Treatment of patients with Coronavirus Disease 2019 (COVID-19) has affected the management of patients with colorectal cancer (CRC). The aim of this study was to compare the diagnosis delay, symptoms, and stage of patients with CRC during the pandemic with a control cohort.
Material and methodsPatients referred to the CRC multidisciplinary team between September 2019 and January 2020 (cohort 1, control group) were compared with those who presented between September 2020 and March 2021 (cohort 2, pandemic group).
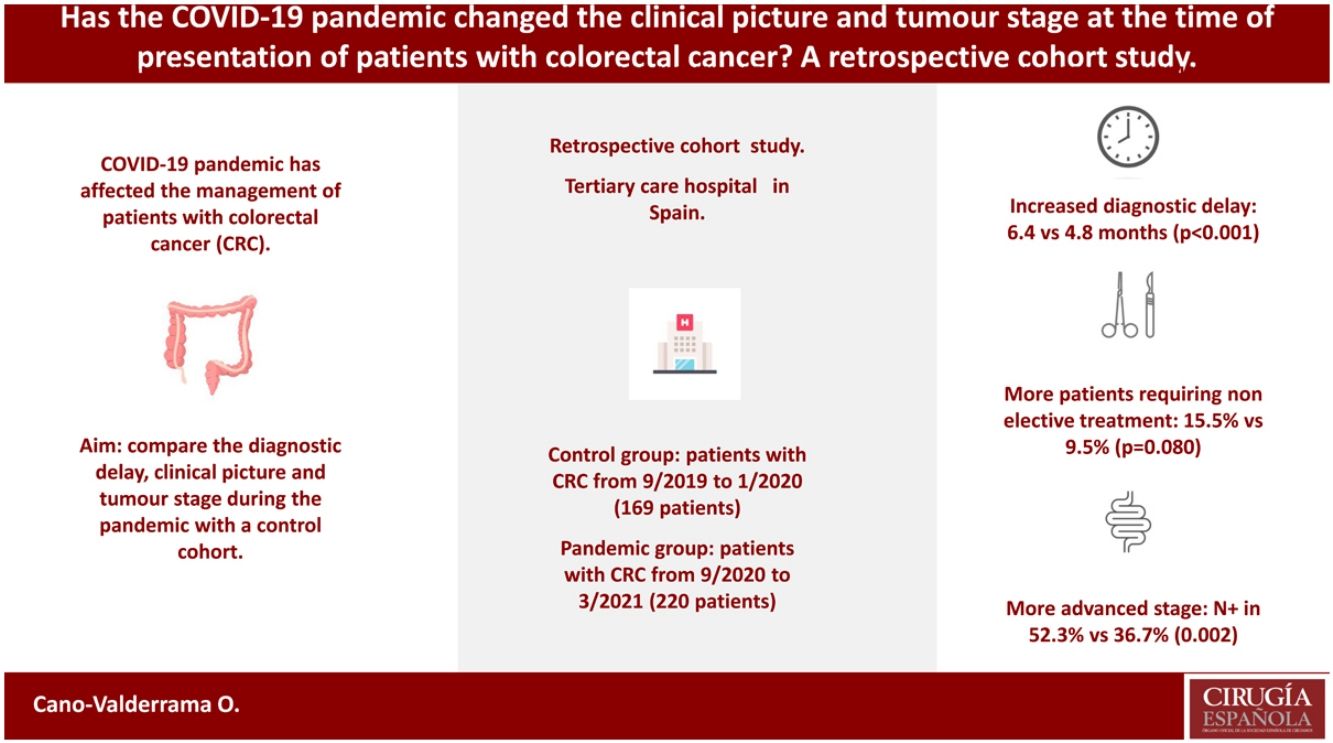
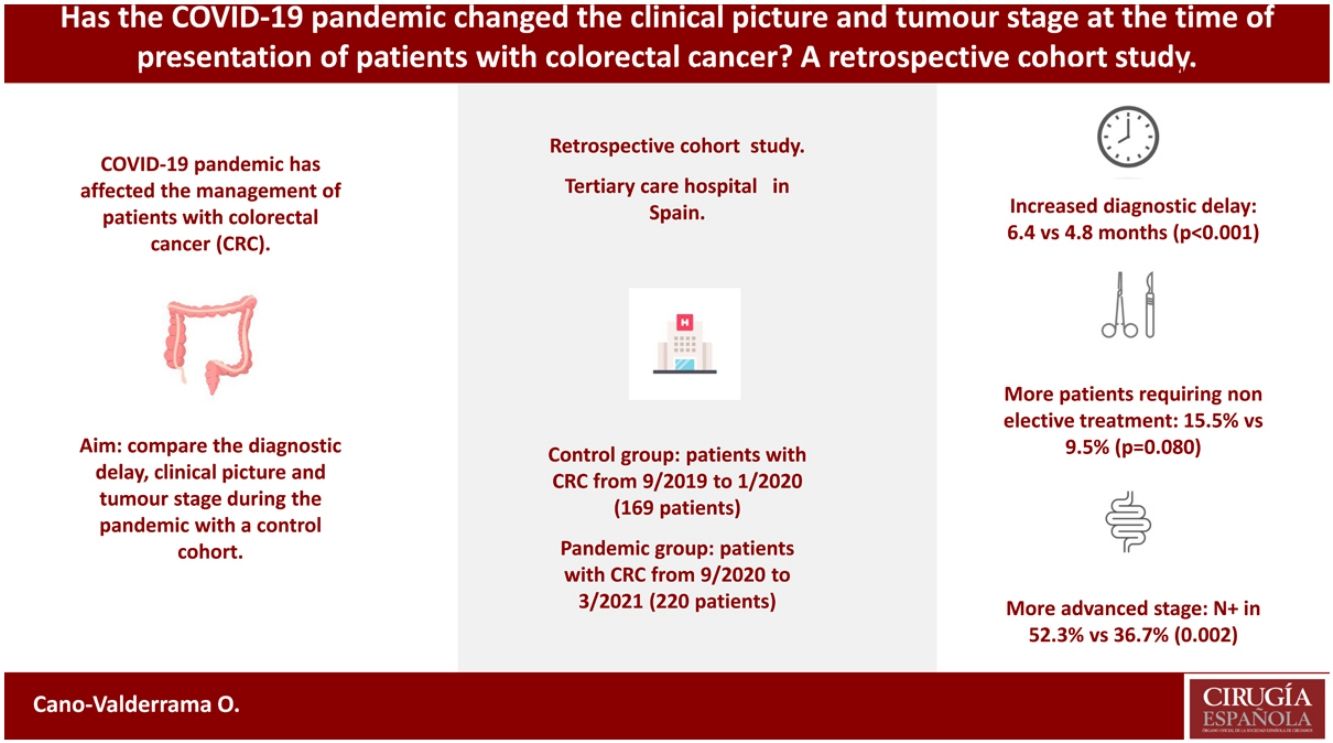
Results389 patients were included, 169 in cohort 1 and 220 in cohort 2. No differences were observed in the main characteristics of the patients. CRC screening and anaemia were the most common causes leading to the diagnosis of the tumour in cohort 1 and 2, respectively (p<0.001). Diagnostic and therapeutic delay was longer in cohort 2 [6.4 (95% CI 5.8–6.9) vs. 4.8 (95% CI 4.3–5.3) months, p<0.001]. More patients required non-elective treatment in the pandemic cohort (15.5% vs. 9.5%, p=0.080). The tumour stage was more advanced in patients in cohort 2 [positive nodes in 52.3% vs. 36.7% (p=0.002), and metastatic disease in 23.6% vs. 16.6% (p=0.087)].
ConclusionCRC patients in the pandemic cohort had a longer diagnostic and therapeutic delay and less patients were diagnosed because of CRC screening. In addition, patients with CRC during the pandemic needed non-elective treatment more frequently than patients in the control cohort, and their tumour stage tended to be more advanced.
La pandemia de la enfermedad por coronavirus 2019 ha afectado al manejo de los pacientes con cáncer colorrectal (CCR). El objetivo de este estudio fue comparar el retraso diagnóstico, la sintomatología y el estadio de los pacientes con CCR durante la pandemia con una cohorte histórica.
Material y métodosLos pacientes valorados en el comité multidisciplinar de CCR entre septiembre de 2019 y enero de 2020 (cohorte 1) se compararon con los presentados entre septiembre de 2020 y marzo de 2021 (cohorte 2).
