Serrated polyps of the colorectum are a heterogeneous group of lesions with potential malignant transformation through the “serrated pathway” of carcinogenesis. The discovery of these lesions has been a paradigm shift in the concept of the adenoma-carcinoma sequence, so that up to 30% of tumors develop through this pathway. The main factors associated with an increased risk of malignancy in serrated polyps are size ≥10mm, multiplicity, sessile serrated adenoma histology, presence of associated dysplasia and proximal location. Current evidence indicates that these lesions should be resected completely, and the patient requires an endoscopic surveillance program. Serrated polyposis syndrome is a clinicopathological entity characterized by multiple and/or large serrated polyps with an increased risk of developing colorectal cancer. These patients and their families, require multidisciplinary assessment in specialized high risk colorectal cancer units.
Los pólipos serrados del colon constituyen un grupo heterogéneo de lesiones con potencial de transformación a cáncer colorrectal a través de la “vía serrada” de la carcinogénesis. El descubrimiento de estas lesiones ha supuesto un cambio de paradigma en el concepto de la secuencia adenoma-carcinoma, de modo que hasta un 30% de los tumores se desarrollan por esta vía. Los principales factores que se asocian a un mayor riesgo de malignizaciónen los pólipos serrados son el tamaño ≥10mm, la multiplicidad, la histología de adenoma serrado sésil, la presencia de displasia asociada y la localización proximal. La evidencia actual indica que estas lesiones deben ser resecadas completamente y que el paciente requiere un programa de vigilancia endoscópica. El síndrome de poliposis serrada es una entidad clínico-patológica asociada a un aumento del riesgo de padecer cáncer colorrectal. Estos pacientes y sus familiares requieren una evaluación multidisciplinar en unidades de alto riesgo de cáncer colorrectal.
Colorectal cancer (CRC) is currently the most frequent neoplasm in Spain when both sexes are included, and it represents the second cause of death by cancer.1–3 These data contrast with the fact that CRC is the paradigm of a preventable neoplasm.4,5 This is because its natural history is known (most CRC originate from a premalignant lesion known as an adenoma), there are many means for its early detection (colonoscopy, fecal occult blood testing) and detection in early phases significantly improves prognosis. In recent decades, the prognosis of patients with CRC has improved, due to screening programs aimed at detecting asymptomatic individuals that present precancerous lesions (polyps) or adenocarcinomas in initial tumor phases, and thus decreasing the incidence and mortality of CRC.3,4,6
Until a few years ago, it was believed that the majority of CRC came from a common precursor lesion known as an adenomatous polyp, or adenoma. This lesion, although benign, can potentially become malignant through the so-called “traditional” carcinogenesis model, related to chromosome instability and initiated by means of the inactivation of the APC gene. Nevertheless, today we know that this model is implicated in only 70%–80% of CRC.7 One of the most significant advances in recent years in the field of digestive oncology related with CRC has been recognizing serrated lesions as precursors to CRC, through the so-called “serrated” carcinogenesis pathway, which is responsible for 20%–30% of all CRC.7–9 These lesions, with specific endoscopic, anatomic and pathologic characteristics that differentiate them from adenomas, have an important clinical relevance, as it has been observed that the majority of interval CRC detected in patients participating in CRC screening programs (tumors detected between 2 colonoscopies) are this type of lesion. In addition, recent studies have demonstrated that patients with multiple and/or large serrated polyps have an increased risk for developing CRC.10–12
The objective of this article is to describe the concept of the serrated carcinogenesis pathway, serrated polyps and serrated polyposis syndrome (SPS), as well as explaining the clinical implications for the diagnosis, treatment and follow-up of these lesions.
Search MethodologyA search was performed in PubMed of scientific articles (original articles and reviews) in English with the following words: “serrated polyps”, “hyperplastic polyposis”, “serrated polyposis” and “hyperplastic polyps”.
