Sarcoidosis is a chronic granulomatous disease that has been linked to exposure to certain environmental antigens, including previous contact with infectious agents, and a variety of other organic and inorganic particles. There is an acute form of presentation of this disease that courses with stereotypical clinical findings known as Löfgren's syndrome. A case is presented of a female patient with all the characteristic features of this syndrome.
La sarcoidosis es una enfermedad granulomatosa crónica que se ha relacionado con la exposición a antígenos ambientales entre los que se incluye el contacto previo con agentes infecciosos y variedad de partículas orgánicas e inorgánicas. Existe una forma de presentación aguda de esta enfermedad que cursa con hallazgos clínicos estereotípicos denominada síndrome de Löfgren. Nosotros presentamos un caso de una paciente con todos los hallazgos característicos de este síndrome.
Sarcoidosis is a multisystem disease of unknown etiology, characterized by the formation of noncaseating granulomas in different body tissues, being the lymph nodes and the lungs the most frequently affected.1 Its etiology is not completely understood. It is known that exposure to different types of organic and inorganic particulate material and to microbial antigens is related to the formation of the granulomas.2 A form of acute presentation of this entity, characterized by the intercurrence of hilar pulmonary adenopathies, erythema nodosum and arthralgias, is known as Löfgren's syndrome. It affects both sexes in a similar proportion (55% men, 45% women), with a higher incidence between 30 and 40 years of age for both sexes, and with a second peak in women between 45 and 65 years of age.3 In Colombia, in 1977, a report of 51 cases of sarcoidosis described 27 cases in men and 24 in women; only one case of a women who started with findings compatible with Löfgren's syndrome was documented.4 Below, we describe the case of a patient who presented at our service with findings characteristic of this entity.
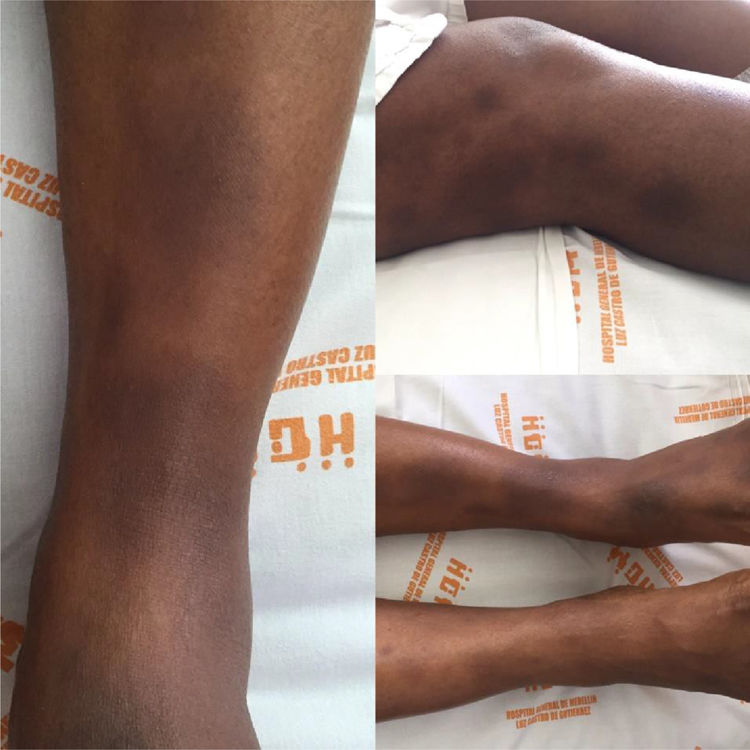
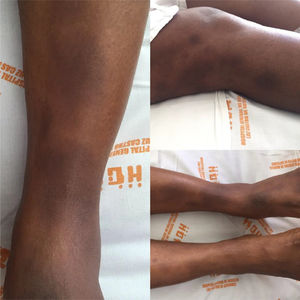
Case reportA 34-year old black women with a personal history of hidroadenitis suppurativa in 2008, who now consults for a clinical picture of 15 days of evolution consisting of generalized osteomyalgia, inflammatory arthralgias in wrists, elbows, knees and ankles, subsequently associated with oligoarthritis of the right wrist and both ankles. Simultaneously, with objective fever of up to 39°C and painful erythematous nodules in lower limbs below the knees and of pretibial localization. Physical examination shows arthritis of the left ankle and multiple erythematous nodules painful to palpation in lower limbs, in the pretibial region, which tend to coalesce. (Fig. 1).
