were 0.952 aw and 25°C, with a maximum growth rate of 9.6mm/day. At 15°C, 0.952 was also the optimum aw level, at which the growth rate was 3.3mm/day (Fig. 2).
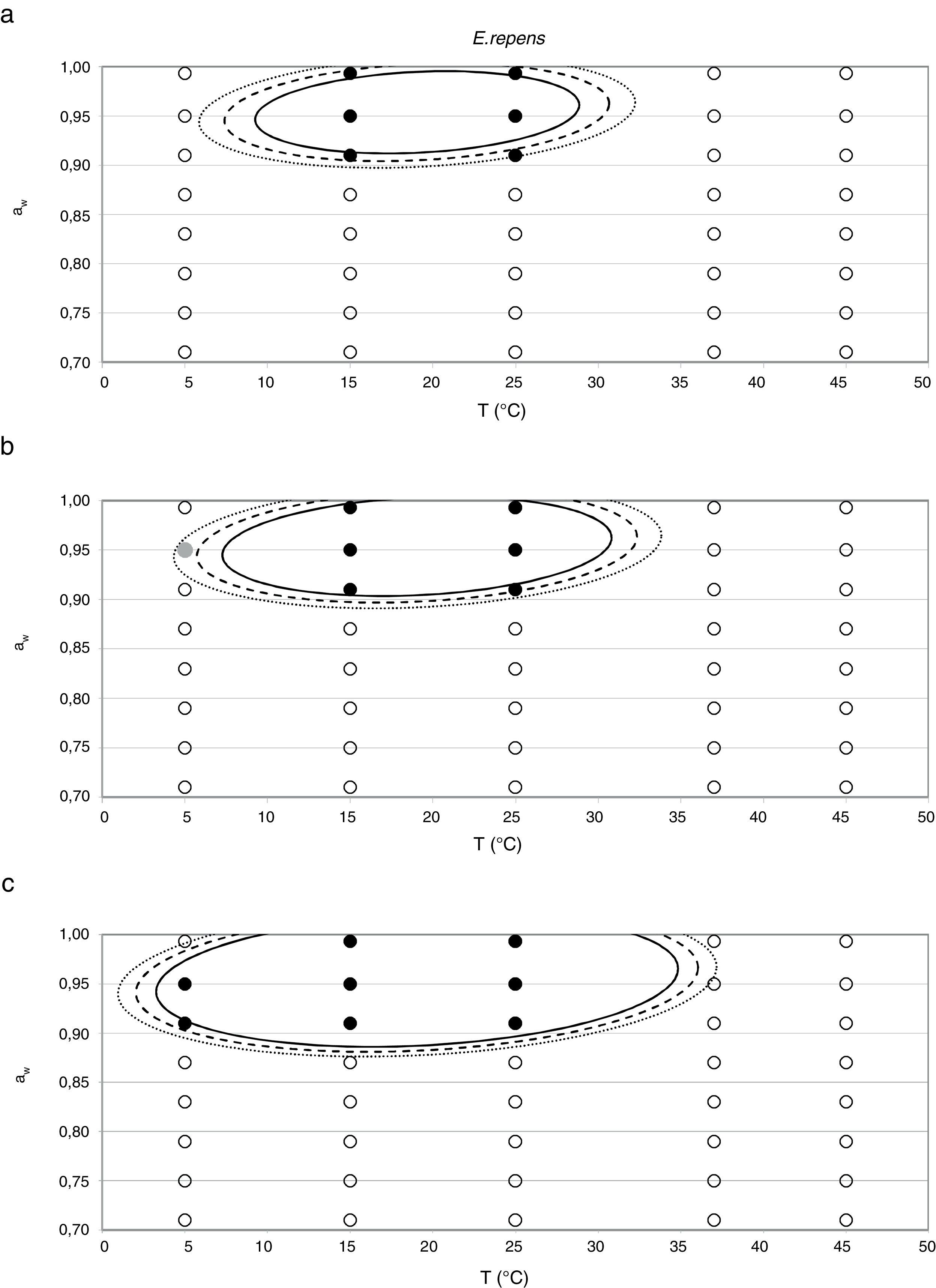
Regarding E. repens, the aw range for growing was again the same as for the germination. At temperatures of 15 and 25°C, the growth only occurred in the range 0.914–0.993 aw. Unlike the species previously discussed, E. repens could grow at 5°C and 0.914 aw and 0.952 aw levels, although at low rates (0.43 and 0.51mm/day, respectively). The optimal aw value to grow was 0.952 at 5, 15 and 25°C, with a maximum growth rate of 13.5mm/day (25°C and 0.952 aw) (Fig. 2).