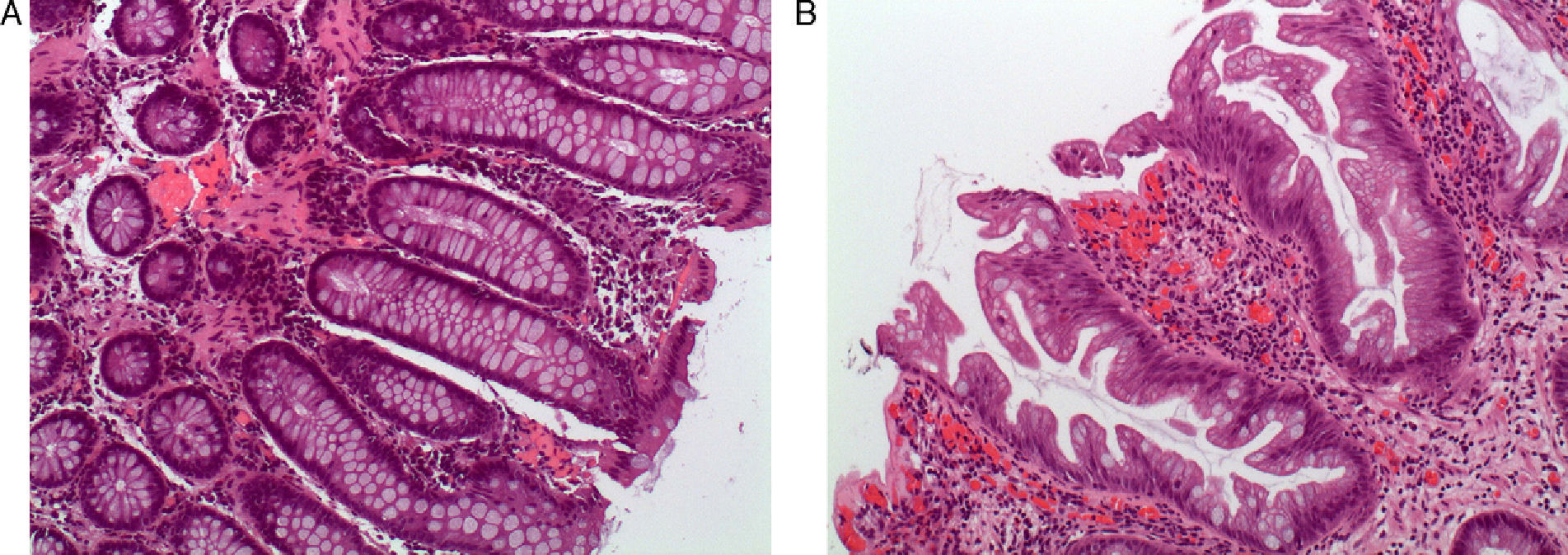
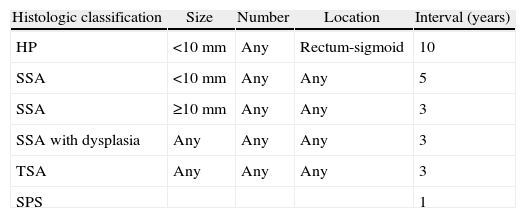
Serrated Polyps: Concept and ClassificationTraditionally, hyperplastic polyps have been considered benign lesions with no risk of neoplastic progression. However, hyperplastic polyps are only a part of the spectrum of serrated polyps, and today we know that some subtypes have the potential to transform into colorectal carcinoma through the serrated pathway.7,8 This group of lesions has a common histologic characteristic, which is the appearance of serrated teeth in the epithelium of the crypt due to an accumulation of colonocytes secondary to inhibited apoptosis (Fig. 1 A and B).
Histologic appearance of serrated polyps: (A) normal colon mucosa (hematoxylin eosin 100×). Note the straight morphology of the crypts in the longitudinal cut; (B) sessile serrated adenoma (hematoxylin eosin 100×). The main characteristic of the serrated polyps was the sawtooth appearance of the crypts in the longitudinal cut. The appearance of the crypt in the cross-sectional cuts is usually star-shaped. Note the presence of serration of the crypt up to its base, typical characteristic of sessile serrated adenoma (original images by Dr. Cuatrecasas).
