Early diagnosis of candidemia is critical for the correct management and treatment of patients.
AimsTo test the efficacy of different blood culture bottles in the growth of Candida strains.
MethodsWe compared the performance of BD BACTEC™ Plus Aerobic/F (Aero) culture bottles with the specific BD BACTEC™ Mycosis IC/F Lytic (Myco) culture bottles using the BD BACTEC™ FX 40 automated blood culture system to determine the mean time-to-detection (TTD) in Candida species. One isolate each of six Candida species was inoculated into blood culture bottles (final concentration, 1–5CFUml−1) and incubated at 37°C until automated growth detection.
ResultsCandida albicans and Nakaseomyces glabratus (Candida glabrata) were detected earlier in the specific culture bottle, whereas Candida tropicalis was detected earlier in the nonspecific bottle; Candida parapsilosis, Pichia kudriavzevii (Candida krusei), and Meyerozyma guilliermondii (Candida guilliermondii) presented similar TTD in both bottles.
ConclusionsOur study suggests the suitability of using both bottles in clinical laboratories for a faster diagnosis and prompt starting of any treatment.
El diagnóstico precoz de la candidemia es fundamental para el correcto manejo y tratamiento de los pacientes.
ObjetivosComprobar la eficacia de diferentes frascos de hemocultivo en el crecimiento de cepas de Candida.
MétodosComparamos el rendimiento de los frascos de cultivo BD BACTEC™ Plus Aerobic/F (Aero) con los frascos de cultivo BD BACTEC™ Mycosis IC/F Lytic (Myco) específicos utilizando el sistema automatizado de hemocultivo BD BACTEC™ FX 40 para determinar la media de tiempo de detección (TTD) de especies de Candida. Se inoculó un aislamiento de cada una de seis especies de Candida en los frascos de hemocultivo (concentración final, 1-5CFUml−1) y se incubaron a 37°C hasta la detección automática del crecimiento.
ResultadosCandida albicans y Nakaseomyces glabratus (Candida glabrata) se detectaron antes en el frasco de cultivo específico, mientras que Candida tropicalis se detectó antes en el frasco no específico; Candida parapsilosis, Pichia kudriavzevii (Candida krusei) y Meyerozyma guilliermondii (Candida guilliermondii) presentaron TTD similares en ambas botellas.
ConclusionesNuestro estudio sugiere el uso simultáneo de ambos frascos en los laboratorios clínicos para un diagnóstico más rápido y un inicio inmediato del tratamiento.
Candida species are the fourth most common cause of bloodstream infections. Candidemia usually occurs in a healthcare setting and results in a high morbidity and mortality (up to 40%) of hospitalized patients.17,19,9 The most common species causing candidemia are Candida albicans, Candida parapsilosis, Candida tropicalis, Nakaseomyces glabratus (Candida glabrata), Pichia kudriavzevii (Candida krusei) and Meyerozyma guilliermondii (Candida guilliermondii).16,4
Risk factors for acquiring candidemia are long periods of hospitalization in intensive care units (ICU), exposure to broad-spectrum antibiotics, use of artificial devices (central venous catheter, intravascular lines, prosthetics, implantable cardiac devices), abdominal viscus loss of integrity, and abdominal surgery.13,10,14 Furthermore, Candida infections often occur in patients with malignancies, immune compromise, or diabetes mellitus.20,2
In view of the severity of candidemia with high mortality of patients, an early diagnosis is the key to a better outcome.12 The “gold standard” test for diagnosing candidemia is blood culture. Automated systems achieve higher rates of sensitivity, and are time-saving.8 Since bacterial or fungal agents can cause sepsis, culture bottles are designed to grow a spectrum of these microorganisms. BD BACTEC™ Plus Aerobic/F (Becton Dickinson, New Jersey, United States - USA) is designed to grow aerobic microorganisms, mostly bacterial, although yeasts can also grow, whereas BD BACTEC™ Mycosis IC/F Lytic (Becton Dickinson, New Jersey, USA) is designed more specifically for fungal growth due to its complementation with saponin 0.24% (blood lysing agent), ferric ammonium citrate, chloramphenicol and tobramycin (Becton Dickinson, New Jersey, USA), limiting bacterial growth and thus increasing the capacity for fungal detection.7,18
Since an early diagnosis of candidemia is correlated with more favorable outcomes and reduction in the total cost of hospitalization, we studied the efficiency of a blood culture method specific to fungi, and compared the results to those obtained with the nonspecific bottle. We compared the performance of BD BACTEC™ Plus Aerobic/F with the BD BACTEC™ Mycosis IC/F Lytic culture bottle, using the automated BD BACTEC™ FX 40 blood culture system in vitro to evaluate the speed of detection of Candida species.