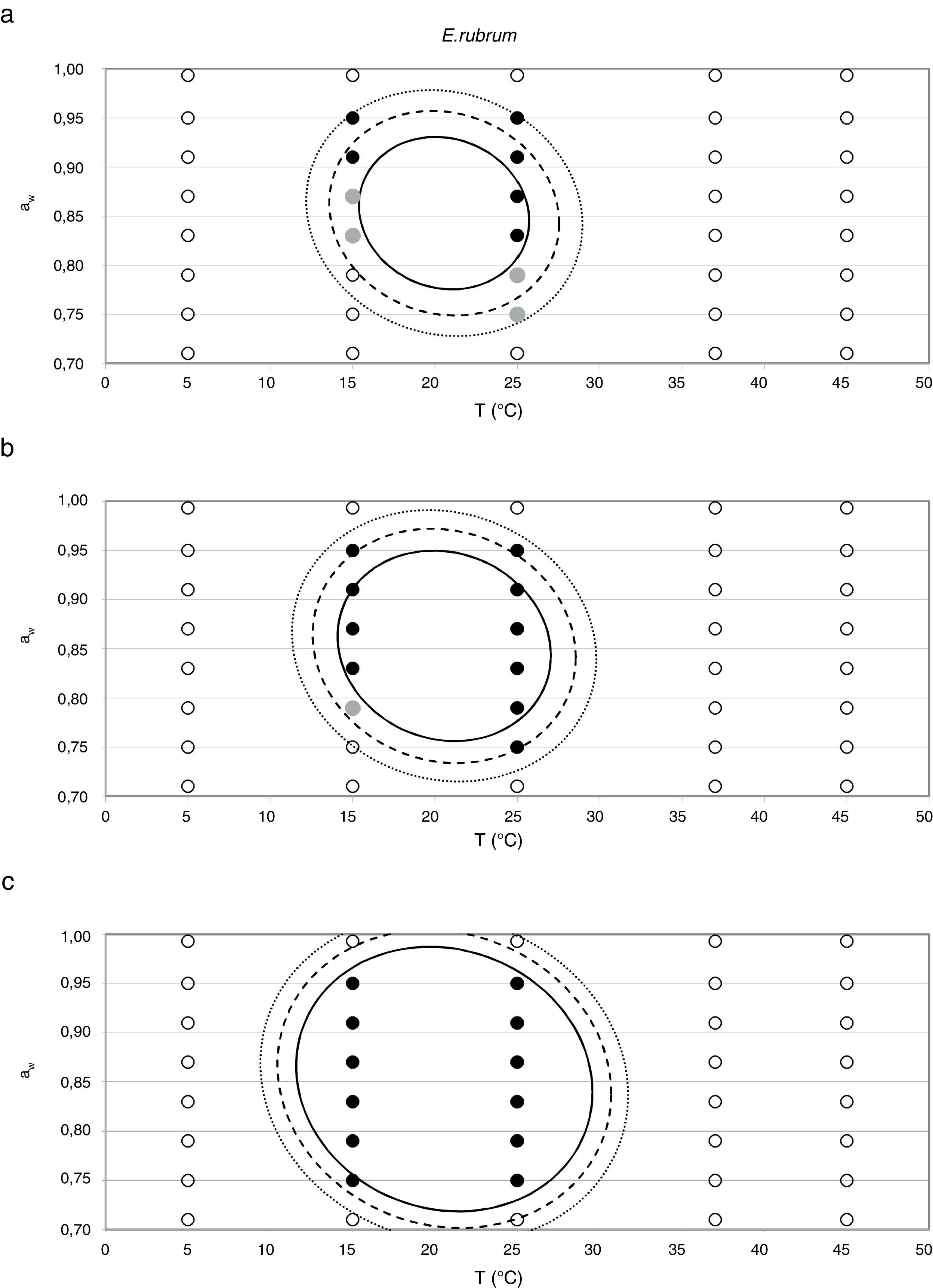
For E. rubrum, growth was registered both at 15 and 25°C in the range 0.750–0.952 aw. The optimal aw values were in the range 0.870–0.914 aw at 15°C (3.6mm/day at both aw levels) and 0.914aw at 25°C, conditions at which the maximum growth rate was registered (8.2mm/day) (Fig. 2). The minimum growth rate for this species was 0.62mm/day and it occurred at 15°C and 0.750 aw.