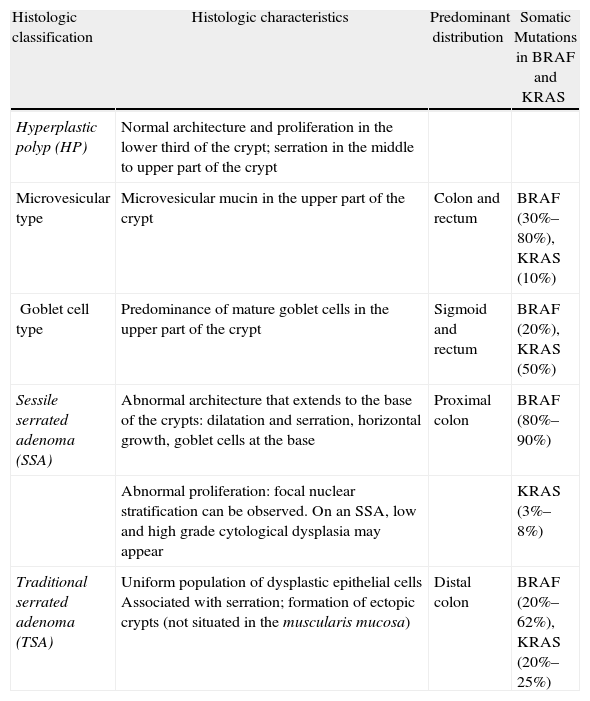
Despite the fact that serrated lesions were described more than 2 decades ago, there has been some controversy about their pathologic classification. Currently, serrated lesions are classified using WHO criteria into 3 categories (Table 1).
Pathologic Classification of Serrated Polyps.
| Histologic classification | Histologic characteristics | Predominant distribution | Somatic Mutations in BRAF and KRAS |
| Hyperplastic polyp (HP) | Normal architecture and proliferation in the lower third of the crypt; serration in the middle to upper part of the crypt | ||
| Microvesicular type | Microvesicular mucin in the upper part of the crypt | Colon and rectum | BRAF (30%–80%), KRAS (10%) |
| Goblet cell type | Predominance of mature goblet cells in the upper part of the crypt | Sigmoid and rectum | BRAF (20%), KRAS (50%) |
| Sessile serrated adenoma (SSA) | Abnormal architecture that extends to the base of the crypts: dilatation and serration, horizontal growth, goblet cells at the base | Proximal colon | BRAF (80%–90%) |
| Abnormal proliferation: focal nuclear stratification can be observed. On an SSA, low and high grade cytological dysplasia may appear | KRAS (3%–8%) | ||
| Traditional serrated adenoma (TSA) | Uniform population of dysplastic epithelial cells Associated with serration; formation of ectopic crypts (not situated in the muscularis mucosa) | Distal colon | BRAF (20%–62%), KRAS (20%–25%) |
This is the most numerous subtype within the serrated lesions (estimated at 80%–90%).13 With endoscopy, these polyps are seen as flat lesions (normally <5mm) that are pale, whitish or a color similar to the surrounding mucosa. They are usually covered by a thin layer of mucus and present a weak vascular network; this is in contrast to adenomas, which are usually hypervascular. Hyperplastic polyps (HP) are usually numerous and located predominantly in the rectum and sigmoid colon. Histologically, they are characterized by retaining normal architecture, with little distortion and proliferation without dysplasia. They present cell accumulations in the shape of sawteeth in the upper and middle part of the crypt, and normal proliferation in the lower crypt. HP are divided into 2 histologic subtypes (microvesicular and rich in goblet cells). Although the microvesicular subtype has been associated with a greater risk of malignization, current data are controversial.7