Material and methodsIsolates from six species of Candida, representing the most common species encountered in blood cultures [C. albicans (number M7122), C. parapsilosis (number M6993), C. tropicalis (number M8459), N. glabratus (C. glabrata) (number M6674), P. kudriavzevii (C. krusei) (number M6666) and M. guilliermondii (C. guilliermondii) (number M7757)], from the fungal collection of the Mycology Laboratory (Faculdade de Medicina – FAMED/Universidade Federal do Rio Grande – FURG) were studied. All the isolates included (one for each species) were previously retrieved from their frozen stocks, subcultured on Sabouraud dextrose agar (SDA) plates, and colonies were identified using matrix-assisted laser desorption ionization-time-of-flight-mass spectrometry (MALDI-TOF-MS, Bruker Corporation®, Billerica, Massachusetts, USA, database flex control version 3.4).
A yeast inoculum from young colonies was standardized (two days on Sabouraud agar at 25°C) using a spectrophotometer (K37-UVVIS, KASVI, São José dos Pinhais, Brazil) (530nm), according to the Clinical and Laboratory Standards Institute document M27-ED4,1 and serial dilutions in sterile saline solution to achieve a concentration of 5–25 colony forming units (CFU) per ml were made. The inoculum concentration of all species was confirmed by plating 0.5ml from the final solution onto Sabouraud dextrose agar plates (KASVI) that were incubated at 35°C until growth for CFU counting.
An aliquot of 0.5ml of the standardized inoculum (5–25CFUml−1) of each Candida species was added to 2ml of fresh uninfected whole blood samples (from healthy volunteers), resulting in a final concentration of 1–5CFUml−1. After homogenization, 1ml of the experimentally infected blood was immediately inoculated into BD BACTEC™ Mycosis IC/F Lytic (Myco), and another 1ml into the BD BACTEC™ Plus Aerobic/F culture (Aero) bottles. The bottles were incubated in the BACTEC™ FX40 at 37°C, with continuous agitation until growth was detected. The time necessary to the BACTEC™ sensor to detect growth was analyzed, and an aliquot of 0.1ml was spread on CHROMagar™ Candida Agar (DIFCO, Michigan, USA), incubated at 35°C for 48h, and resubmitted to MALDI-TOF-MS to confirm that the growth detected was pure and corresponded to the Candida species added, validating the experiment. All assays were performed in biological duplicates through two independent experiments performed on different days.
The time-to-detection (TTD) for each Candida species was registered through the BD System BACTEC™ FX 40 equipment, and the mean from both experiments was calculated. The results obtained with both the BD BACTEC™ Mycosis IC/F Lytic (Myco) and BD BACTEC™ Plus Aerobic/F (Aero) culture incubation system were compared using the Student's t test with SPSS 25.0® software (IBM, Chicago, USA). p-Values ≤0.05 were considered statistically significant.
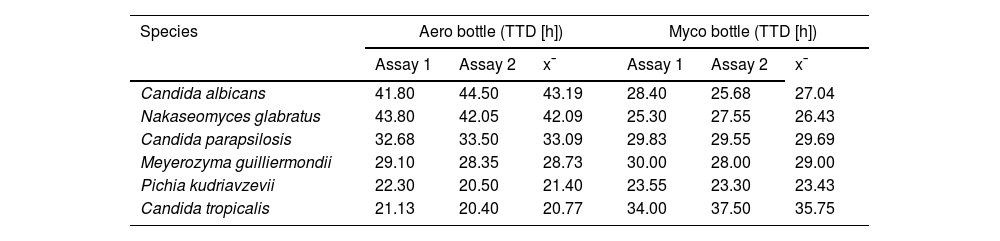
ResultsCandida was detected in all the blood samples experimentally infected in less than 45h of incubation in both culture bottles (Myco and Aero). There were no significant differences in TTD results between the duplicate experiments (Table 1).