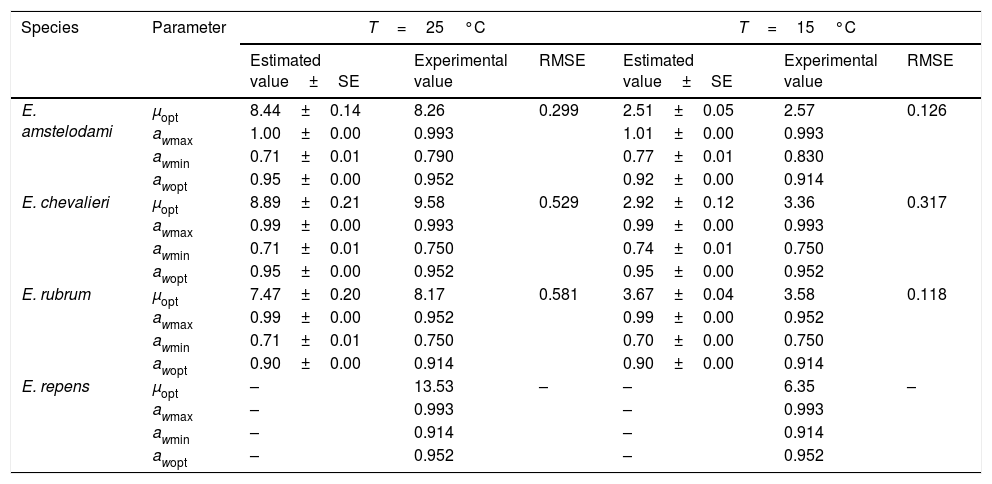
Secondary modelSeveral existing secondary kinetic models were evaluated (data not shown) to describe fungal growth response in the assayed environmental conditions (Davey, Miles, Gibson, Parra and polynomial models),19 obtaining the best fit to the experimental data when applying the Rosso cardinal model at fixed temperatures of 15 and 25°C. In this model it is common to substitute awmax for 1,42 since aw cannot exceed this value, and most bacteria and several fungal species have no upper limit to grow. However, as this was not the case with xerophilic fungi, this parameter was left to be estimated by the model. The cardinal values of environmental factors obtained (minimum, maximum and optimum) by the secondary cardinal model for each species are shown in Table 1, while graphics of the adjusted models (solid and dashed lines) and experimental data (points) are presented in Fig. 2. The root mean square error (RMSE) was calculated to evaluate the performance of the predictive model for each studied species (Table 1). This parameter determines the average deviation between the observed values and the predicted ones. According to RMSE values, the model was adequate to fit the experimental growth rate data for E. amstelodami and E. chevalieri, at both temperatures, although better estimations were obtained at 15°C than at 25°C for all the tested species. The highest RMSE and, consequently, the poorest fit comparatively among species, was obtained for E. rubrum at 25°C. It was not possible to apply the Rosso cardinal model to E. repens due to insufficient growth data.