ResultadosTrescientos ochenta y nueve pacientes fueron incluidos, 169 en la cohorte 1 y 220 en la cohorte 2. El cribado del CCR y la anemia fueron las causas que llevaron al diagnóstico en más pacientes en la cohorte 1 y 2, respectivamente (p<0,001). El retraso diagnóstico y terapéutico fue mayor en la cohorte 2 (6,4 [IC 95%: 5,8-6,9] vs. 4,8 [IC 95%: 4,3-5,3] meses, p<0,001). En la cohorte pandémica hubo más pacientes que requirieron tratamiento urgente (15,5% vs. 9,5%, p=0,080). El estadio tumoral fue más avanzado en la cohorte 2 (ganglios positivos en el 52,3% vs. 36,7% [p=0,002] y enfermedad metastásica en el 23,6% vs. 16,6% [p=0,087]).
ConclusiónLos pacientes con CCR en la cohorte pandémica tenían un retraso diagnóstico y terapéutico más largo, y menos pacientes fueron diagnosticados en el cribado de CCR. Además, los pacientes con CCR durante la pandemia necesitaron tratamiento urgente con más frecuencia y su estadio tumoral fue más avanzado.
On 11 March 2020, the World Health Organization declared the coronavirus disease 2019 (COVID-19) caused by the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a pandemic. This pandemic overwhelmed the health systems of many countries all around the world. Moreover, the extra healthcare burden caused by the pandemic forced hospitals to focus their resources on the treatment of patients with SARS-CoV-2 infections. Therefore, the treatment of patients with other diseases, such as colorectal cancer (CRC), was also affected.
Several authors have published their experience with the treatment of patients with CRC during the pandemic,1–4 with most centres observing a drop in the number of patients with CRC diagnosed or treated during the pandemic.5–18 One of the main reasons for this reduction was that most CRC screening programmes were cancelled during the pandemic lockdown.19–21 Moreover, endoscopy services also reduced their activity and fewer colonoscopies were performed during this time to diagnose patients with suspected CRC.22,23
A relationship between long-term oncological results and treatment delay for CRC patients has previously been described.24,25 Therefore, some authors have expressed their concern about how CRC diagnosis and treatment delay could have impacted tumour stages at presentation as well as long-term mortality.26 The aim of this study was to analyse the diagnosis and treatment delay as well as the tumour stage of patients diagnosed with CRC and treated during the pandemic in a tertiary care hospital in Spain. The results of this cohort were compared with a cohort of patients with CRC that were treated in the same centre before the pandemic started.
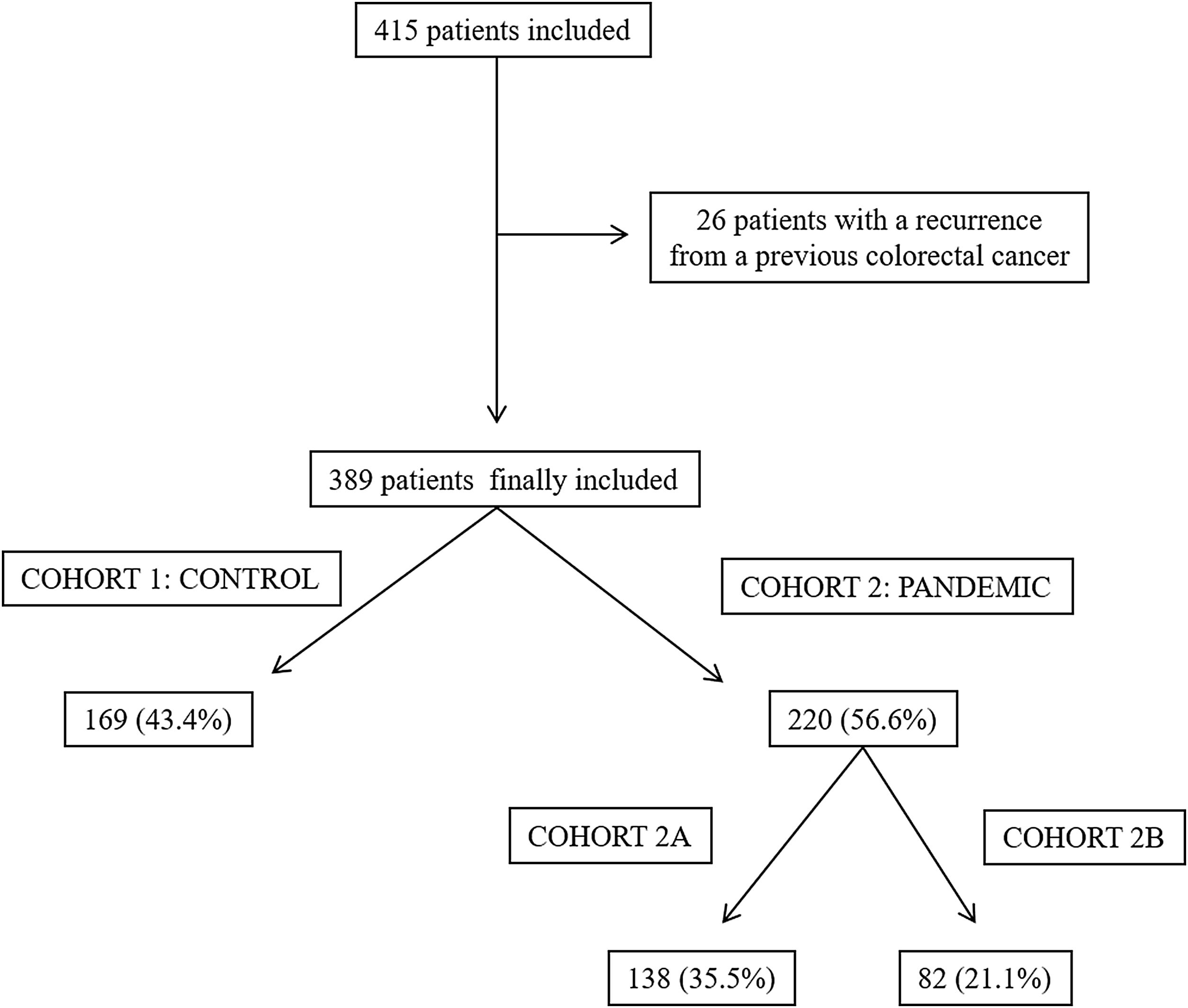
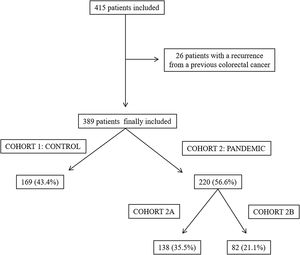