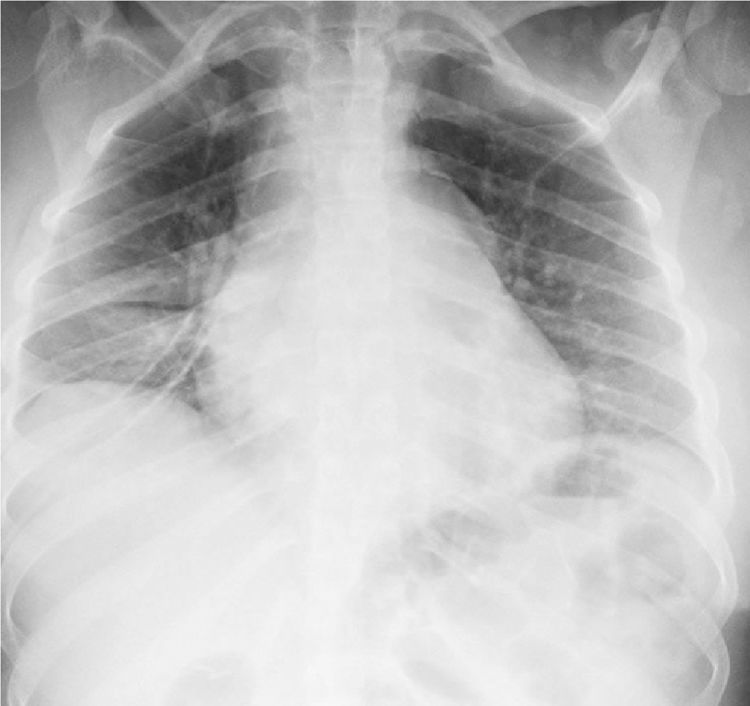
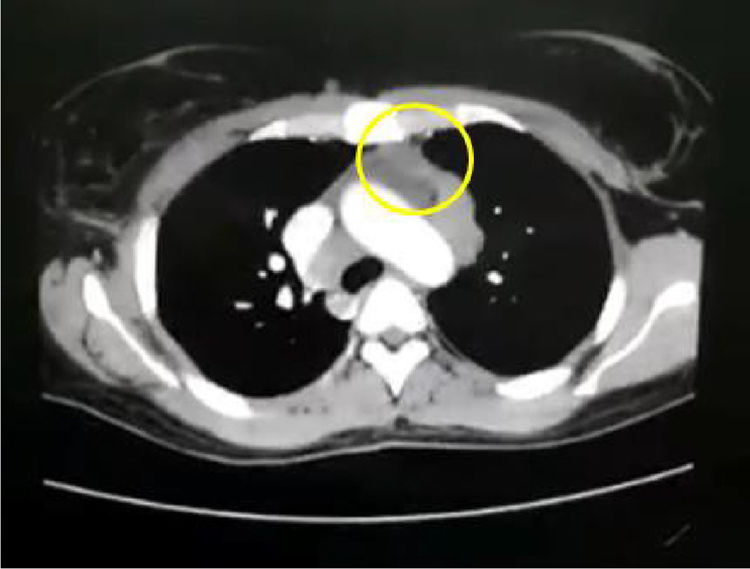
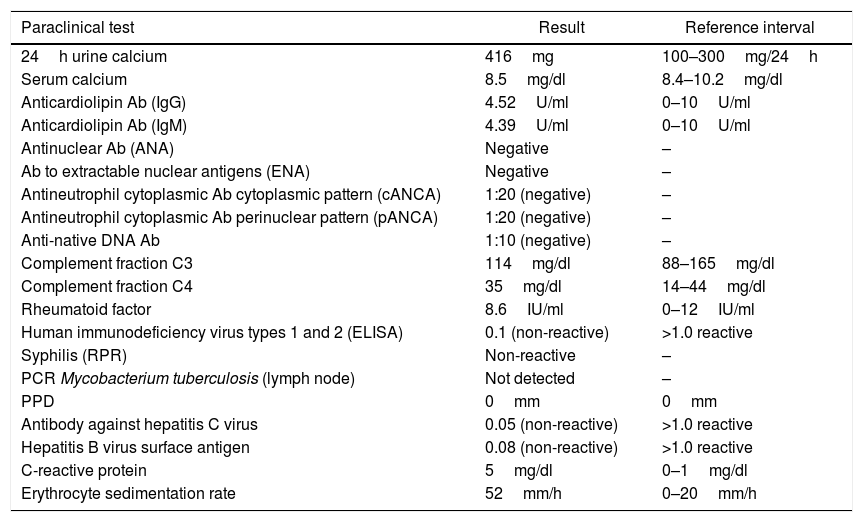
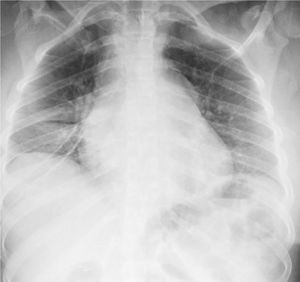
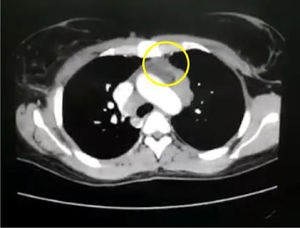
In the paraclinical exams, the only relevant findings are a mediastinal widening observed in the chest X ray (Fig. 2), secondary to multiple adenopathies of paratracheal, right hilar and paraesophageal locations and in station 6, described in the chest CT scan (Fig. 3), and hypercalciuria corresponding to a value of 416mg in 24h. Other autoimmune processes are ruled out with negativity for anticardiolipin IgG and IgM antibodies, antinuclear antibodies (ANA), extractable nuclear antigen antibodies (ENA), antineutrophil cytoplasmic antibodies (ANCA) perinuclear and cytoplasmic staining, anti-native deoxyribonucleic acid (anti-DNA) antibodies, and rheumatoid factor. Infectious diseases such as human immunodeficiency virus (HIV), syphilis and hepatitis B and C viruses are also excluded (Table 1).
Laboratory test results.
| Paraclinical test | Result | Reference interval |
|---|---|---|
| 24h urine calcium | 416mg | 100–300mg/24h |
| Serum calcium | 8.5mg/dl | 8.4–10.2mg/dl |