Cardinal values of water activity (minimum, maximum and optimum) and growth rate (optimum) predicted by secondary cardinal models, experimental values and RMSE obtained for Eurotium species from animal feeds.
| Species | Parameter | T=25°C | T=15°C | ||||
|---|---|---|---|---|---|---|---|
| Estimated value±SE | Experimental value | RMSE | Estimated value±SE | Experimental value | RMSE | ||
| E. amstelodami | μopt | 8.44±0.14 | 8.26 | 0.299 | 2.51±0.05 | 2.57 | 0.126 |
| awmax | 1.00±0.00 | 0.993 | 1.01±0.00 | 0.993 | |||
| awmin | 0.71±0.01 | 0.790 | 0.77±0.01 | 0.830 | |||
| awopt | 0.95±0.00 | 0.952 | 0.92±0.00 | 0.914 | |||
| E. chevalieri | μopt | 8.89±0.21 | 9.58 | 0.529 | 2.92±0.12 | 3.36 | 0.317 |
| awmax | 0.99±0.00 | 0.993 | 0.99±0.00 | 0.993 | |||
| awmin | 0.71±0.01 | 0.750 | 0.74±0.01 | 0.750 | |||
| awopt | 0.95±0.00 | 0.952 | 0.95±0.00 | 0.952 | |||
| E. rubrum | μopt | 7.47±0.20 | 8.17 | 0.581 | 3.67±0.04 | 3.58 | 0.118 |