Material and methodsA retrospective cohort study was performed in a tertiary care hospital in Spain, which had a reference population of 433,514. The treatment of all the patients diagnosed with CRC that this centre were discussed at a multidisciplinary team meeting. All the patients presented at this meeting between September 2019 and January 2020 were included in the control cohort (cohort 1) while patients who presented between September 2020 and March 2021 formed the pandemic cohort (cohort 2). In turn, the pandemic cohort comprised two sub-cohorts: cohort 2A included the patients presented at the multidisciplinary team meeting between September 2020 and December 2020 and cohort 2B included the patients presented between January 2021 and March 2021. Both these sub-cohorts were compared with the control cohort. The division of the pandemic cohort in two sub-cohorts was performed to assess whether there were changes in the results depending on the time of evolution of the pandemic. Each sub-cohort was always compared with the control cohort. Patients with a recurrence from a previously treated CRC were excluded from both groups in this study.
Information about the sociodemographic features, main comorbidities, American Society of Anaesthesiology Classification (ASA), clinical symptoms of CRC, diagnosis and treatment delay, participation in the CRC screening programme, and tumour stage according to the AJCC (8th Edition) were collected using patient electronic medical record data. Diagnosis and treatment delay (DTD) was calculated as the sum of the pre-diagnosis delay (PDD) time (the time from the onset of symptoms to the diagnosis of CRC by colonoscopy or computerised tomography), diagnosis delay (DD) time (from diagnosis to a therapeutic decision, i.e., the time used to perform the requisite complementary tests), and therapeutic delay (TD) time (the time from the therapeutic decision until the day that treatment was initiated).
Colorectal cancer screening programmeThe CRC screening programme in our region was cancelled on 13 March 2020 and was reinstated on 11 May 2020. First, patients with a positive faecal immunochemical test result who could not undergo a colonoscopy because of the pandemic were called for endoscopic evaluation. Then, from 28 May 2020, new appointments were arranged to continue the CRC screening programme with faecal immunochemical tests.21
Statistical analysisQuantitative variables were expressed as the mean [95% confidence interval (CI)] and qualitative data were shown as the number of cases (percentage). The quantitative and qualitative variables were compared using Student t test for independent samples and χ2 tests as appropriate; p values under 0.05 were considered statistically significant. Stata® 13.1 software (StataCorp, TX, USA) was used for all the statistical analyses.