| Anticardiolipin Ab (IgG) | 4.52U/ml | 0–10U/ml |
| Anticardiolipin Ab (IgM) | 4.39U/ml | 0–10U/ml |
| Antinuclear Ab (ANA) | Negative | – |
| Ab to extractable nuclear antigens (ENA) | Negative | – |
| Antineutrophil cytoplasmic Ab cytoplasmic pattern (cANCA) | 1:20 (negative) | – |
| Antineutrophil cytoplasmic Ab perinuclear pattern (pANCA) | 1:20 (negative) | – |
| Anti-native DNA Ab | 1:10 (negative) | – |
| Complement fraction C3 | 114mg/dl | 88–165mg/dl |
| Complement fraction C4 | 35mg/dl | 14–44mg/dl |
| Rheumatoid factor | 8.6IU/ml | 0–12IU/ml |
| Human immunodeficiency virus types 1 and 2 (ELISA) | 0.1 (non-reactive) | >1.0 reactive |
| Syphilis (RPR) | Non-reactive | – |
| PCR Mycobacterium tuberculosis (lymph node) | Not detected | – |
| PPD | 0mm | 0mm |
| Antibody against hepatitis C virus | 0.05 (non-reactive) | >1.0 reactive |
| Hepatitis B virus surface antigen | 0.08 (non-reactive) | >1.0 reactive |
| C-reactive protein | 5mg/dl | 0–1mg/dl |
| Erythrocyte sedimentation rate | 52mm/h | 0–20mm/h |
Ab: antibody; ELISA: enzyme-linked immunosorbent assay; PCR: polymerase chain reaction; PPD: purified protein derivative; RPR: rapid plasma reagin.