| awmax | 0.99±0.00 | 0.952 | 0.99±0.00 | 0.952 | |||
| awmin | 0.71±0.01 | 0.750 | 0.70±0.00 | 0.750 | |||
| awopt | 0.90±0.00 | 0.914 | 0.90±0.00 | 0.914 | |||
| E. repens | μopt | – | 13.53 | – | – | 6.35 | – |
| awmax | – | 0.993 | – | 0.993 | |||
| awmin | – | 0.914 | – | 0.914 | |||
| awopt | – | 0.952 | – | 0.952 | |||
For E. amstelodami, the cardinal parameters estimated by the model were in agreement with the experimental data, except for the minimum aw for growing, which was lower than the experimental value at both temperatures (Table 1). The same was observed for E. chevalieri at 25°C, while at 15°C the predictions were in good agreement with the measured values. For E. rubrum, however, cardinal values estimated by the model differed from the experimental ones, except for the optimum aw to grow (awopt) and the maximum growth rate (μopt) at 15°C.
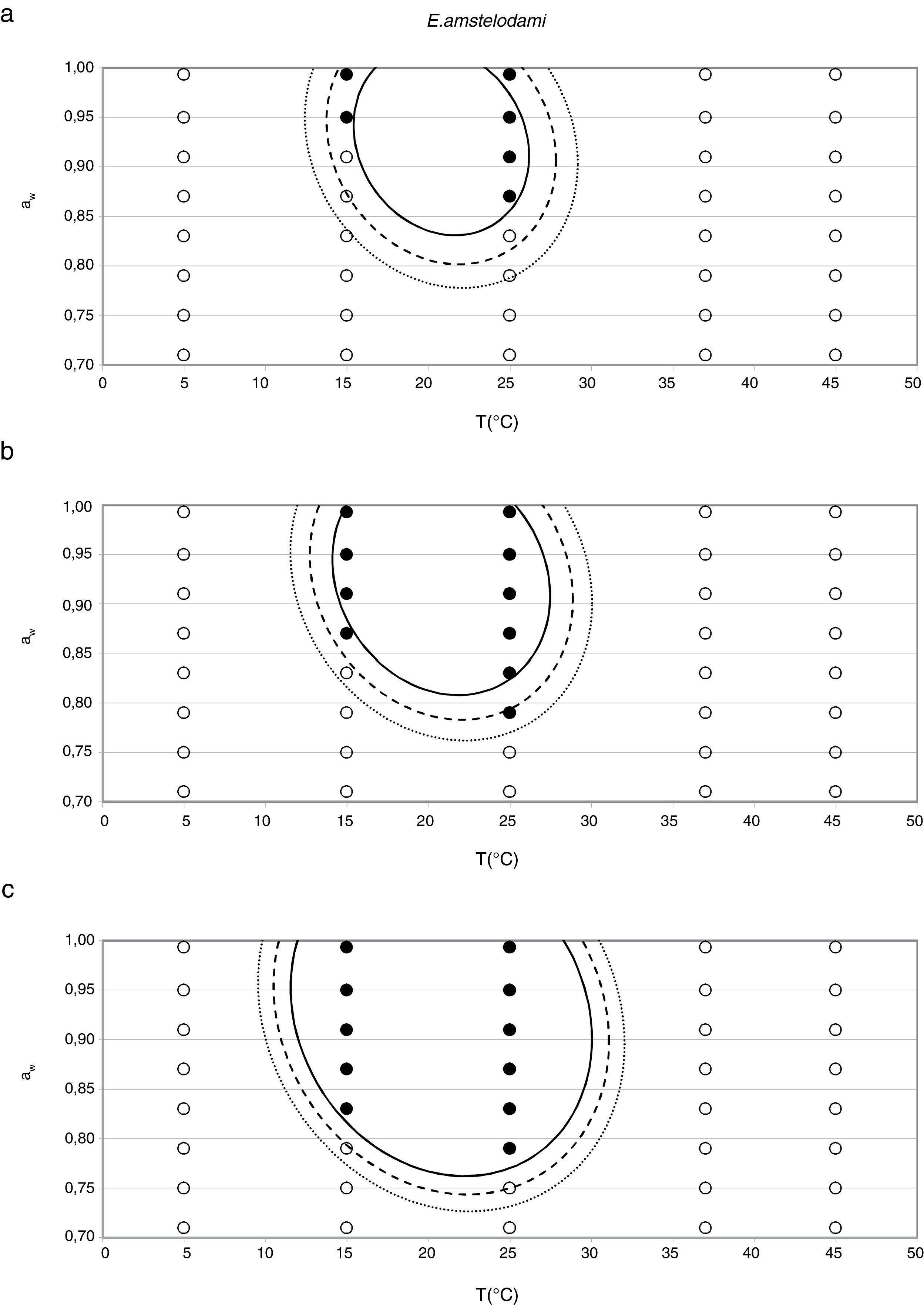
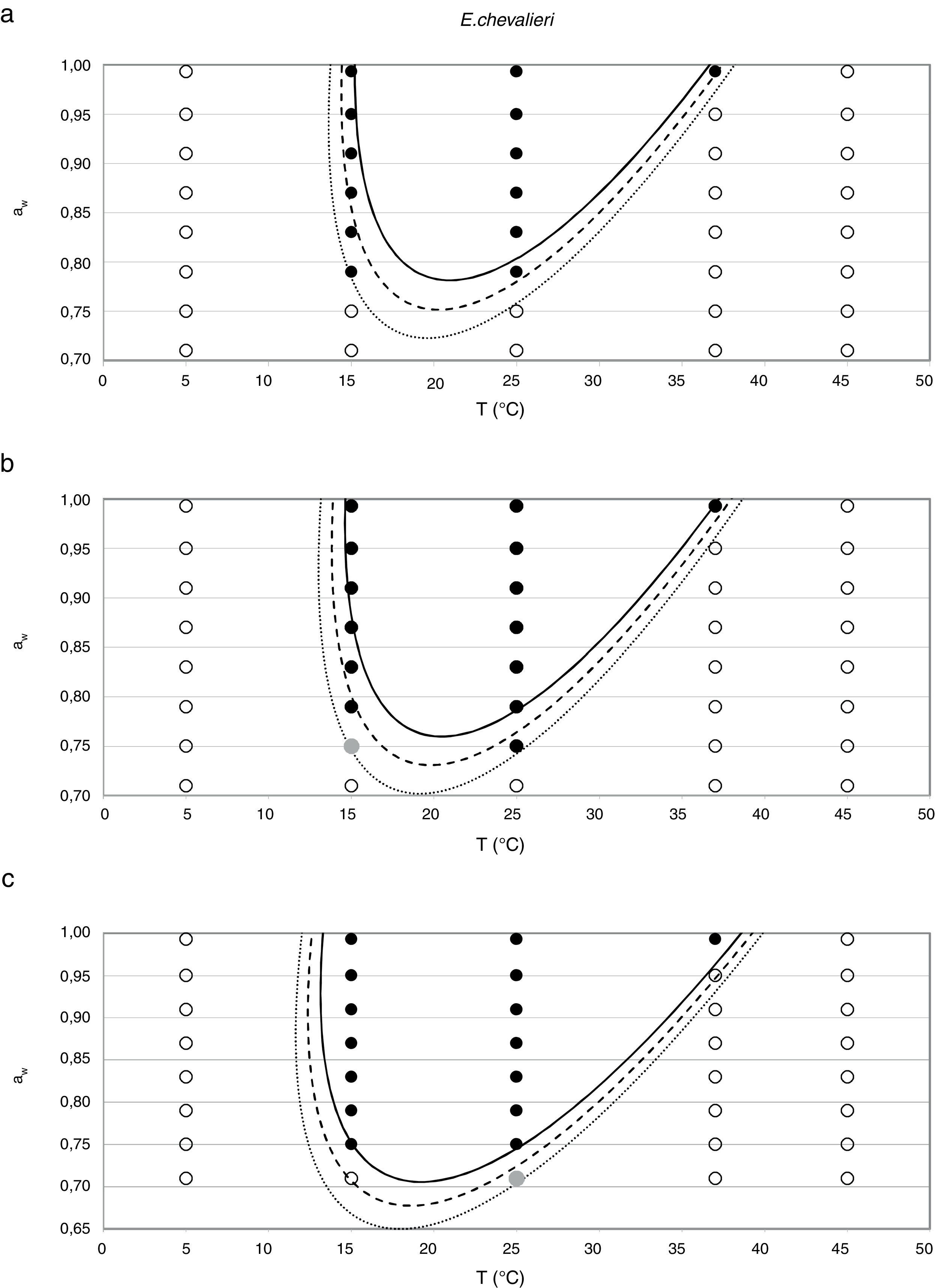