The authors declare that they have no conflicts of interest. This study was not supported by any funding sources. All the procedures performed in studies involving human participants were done so in accordance with the ethical standards of our institution and national research committee and with the 1964 Helsinki declaration and its later amendments. For this type of study, no formal consent was required. This research was approved by our Institutional Review Board (Code 2021/173).
ResultsA total of 415 patients were initially considered in this study; however, 26 of them were excluded as they had a recurrence from a previously treated CRC. Finally, 389 patients were included, 169 (43.4%) in cohort 1 (the control group) and 220 (56.6%) in cohort 2 (the pandemic group). In the latter group, 138 patients were included in cohort 2A and 82 were included in cohort 2B (Fig. 1).
The mean age of the patients included was 70.8 (95% CI [69.7–71.9]) years and 242 (62.2%) of them were male; 211 (54.2%) patients were ASA grade I or II. Anaemia was the most common symptom that led to the diagnosis of the tumour in cohort 2 (23.7%). The mean DTD was 5.7 (95% CI [5.3–6.1]) months, with the mean PDD, DD, and TD being 3.7 (95% CI [3.3, 4.1]), 1.0 (95% CI [0.9–1.1]), and 1.0 (95% CI [0.9–1.0]) months, respectively. The rectum was the most frequent tumour location (132 patients, 33.9%). Moreover, 27 (6.9%) patients required stent placement because of large bowel obstruction and 50 (12.9%) underwent non-elective treatment (stent placement or acute care surgery); 47.3% of the patients had an advanced tumour (stage III or IV).
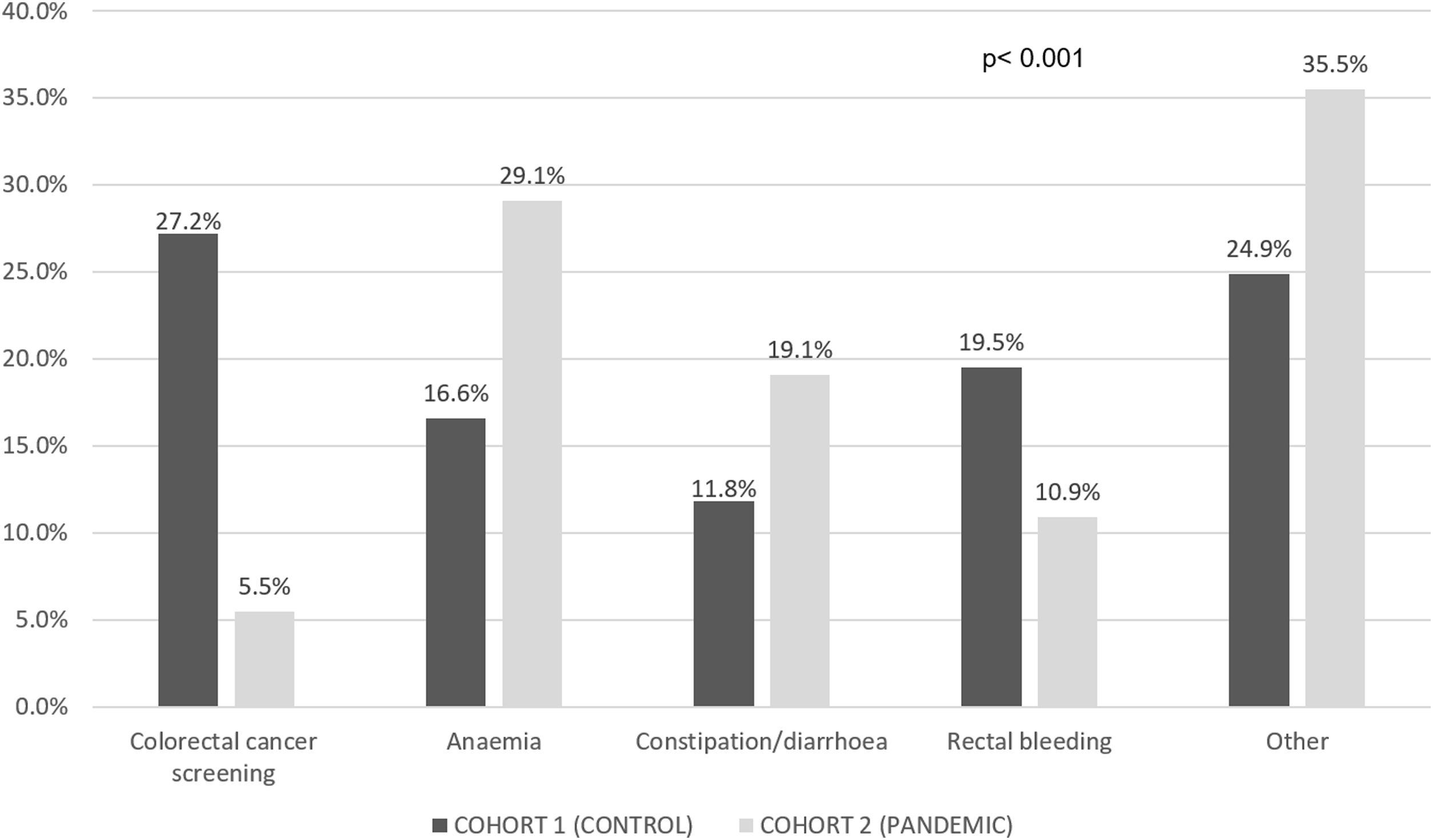
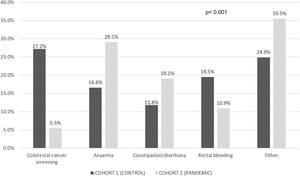
Table 1 compares the main characteristics of the patients in cohorts 1 and 2 and Fig. 2 shows the symptoms that led to the CRC diagnosis in each of the groups.