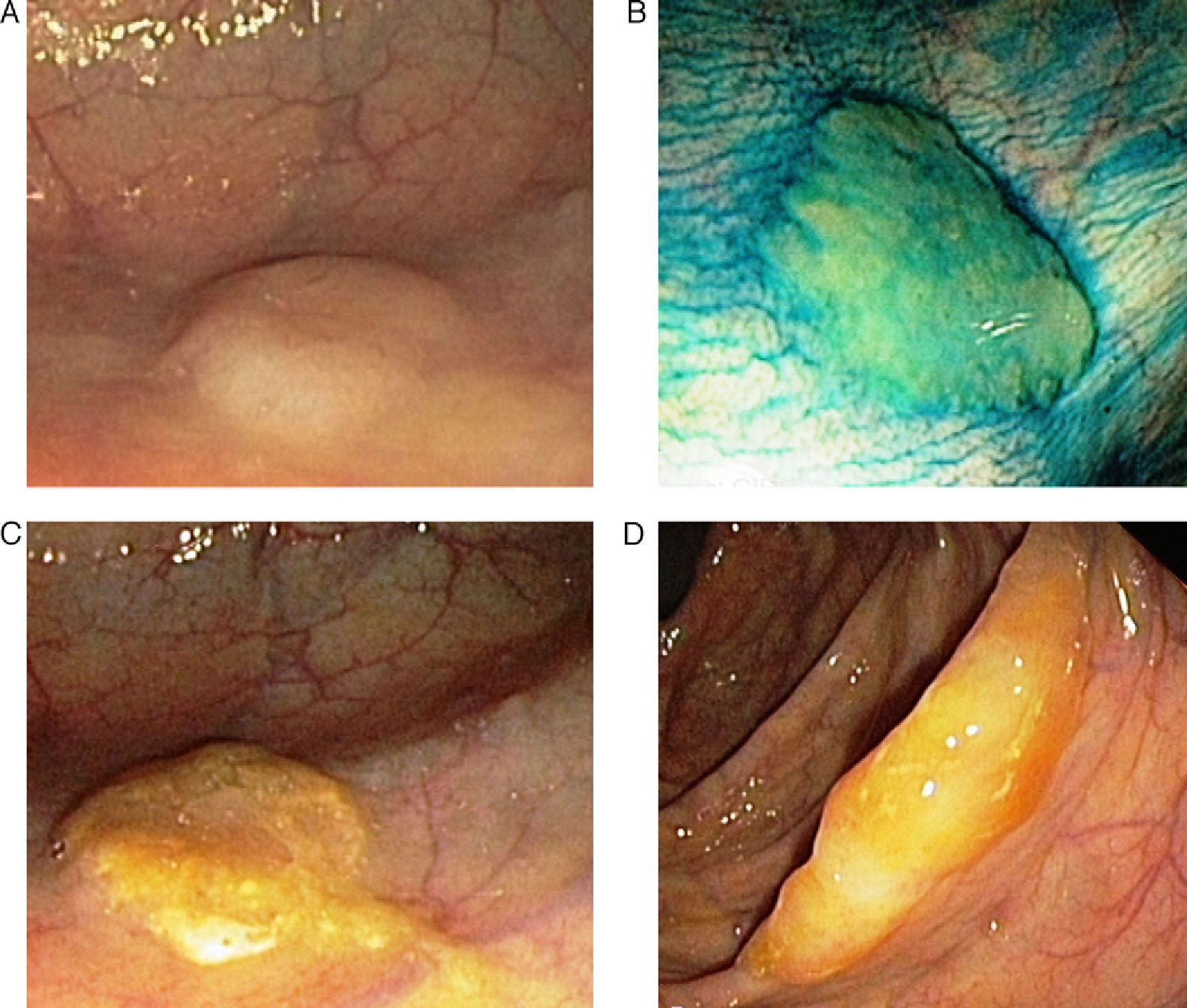
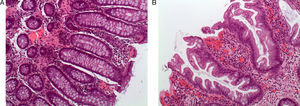
Sessile Serrated Polyp or AdenomaThese represent 15%–20% of serrated lesions and are considered a key preneoplastic lesion in the serrated pathway to carcinogenesis.7,8,13,14 Macroscopically, their appearance is of flat or low lesions, greater than 5mm, usually covered with a layer of mucus and found predominantly in the proximal colon.15 These characteristics and the common location in the right colon are the reason why this type of lesion frequently goes unnoticed (Fig. 2).16
Endoscopic appearance of the serrated polyps: (A) flat polyp located in the right colon. The loss of the vascular pattern is very characteristic as the only sign of the presence of a serrated polyp; (B) the application of chromoendoscopy with indigo carmine makes it easier to determine the lesion type and its limits; (C and D) flat polyps in the right colon. The presence of a mucus plug on the surface of the polyp is a very typical characteristic of serrated polyps (original images from the archives of the Endoscopy Unit at the Hospital Clínic in Barcelona).
When examined under a microscope, the crypt presents normal architecture, is dilated and shows disorderly growth. These alterations extend to the lower part, where sawteeth are observed at their base. There is a characteristic presence of crypts that grow parallel to the muscularis mucosae with an inverted “T” or “L” shape. Due to this characteristic, if the endoscopic biopsy is not deep enough to evaluate the base of the crypt, the differential diagnosis between HP and sessile serrated adenoma or polyp (SSA) may be difficult. SSA without cytological dysplasia should be distinguished from those that present dysplasia, which, as in adenomas, may be low and high grade.