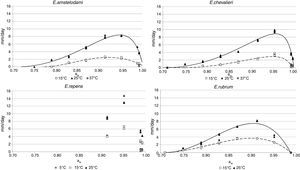
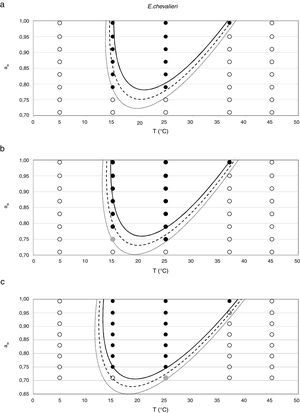
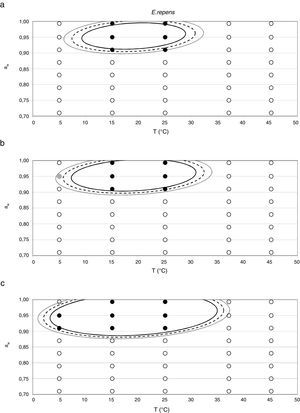
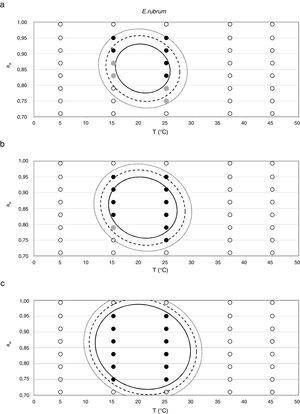
Probability modelTable 2 shows second order logistic regression models. Models were generated including linear terms, quadratic (except the quadratic term of time), and interactions terms, for each of these four species. As all the estimated parameters were significant (p<0.001), no backward elimination of terms was done. Figs. 3–6 show the predicted growth/no-growth boundaries and growth/no-growth cases at probabilities 0.1, 0.5 and 0.9, for time periods of 7, 30 and 90 days. A high percentage of agreement between the experimental data and model fit can be observed. All cases contained in the area P>90% have shown 100% growth (all replicates showed growth at the reported time); 59% of the cases in the area 10<P<90 showed 100% growth, and there were no-growth cases in the P<10% region.