Comparison of the sociodemographic characteristics of patients in cohort 1 (the control group) and cohort 2 (the pandemic group).
| Cohort 1 (control) | Cohort 2 (pandemic) | p | |
|---|---|---|---|
| N | 169 | 220 | – |
| Mean age (years) | 70.0 (95% CI [68.3–71.6]) | 71.4 (95% CI [69.9–73.0]) | 0.196 |
| Gender (% male) | 64 (37.9%) | 83 (37.7%) | 0.977 |
| Hypertension (%) | 84 (49.7%) | 111 (50.5%) | 0.883 |
| Dyslipidaemia (%) | 74 (43.8%) | 98 (44.6%) | 0.881 |
| Obesity (%) | 40 (23.7%) | 56 (25.5%) | 0.686 |
| Diabetes (%) | 35 (20.7%) | 44 (20.0%) | 0.863 |
| Ischaemic heart disease (%) | 21 (12.4%) | 15 (6.8%) | 0.059 |
| Chronic pulmonary obstructive disease (%) | 13 (7.7%) | 16 (7.3%) | 0.876 |
| Chronic kidney disease (%) | 6 (3.6%) | 10 (4.6%) | 0.624 |
| ASA (% ASA III or IV) | 77 (45.6%) | 101 (45.9%) | 0.946 |
ASA: American Society of Anaesthesiology Classification.
More patients suffered anaemia and constipation or diarrhoea in the pandemic cohort [35.5% vs. 24.3% (p=0.018) for anaemia and 45.9% vs. 33.1% (p=0.011) for constipation or diarrhoea] compared to the control group. Table 2 compares the DTD in both cohorts. PDD was longer in the pandemic cohort even if asymptomatic patients were excluded from the analyses (4.8 [95% CI 4.3–5.4] vs. 3.5 [95% CI 2.9–4.1] months, p=0.002).
Comparison of the diagnosis and therapeutic delay, pre-diagnosis delay, diagnosis delay, and therapeutic delay in both cohorts.
| Cohort 1 (control) | Cohort 2 (pandemic) | p | |
|---|---|---|---|
| DTD (months) | 4.8 (95% CI [4.3–5.3]) | 6.4 (95% CI [5.8–6.9]) | <0.001 |
| PDD (months) | 2.6 (95% CI [2.1–3.1]) | 4.6 (95% CI [4.0–5.1]) | <0.001 |
| DD (months) | 1.0 (95% CI [0.9–1.2]) | 0.9 (95% CI [0.8–1.1]) | 0.391 |
| TD (months) | 1.2 (95% CI [1.1–1.3]) | 0.8 (95% CI [0.8–0.9]) | <0.001 |
DTD: diagnosis and therapeutic delay; PDD: pre-diagnosis delay; DD: diagnosis delay; TD: therapeutic delay.