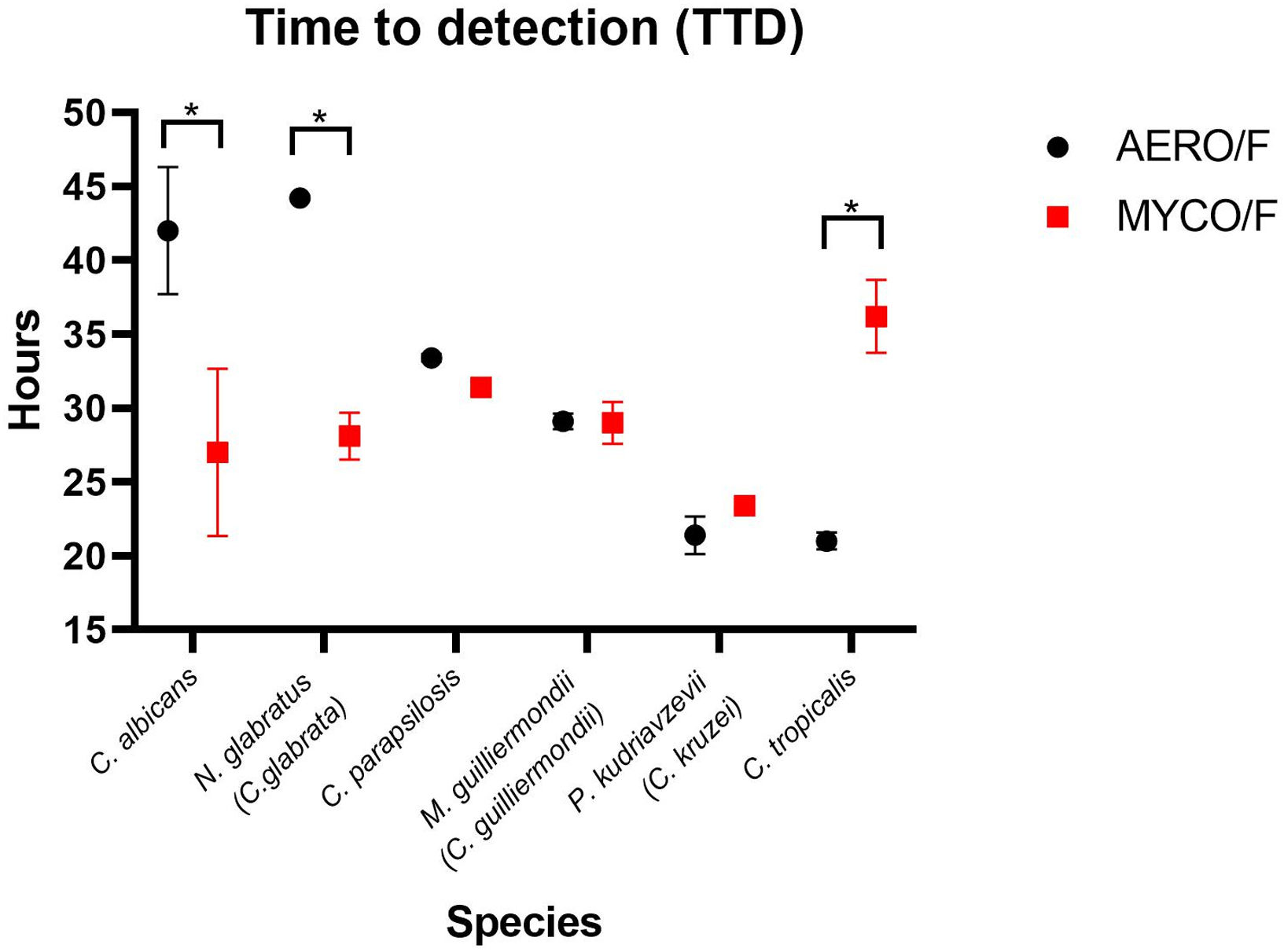
Time-to-detection (TTD) in hours obtained from two blood cultures each of Candida albicans, Nakaseomyces glabratus (Candida glabrata), Candida parapsilosis, Meyerozyma guilliermondii (Candida guilliermondii), Pichia kudriavzevii (Candida krusei), and Candida tropicalis in BD BACTEC™ Plus Aerobic/F (Aero) or BD BACTEC™ Mycosis IC/F Lytic (Myco) bottles.
| Species | Aero bottle (TTD [h]) | Myco bottle (TTD [h]) | ||||
|---|---|---|---|---|---|---|
| Assay 1 | Assay 2 | x¯ | Assay 1 | Assay 2 | x¯ | |
| Candida albicans | 41.80 | 44.50 | 43.19 | 28.40 | 25.68 | 27.04 |
| Nakaseomyces glabratus | 43.80 | 42.05 | 42.09 | 25.30 | 27.55 | 26.43 |
| Candida parapsilosis | 32.68 | 33.50 | 33.09 | 29.83 | 29.55 | 29.69 |
| Meyerozyma guilliermondii | 29.10 | 28.35 | 28.73 | 30.00 | 28.00 | 29.00 |
| Pichia kudriavzevii | 22.30 | 20.50 | 21.40 | 23.55 | 23.30 | 23.43 |
| Candida tropicalis | 21.13 | 20.40 | 20.77 | 34.00 | 37.50 | 35.75 |
x¯: mean value.
A significant decrease in TTD was observed in the Myco bottle when compared with the Aero bottle in the case of blood samples infected with C. albicans (mean of 27h versus mean of 43h; p=0.02) and with N. glabratus (26h versus 48h; p=0.018). The TTD of C. parapsilosis, M. guilliermondii and P. kudriavzevii was similar in both culture bottles (p>0.05), whereas the growth of C. tropicalis was detected earlier (p=0.014) in the Aero culture than in the Myco one (mean of 21h versus 36h) (Fig. 1).