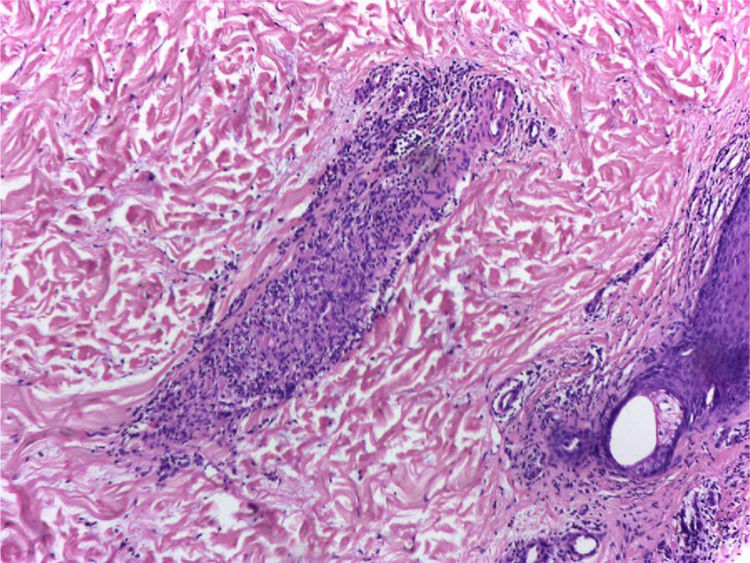
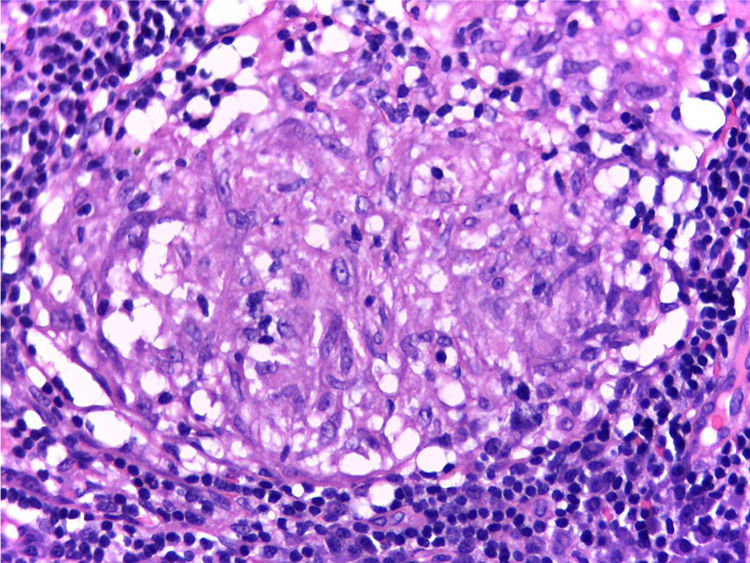
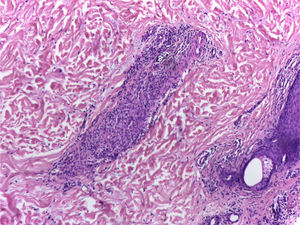
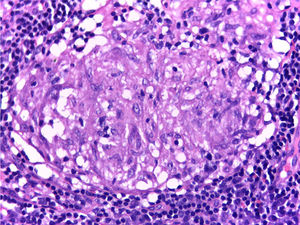
It is performed a skin biopsy, which reports an inflammatory process of septal localization in subcutaneous cellular tissue, characterized by the accumulation of lymphocytes, plasma cells and multinucleated giant cells, as well as few neutrophil polymorphonuclear cells, findings consistent with erythema nodosum (Fig. 4). Subsequently, a mediastinal adenopathy is resected by thoracoscopy, observing a chronic granulomatous inflammation without caseous necrosis (Fig. 5); both diseases with studies negative for infections and neoplasms, thus confirming the diagnosis of Löfgren's syndrome.
During the hospitalization, it is decided to initiate management with colchicine and chloroquine in order to control the symptoms of the patient and to improve hypercalciuria. Due to the satisfactory evolution, it is decided to discharge the patient adding steroids to her treatment.
DiscussionSarcoidosis is a disease difficult to diagnose due to the possible differentials with similar clinical characteristics, the absence of a specific diagnostic test and the different conditions that may present similar histopathological findings. Among the possible differential diagnoses we find etiologies that can cause a granulomatous reaction (including infections by mycobacteria, fungi, malignancy, inflammatory bowel disease, granulomatosis with polyangiitis, lymphogranulomatosis, giant cell arteritis, lupus erythematosus, polyarteritis nodosa, eosinophilic granulomatosis with polyangiitis, among others).2,5 It is important to inquire about the exposure to occupational and environmental particles in order to exclude entities such as berylliosis, which can go unnoticed in 40% of cases, and other entities such as silicosis and asbestosis.6 The interaction with organic aerosols plays a very important role when it comes to rule out other diseases, because it is a potential cause of hypersensitivity pneumonitis, thus causing respiratory symptoms similar to those of sarcoidosis.6
Although there are no specific studies to make the diagnosis of sarcoidosis, several tests to attempt to exclude other causes or to evaluate the activity of the disease have been developed. The angiotensin-converting enzyme is elevated in 60% of patients with sarcoidosis of acute presentation and in 10% of patients with chronic sarcoidosis. In addition, levels higher than twice the normal limit decrease the probability of diseases, as neoplastic causes (lymphoma).7 Unfortunately, due to the lack of specificity and sensitivity of this test,8 added to the polymorphism of the gene that synthetizes this enzyme, it is not useful as a screening test.6
One of the acute forms that this disease may exhibit is Löfgren's syndrome, originally described by Löfgren and Lundback in 1952.9 Tipically, it includes concurrently erythema nodosum (the most frequent form in women).1 which was reported in 58% of the patients studied in a series of cases in the Karolinska University Hospital in Sweden; inflammatory arthralgias, which were associated with erythema nodosum in 79.8% of the patients of this series, and bilateral hilar adenopathies, found in 100% of the patients with acute presentation.10 When the elements that make up the syndrome are found, the specificity for the diagnosis of sarcoidosis is up to 95%11; however, this is a diagnosis of exclusion and it does not represent the only identifiable cause of erythema nodosum, being, in most cases, idiopathic.12 The triad can be present from the beginning in 60% of cases, recurrences are infrequent (17%) and there is usually no serious organ involvement, which is why it is considered a relatively benign form of presentation of sarcoidosis.13,14
Erythema nodosum is a form of neutrophilic panniculitis whose etiology is not completely understood; however, it has been linked to the deposition of immune complexes in the septal veins of subcutaneous fat.15 Its link with sarcoidosis as a genetic variation of the LTA/TNF locus has been described in Caucasian population of the ACCESS study.16