Stent placement was required in 19 (8.6%) and 8 (4.7%) patients because of large bowel obstruction in the pandemic and control cohorts, respectively (p=0.133). In addition, more patients required non-elective treatment (stent placement or acute care surgery) in cohort 2 (15.5% vs. 9.5%, p=0.080). Tumour stage was more advanced for patients included in the pandemic cohort, as observed in the tumours (T3 or T4 in 74.7% vs. 61.5%, p=0.006), lymph nodes (N1 or N2 in 52.3% vs. 36.7, p=0.002), and metastases (M1 in 23.6% vs. 16.6%, p=0.087) TNM staging. Table 3 compares the tumour staging for cohorts 1 and 2.
Table 4 compares the diagnosis and therapeutic delay, stent placement, non-elective treatment, and tumour stage in the control group and cohort 2a (early pandemic) and cohort 2b (late pandemic).
Comparison between the results in cohort 1 (control), cohort 2a (early pandemic), and cohort 2b (late pandemic).
| Cohort 1 (control) | Cohort 2a (early pandemic) | p (cohort 1 vs. cohort 2A) | Cohort 2b (late pandemic) | p (cohort 1 vs. cohort 2a) | |
|---|---|---|---|---|---|
| DTD (months) | 4.8 (95% CI [4.3–5.3]) | 6.7 (95% CI [6.0–7.5]) | <0.001 | 5.7 (95% CI [4.8–6.6]) | 0.053 |
| PDD (months) | 2.6 (95% CI [2.1–3.1]) | 4.9 (95% CI [4.1–5.6]) | <0.001 | 4.1 (95% CI [3.3–4.9]) | 0.001 |
| DD (months) | 1.0 (95% CI [0.9–1.2]) | 1.0 (95% CI [0.8–1.2]) | 0.908 | 0.8 (95% CI [0.6–1.0]) | 0.1 |
| TD (months) | 1.2 (95% CI [1.1–1.3]) | 0.9 (95% CI [0.8–1.0]) | <0.001 | 0.8 (95% CI [0.6–0.9]) | <0.001 |
| Stent placement (%) | 8 (4.7%) | 9 (6.5%) | 0.497 | 10 (12.2%) | 0.038 |
| Non-elective treatment (%) | 16 (9.5%) | 16 (11.6%) | 0.545 | 18 (22.0%) | 0.008 |
| T stage (T3–T4%) | 102 (61.5%) | 95 (70.4%) | 0.106 | 67 (81.7%) | 0.002 |
| N stage (N1–N2%) | 62 (36.7%) | 65 (47.1%) | 0.066 | 50 (61.0%) | <0.001 |
| M stage (M1%) | 28 (16.6%) | 27 (19.6%) | 0.496 | 25 (30.5%) | 0.012 |
| Tumour stage (stage III–IV%) | 66 (39.1%) | 66 (47.8%) | 0.123 | 52 (63.4%) | <0.001 |
DTD: diagnosis and therapeutic delay; PDD: pre-diagnosis delay; DD: diagnosis delay; TD: therapeutic delay.
Since the start of the COVID-19 pandemic, healthcare systems have been under great pressure. Therefore, the diagnosis and treatment of patients with non-COVID-related diseases have also been affected. In our study, patients with CRC diagnosed during the pandemic had a longer DTD caused by a delay between the onset of symptoms and a CRC diagnosis. This finding is worrying, especially when considering that the tumour stage was also more advanced in the pandemic cohort. Thus, it will be important for healthcare systems to adjust their resources and structures to ensure that patients with CRC are always accurately diagnosed and treated in a timely manner.
No differences were observed when we compared the characteristics of the patients included in the control and pandemic cohorts indicating, that both groups were comparable. It is possible that high-risk patients (for example older patients or patients with a higher ASA) may have been afraid of SARS-CoV-2 infection and thus, could have delayed seeking medical advice during the pandemic after developing symptoms of CRC. However, our results suggest that did not happen and that high-risk patients still asked for medical advice and treatment at the same rate as other patients.