Second order logistic regression models for Eurotium species.
| Species | Parameter | Estimated value±SE |
|---|---|---|
| E. amstelodami | Intercept | 377±17 |
| t | −0.073±0.005 | |
| aw | −682±39 | |
| T | −6.4±0.2 | |
| aw2 | 346±20 | |
| T2 | 0.109±0.001 | |
| awT | 1.9±0.2 | |
| E. chevalieri | Intercept | 74±5 |
| t | −0.068±0.003 | |
| aw | −99±14 | |
| T | −0.80±0.08 | |
| aw2 | 95±9 | |
| T2 | 0.127±0.005 | |
| awT | −5.8±0.3 | |
| E. rubrum | Intercept | 395±15 |
| t | −0.066±0.003 | |
| aw | −807±29 | |
| T | −5.3±0.1 | |
| aw2 | 457±16 | |
| T2 | 0.102±0.001 | |
| awT | 1.3±0.1 | |
| E. repens | Intercept | 2809±97 |
| t | −0.104±0.008 | |
| aw | −5951±432 | |
| T | 2.3±0.3 | |
| aw2 | 3168±229 | |
| T2 | 0.058±0.003 | |
| awT | −4.8±0.4 | |
p value<0.001.
According to the data obtained in this study for E. amstelodami, minimum values of water activity and temperature at which germination and growth occurred were 0.790 aw at 25°C, and 0.830 aw at 15°C, showing the highest growth rate at 0.952 aw and 25°C. Other authors reported an optimal temperature to grow at 33–35°C,16 and a maximum of 43–46°C.9 El Halouat and Debevere17 reported that the germination of E. amstelodami isolated from prunes in synthetic medium under modified atmosphere occurred at temperatures as high as 40°C at 0.83, 0.92 and 0.95 aw. However, in our study, growth at 37°C was only detected at 0.989 aw, and no growth was observed at 45°C. Armolik and Dickson5 reported growth of E. amstelodami from stored grain at 25°C and 0.75 aw, a lower aw value than that observed in the present work. In addition, Abellana et al.2 reported growth of Eurotium species from bakery products, including E. amstelodami, on flow wheat-sucrose agar at 0.775 aw and temperatures above 20°C. It seems that the aw and temperature ranges of E. amstelodami from food origin is wider than for the animal feed strain used in the present work.
Regarding E. chevalieri, the minimum aw value at which germination and growth occurred was 0.750, both at 25 and 15°C; maximum growth was registered at 0.952 aw and 25°C. Information from the literature indicates that the optimum temperature for growing is in the range 30–35°C16 with a maximum at 40–43°C.9 In the present study the growth at 37°C only occurred at the two highest aw levels, and no growth was registered at 45°C. Ayerst6 reported the minimum growth at 0.71 aw and 42°C, while Abellana et al.3 determined that an E. chevalieri strain from bakery products was able to grow at 0.75 aw at 30°C. However, both Abellana et al.3 and Marín et al.30 found that E. chevalieri could not grow on sponge cake analogue medium at 0.75 aw and 25°C; meanwhile a strain of E. chevalieri isolated from milk jam was able to grow at 0.74 aw at this temperature.12 Even though literature data differ on the growing extreme conditions, the strain isolated from animal feeds was able to grow in a more restricted aw and temperature range than most of the reported strains from foods.