Mean of time-to-detection (TTD) in hours of Candida albicans, Nakaseomyces glabratus (Candida glabrata), Candida parapsilosis, Meyerozyma guilliermondii (Candida guilliermondii), Pichia kudriavzevii (Candida krusei) and Candida tropicalis in BD BACTEC™ Plus Aerobic/F (Aero) and BD BACTEC™ Mycosis IC/F Lytic (Myco) bottles. C. albicans and N. glabratus were detected earlier in the Myco bottles than in the Aero bottles, p=0.02 and p=0.018, respectively. TTD of C. parapsilosis, M. guilliermondi and P. kudriavzevii was similar in both bottles (p=0.3; p=0.8 and p=0.16, respectively). C. tropicalis was detected earlier in the Aero bottles (p=0.014). *Statistical difference.
Our study showed significant differences in the TTD when comparing the growth of several Candida species from blood experimentally infected in specific (Myco) and nonspecific (Aero) blood culture bottles. These differences in yeast detection between bottles could directly impact the patient's outcome, since a delay of 24–48h in the diagnosis increases the mortality rate almost 100% (23.6–41.4%).5
In our study, C. albicans was detected ∼17h earlier in the specific bottle for fungal blood culture, which is consistent with the findings of Nawrot et al.,11 who reported a similar TTD (14.6h) for C. albicans, a time that was shorter as well than the TTD obtained with the nonspecific bottles. Similarly, N. glabratus was detected 21.8h earlier approximately using the specific culture bottles, and this finding aligns with the results reported by Posteraro et al.,15 who described a similar TTD of 14h for this species.
This difference between the two bottles may be attributed to the presence of saponins in the Myco bottles, which enhances yeast recovery due to blood lysis and, consequently, accelerates fungal detection. Moreover, the presence of ferric ammonium citrate in the Myco bottles provides an iron source for fungi, and the medium includes carbohydrate and/or protein sources as well (according to Becton Dickinson, New Jersey, USA) to accelerate yeast growth.8,6
Many hospitals do not have access to or do not use the specific blood culture bottle for growing fungi in routine clinical diagnosis. Considering the difference (TTD) found in our study among Candida species, a local evaluation of the epidemiology in each center is necessary to analyze the cost–benefit of using both bottles. In addition, a factor that should also be considered is the volume of blood that is required for inoculating two bottles, particularly an issue in neonatal ICUs, considering the difficulty of obtaining sufficient blood from neonatal patients.
C. tropicalis was detected approximately 15h later in the specific blood bottles in our study, in agreement with previously reported data (16.3h later) by Jekarl et al.7 On the other hand, C. albicans (the agent of ∼50% of candidemia cases in Brazil),3 plus the second most prevalent agent among Candida-related species, N. glabratus, were detected significantly earlier in the fungal-specific bottle. The probable impact in reducing mortality rates may justify the importance of using both bottles (Aero and Myco) routinely in the hospital.
This would improve the diagnosis of candidemia and allow a more rapid clinical intervention, resulting in a favorable outcome of the patients. The usage of both bottles would also lead to a shorter period of hospital stay and, consequently, a reduction in the cost of hospitalization, off setting any increased diagnostic laboratory costs. A limitation of our study was the use of a less-than-minimum volume (3ml) of blood sample than that suggested by the manufacturers; however, we tried to reproduce the usual situation encountered with blood samples from patients in neonatal ICU. Another limitation was testing only one isolate for each species; thus, we suggest including more isolates per species in future studies, considering the biological differences that clinical strains can present. Further studies should also evaluate the time-of-detection of mixed species of Candida, as well as mixed bacteria and Candida, using the Aero and Myco bottles, to simulate mixed infections.
FundingNo funding was provided.
Authors’ contributionsAll authors contributed significantly to the study, covering conception, design, data acquisition, analysis, interpretation, article drafting, critical content revision, and final version approval.
Melissa Orzechowski Xavier: Conceptualization, Methodology, Supervision, Validation, Software, Writing – reviewing and editing; Leandre Carmem Wilot and Vanice Rodrigues Poester: Formal analysis, Writing – original draft preparation, Visualization, Investigation; Karine Ortiz Sanchotene, Bruna Muradás Esperon, Mariana Rodrigues Trápaga and David A. Stevens: Writing – reviewing and editing.
Competing interestsThe authors declare that, to their knowledge, they do not have competing financial interests or personal relationships that could have influenced the work reported in this paper.
The authors would like to thank the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPQ - number 316067/2021-0) for their support.