CRC screening was the most common cause of diagnosis for the control cohort while patients diagnosed during the pandemic most frequently referred anaemia, constipation, or diarrhoea. Thus, the closure of our screening programme was probably one of the main causes of delayed CRC diagnosis. Indeed, CRC screening programmes were cancelled nationally during the pandemic and gradually restarted their activity.16–18 Moreover, the effect of the screening programmes cancellation could be longer than the time they were closed; as the restart of the programme was gradual and many patients could have decided not to be included in the programmes to avoid unnecessary exposition to the virus. Some authors have analysed the impact that the cancellation of these CRC screening programmes may have had on CRC. For example, Ricciardiello et al. built a procedural model and concluded that delaying CRC screening by 4–6 months would increase the number of advanced CRC cases, as well as mortality if postponed by more than 12 months.27 Another study using microsimulation models found that suspending screening for six-months could increase the CRC incidence and mortality rates.28 Therefore, it is of utmost importance that CRC screening programmes are fully reinstated and that health authorities make sure that the public knows that these programmes are working and how important they are.
In this study, total DTD was longer in the pandemic cohort, which was worrying because some authors have described a relationship between the time to treatment initiation and mortality.24 The most important reason for a diagnosis delay was a longer time between the onset of symptoms and a CRC diagnosis. Cancellation of CRC screening programmes could be one of the reasons for this delay, but other factors may also be involved. One of these elements could be primary care attention. During the pandemic, general practitioners had to attend many patients with COVID-19 and so, they had less time for patients with other pathologies. Moreover, many general practitioner consultations were carried out by telephone or video call. This meant that patients were not properly examined and important findings may have been omitted. For example, rectal bleeding could be wrongly attributed to proctological disorders without a proper assessment. Given the importance of resolving the origin of symptoms suspected of CRC as soon as possible, the availability of primary care faculties and resources must be adjusted in the future to ensure that all patients, including those without COVID-19, are properly assessed.
In contrast to the delay in diagnosing CRC, the time from diagnosis to the therapeutic decision was unchanged during the pandemic, while the time taken to treat patients was even somewhat shortened. This was because oncological patients were considered a priority and therefore, they underwent surgery even during the busiest periods of the pandemic. This finding will be different in regions with a higher incidence of the pandemic or if clean circuits could not be implemented to go on with the oncologic surgery.
However, the delay in diagnosis had implications on the percentage of patients requiring non-elective treatment (stent placement or acute care surgery). Although these differences were not statistically significant when the control and pandemic groups were compared, a statistically significant increase was observed when cohort 1 was compared with cohort 2B. This finding suggests that the effect of CRC diagnosis delay took some time to become clinically significant and so, more time might be needed to fully assess the real consequences of this delay. Similar results were also found by other authors16,29,30; for example, Mizuno et al. observed an increase of obstructive CRCs31 and Shinkwin et al. reported a higher-risk of emergency presentations during the pandemic.32 Dividing the pandemic cohort in two sub-cohorts we could observe that patients with CRC didn’t present in a homogeneous way during all the pandemics. Therefore, more studies will be needed to assess how the evolution of the pandemic affected the tumour stage and clinical picture of patients with CRC.
Finally, we also analysed tumour stage in this study. During the pandemic, there was a higher percentage of patients with advanced tumours (T3 or T4 tumours, and patients with positive nodes and metastatic disease). Once again, this difference was more significant for the late pandemic cohort (Cohort 2B). Considering the importance of tumour stage on long-term survival, stage migration as a result of the pandemic could worsen long-term survival in patients diagnosed with CRC. Indeed, studies analysing this same issue early in the pandemic have already observed similar, but less significant, differences.6,8,13,14,29,32
The main limitation of this current study was its retrospective design. Nevertheless, both the control and pandemic cohorts were comparable, and no differences were observed in the main patient characteristics. The second limitation was that long-term oncological results could not be assessed because of our short patient follow-up time. Although tumour stage is an important factor, a longer follow-up will be needed to understand the real impact of the COVID-19 pandemic on disease recurrence and survival in patients with CRC. Finally, we observed that non-elective treatment, stent placement, and advanced tumour stage was more frequent in the late pandemic cohort (Cohort 2B). This suggests that the effects of the pandemic are still unfolding and so, its effects on patients with CRC should be assessed over the coming months and years.
In conclusion, colorectal cancer patients in the COVID-19 pandemic cohort had a longer DTD than patients in the control cohort because of the longer time from the onset of symptoms to the CRC diagnosis. Patients diagnosed as a result of the CRC screening programme decreased during the pandemic. Moreover, more patients with CRC needed stent placement and non-elective treatment during the pandemic than those in the control cohort. Finally, tumour stage was more advanced in patients diagnosed with CRC during the pandemic.
FundingThis study was not supported by any funding sources.
Conflicts of interestThe authors declare that they have no conflicts of interest.