Sarcoidosis, meanwhile, is a systemic granulomatous disease, whose etiology is not fully understood. It has been proposed that, being the lungs, the eyes and the skin the most affected organs, there is an association between airborne antigens and the genesis of the disease. It seems that various infectious agents and organic or inorganic agents have the ability to deposit non-degradable antigens that induce a response against self-antigens. This would cause activation of T cells by antigen-presenting cells, with subsequent recruitment of macrophages that, when compacted, form epithelioid cell granulomas.1Mycobacterium tuberculosis antigens have been linked as potential causative agents of sarcoidosis,17 although in this patient it was not demonstrated infection thereof. Therefore, studies should be always performed to rule out active tuberculosis infection that may simulate the disease or contraindicate immunosuppression. Usually, the purified protein derivative test (PPD) or interferon gamma release assays (IGRA)5 have been proposed as a part of the screening. However, it has been found that the mycobacterial ESAT-6 and KatG peptides induce greater production of gamma interferon by the T cells compared with the PPD,18 and in different series have been reported false negatives of PPD versus IGRA that suggest a state of anergy towards these protein derivatives in the disease,19,20 with a performance of IGRA that seems not to be affected by the treatment or the activity of the sarcoidosis,21,22 reason by which the literature suggests to prefer IGRA rather than PPD in case of screening for tuberculosis.
An important associated finding, documented in our patient, was the hypercalciuria, which can be found in between 40 and 62% of cases of sarcoidosis; hypercalcemia is less frequent and is usually asymptomatic, affecting about 5% of patients.23,24 Calcium disorders in the context of sarcoidosis are apparently favored by the conversion of 25-hydroxyvitamin-D into 1.25 hydroxyvitamin-D, by the expression of alpha-1-hydroxylase in the granulomas. The presence of a peptide similar to parathormone originated in the same way in the granulomas has also been proposed.24,25
The treatment of sarcoidosis depends on the severity of the manifestations and the organs involved.26 When the affection is mainly cutaneous, topical steroids and tacrolimus are indicated. As it is considered that there is a greater systemic impact, immunomodulatory (chloroquine, hydroxychloroquine) and immunosuppressant (prednisone, methotrexate, azathioprine, mycophenolate mofetil) drugs, and even anti-tumor necrosis factor biological drugs (adalimumab, infliximab) can be used,26,27 being the latter reserved for patients with pulmonary or extrapulmonary sarcoidosis who have not responded to previous treatments.28,29 In the case of hypercalciuria, management has generally been based on steroids and antimalarials,30 which were administered to our patient. In addition, the use of colchicine has been described in patients with Löfgren's syndrome and erythema nodosum.28
The importance of this report lies in attracting the attention of the clinician to an uncommon disease such as sarcoidosis and, in its context, Löfgren's syndrome. In these cases it is possible to favorably impact the patient's health with a therapy based on basic drugs,3 with a satisfactory clinical response.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of dataThe authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consentThe authors have obtained the written informed consent of the patients or subjects mentioned in the article. The corresponding author is in possession of this document.
Conflict of interestThe authors declare they do not have any conflict of interest.
To Dr. Sebastián Herrera Uribe, MD, Rheumatologist of the General Hospital of Medellin, for the facilities for the investigation of the case and the clinical contributions.
Please cite this article as: Montoya Castillo M, Herrera Uribe S, Berlinghieri Pérez JD. El síndrome de Löfgren como presentación aguda de la sarcoidosis. Rev Colomb Reumatol. 2018;25:126–131.