E. repens showed the highest growth rate at 0.952 aw and 25°C, while 0.91 was the minimum aw value at which germination and growth was observed, both at 15 and 25°C. This was the only species able to grow at 5°C, but unlike the two previous mentioned ones, it did not grow at 37°C. According to González et al.21 and Panasenko,33E. repens grows in the range of temperatures from 4–5 to 38–40°C, with the optimum temperature in the range 25–27°C. Several authors have reported 0.72 aw as the minimum value at which germination occurs at temperatures of 20–25°C.5,28 Meanwhile, Gock et al.20 reported a minimum aw for germination and growth of 0.74 at 25°C for an isolate from dried prunes. According to Dagnas et al.,14E. repens isolated from bakery products showed estimated values of optimum temperature and aw of 29°C and 0.91, respectively, and could grow in the temperature range 0–35°C. More recently, Dagnas et al.15 estimated a minimum aw of 0.74 for this species. The narrow range of aw levels at which the animal feed strain was able to grow differs from most reports in the literature and from the other Eurotium species studied.
Minimum aw values of 0.70–0.72 have been reported in the germination of E. rubrum at 25°C,5,20 with a minimum growth temperature of 5°C and an optimum of 25–27°C.33 Gock et al.20 reported minimum growth parameters of 0.74 aw and 25°C on synthetic medium for isolates from prunes. The E. rubrum isolate from animal feeds used in the present study could not grow below 0.750 aw at any of the tested temperatures. The highest growth rate for this strain was observed at 0.914 aw at 25°C, which is similar to that reported by Wheeler et al.44 for dried fish strains (0.91–0.94).
These wide discrepancies could be due to geographical variabilities among isolates, nutritional differences of the substrates used to assess growth,34 and climate adaptation. However, in general, isolates from food products were able to germinate and grow in a wider range of aw and temperature than those from animal feeds evaluated in the present work. Thus, it is relevant to understand the ecophysiology of these particular strains in order to prevent fungal contamination of animal feeds and reduce the costs associated with their spoilage.
In general terms, the cardinal model was adequate for the estimation of the awopt and μopt, the latter with the only exception of E. rubrum at 25°C. However, limiting conditions were poorly predicted by this model, especially the awmin for growing, which was underestimated in all three Eurotium species at both temperatures, except for E. chevalieri at 15°C. Regarding the growing-upper aw limit, the model could appropriately estimate it when this parameter was close to 1, but it failed to provide a suitable estimation when an aw limit for growing existed, as the case with E. rubrum.
Since for spoiling xerophilic fungi predicting the restricting environmental conditions are more relevant than the optimal ones for growing, probabilistic models were also developed for each Eurotium species with the aim to predict the growth/no-growth boundaries. As no-growth data sets are useful for the construction of these models, both aw and T terms were included in the probability equation. The model showed an adequate fit to the experimental data and could accurately predict growth/no-growth cases in the range under study (Figs. 3–6). Validation with literature data is difficult due to the high variability observed between the strains isolated from different sources and geographical origins. Additionally, in some cases, the range of environmental conditions studied does not include minimum, maximum or optimum values to grow. Comparison of the minimum aw predicted by probability models for the different Eurotium species with previously published data shows certain agreement. For E. amstelodami, minimum predicted aw was 0.82 at 15°C and 0.76 at 25°C, values in agreement with Abellana et al.2,3 for strains from bakery products (awmin 0.80–0.825 at 15°C, and 0.75–0.80 at 25°C). The minimum aw reported by Char et al.12 for E. chevalieri on milk jam (<0.74) shows a better agreement with the one predicted by the model than those reported by Abellana et al.2,3 (0.825–0.875 at 15°C, and 0.775–0.80 at 25°C), as well as the minimum aw (≤0.74 reported by Gock et al.20 for E. rubrum in malt extract agar. The predicted cardinal for E. repens did not show agreement with literature data,14,20 as it was expected by the difference observed between this strain and most food strains reported elsewhere.
The data reported in the present study are relevant as a contribution to the understanding of the ecophysiological behaviour of these four Eurotium species isolated from animal feeds. The results presented here can be applied, after validation in the respective substrates, to predict Eurotium spp. growth in feeds and similar matrices. This information will be useful to develop control strategies to prevent fungal spoilage of feeds.
Conflict of interestThe authors declare that they have no conflicts of interest.
This work was supported by UNQ, UNRN, CONICET, and ANPCyT. G. Pose, A. Patriarca and A. Pardo are members of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET). Engineer G. Pacheco is acknowledged for his assistance.