Traditional Serrated AdenomaThis is a rare subtype (1%–6%)14 that is usually pedicled and more common in the left colon (60%). These adenomas present very prominent sawteeth and cytological dysplasia similar to adenomas, which differentiates them from SSA.
Even though there is consensus among pathologists regarding this classification, there is still important interobserver variability17 and SSA are often classified as hyperplastic polyps.
Serrated Pathway of CarcinogenesisThe genetic and epigenetic molecular characterization of serrated adenocarcinomas and polyps in recent years, together with their improved histological characterization, have provided convincing evidence in favor of the so-called serrated pathway to pathogenesis, and its distinction from classic adenoma-carcinoma sequence.7 For example, unlike the classic carcinogenesis pathway where the loss of heterozygosity or alleles in genes APC and p53 is the main characteristic, this is a rare phenomenon in serrated lesions. The molecular alterations of the serrated pathway include: (1) activation of the MAPK signaling pathway (mutation in BRAF and KRAS); (2) epigenetic silencing of genes through the hypermethylation of their promoter regions; and (3) acquisition of the microsatellite instability (MSI) phenotype secondary to MLH1 promoter methylation, part of the DNA repair gene family, whose germinal mutational is responsible for Lynch syndrome. Based on the molecular and histologic correlation studies, 2 subtypes of the serrated pathway to carcinogenesis are recognized: the “sessile serrated pathway” and the “alternative serrated pathway”. The sessile serrated pathway is characterized by presenting mutations in the proto-oncogene BRAF as the earliest alteration and by showing the so-called CpG Island Methylator Phenotype (CIMP-high), characterized by the aberrant methylation of tumor suppressor genes, which would contribute to neoplastic progression. This pathway is characterized by the presence of MSI secondary to MLH1 methylation. The central preneoplastic lesion in this pathway is SSA, and typically CRC appears more frequently in the proximal colon. The alternative serrated pathway is characterized by KRAS proto-oncogene mutation, the presence of an attenuated methylator phenotype (CIMP-low), and frequent methylation of the MGMT promoter. Histologic and molecular correlation in this pathway is lower than in the sessile serrated pathway, and the preneoplastic lesion is believed to be traditional serrated adenoma (TSA).
Serrated Polyposis SyndromeSPS, formerly known as hyperplastic polyposis syndrome, is phenotypically characterized by the presence of multiple serrated and/or large-sized polyps in the colon, which predisposes to the development of CRC.8,16,18,19 Although its actual incidence is unknown, screening studies in populations at moderate risk with sigmoidoscopy have estimated an incidence of 1/3000 sigmoidoscopies.20 Furthermore, 2 recent studies in screening populations based on fecal occult blood testing have observed that between 1/151 and 1/294 individuals with positive fecal tests who undergo colonoscopy are diagnosed with SPS.21,22 These data suggest that the prevalence is greater than that published. This is due to the difficult endoscopic detection of serrated lesions, lack of consensus in their classification by pathologists and lack of knowledge of this entity in the medical community as they frequently go unnoticed.13,15,23,24
In accordance with the WHO definition, the diagnosis of SPS is established by the presence of the following criteria:
- a.
Presence of at least 5 serrated polyps proximal to the sigmoid, 2 of them greater than or equal to 10mm.
- b.
Any number of serrated polyps proximal to the sigmoid colon in an individual with first-degree family history (parents, siblings, children) of SPS.
- c.
More than 20 serrated polyps of any size distributed along the colon.
Even though this classification was established arbitrarily, it has been very useful for standardizing clinical diagnosis. Within SPS, 3 different phenotypes are recognized: proximal, distal and mixed. The proximal phenotype is characterized by the presence of multiple SSA located mainly in the proximal colon, the distal phenotype by the presence of multiple HP in the rectosigmoid colon, and the mixed type by the presence of serrated polyps distributed throughout the colon. Despite these differences, there have been no reports of higher risk for developing CRC according to phenotype.25 On the other hand, the presence of synchronous adenomas is very frequent in patients with SPS (some 70% of cases).6,10 Unlike family-related adenomatous polyposis, there is no increased risk of gastroduodenal lesions or extracolonic neoplasms in SPS.26 Age at the diagnosis of SPS is usually 50–60, and there is a clear association with smoking.26
Currently, we do not understand the genetic factor involved in SPS, although it shows characteristics suggestive of genetic predisposition, including the multiplicity of lesions, early age at diagnosis and the greater prevalence of a family history of neoplasia. Thus, up to 50% of patients with SPS present a family history of CRC and the risk of CRC in first-degree family members is multiplied by 5 compared to the general population.27 However, there are rarely several cases of SPS within one same family. In a small proportion of patients with SPS who present concomitant adenomatous polyposis, biallelic germinal mutations have been identified in MUTYH.28
Clinical Importance of Serrated Polyps and Serrated Polyposis SyndromeThere are 3 main reasons that involve serrated polyps in colorectal carcinogenesis. First of all, SPS has been associated in different studies with the development of CRC. A recent study with a large cohort of patients with SPS has shown that around 35% of patients develop CRC and, furthermore, that the number of HP and SSA correlates with the presence of CRC.10 Second, a clinical and molecular relationship has been established between serrated polyps and sporadic CRC, especially those tumors with MSI.29 Serrated polyps, especially SSA, are usually located in the proximal colon and share molecular characteristics with sporadic cancer with MSI (caused by the somatic methylation of the MLH1 gene29–31), such as the CIMP phenotype and the presence of mutations in the BRAF gene.7 These 2 characteristics, as commented previously, constitute the molecular base of the serrated pathway. Last of all, it has recently been described that patients with sporadic serrated polyps (those that occur in the absence of SPS) have a greater risk of synchronous and metachronous colorectal neoplasm. The higher risk of CRC in serrated polyps is related with multiplicity, the histology of SSA, size ≥10mm and proximal localization; these characteristics define serrated polyps as high risk and at the same time make more probable the presence of conventional adenomas as well as advanced synchronous neoplasms (including advanced adenomas and CRC). Thus, despite limited current evidence, endoscopic surveillance is recommended in patients with this type of lesions.11,12,14,32,33
Although there are data supporting the faster progression of serrated lesions,10,34 their natural history continues to be unknown, especially with regards to their growth rate and the incidence of progression to cancer. In SPS, there are several studies that show a relation with the development of CRC. In case series, between 25% and 70% of patients with SPS presented CRC on diagnosis or during follow-up.10,18,20,35–40 In the longest series, with a cohort of 77 patients, 35% of the subjects developed CRC (28.5% on diagnosis and 6.5% during follow-up).10
Serrated polyps have been related with the appearance of interval CRC in population CRC screening programs. Interval CRC appears between 2 endoscopic screening examinations and means that the early detection of these lesions has failed. Interval CRC shares the same clinical and molecular characteristics as serrated polyps, proximal location and molecular alterations of the serrated pathway (mutations in BRAF and CIMP genes). Therefore, it has been suggested that one of the main causes of interval CRC are unnoticed serrated polyps.41
Lastly, several studies have demonstrated that the use of chromoendoscopy or narrow band imaging (endoscopic mapping techniques based on the use of coloring agents or on light spectrum modification) make it easier to detect serrated polyps, both in moderate risk populations as well as in patients with SPS.23,24,33,42–44 Thus, most authors recommend the use of these techniques for optimizing detection rates, especially in patients with SPS (Fig. 2B).
Therapeutic Management of Serrated Polyps and Serrated Polyposis Syndrome (SPS)Sporadic Serrated PolypsCurrent evidence recommends complete resection of serrated polyps.8,10–12,14,15 The only exception are small-sized HP (<5mm) located in the rectosigmoid, which have not been demonstrated to be associated with an increase in the number of proximal adenomas or CRC, and their resection is therefore not formally recommended. Any serrated polyp proximal to the sigmoid colon should be considered a potentially premalignant lesion and their complete resection is therefore recommended. In cases where it is uncertain whether the resection has been complete (which frequently occurs in large, flat lesions located in the proximal colon) it is recommended to repeat the exploration in 3–6 months in order to observe the base of the resection.33
In addition to endoscopic treatment, there are different clinical scenarios in which surgical treatment can be indicated:
- 1)
Unresectability – In the case that a serrated polyp is considered endoscopically unresectable, usually due to size and/or location, segmental surgical resection should be considered, keeping in mind factors related with the polyp (histology of SSA or TSA, presence of dysplasia) and the patient (age, comorbidity).45
- 2)
Multiplicity – The individuals that present multiple serrated polyps in the proximal colon (without meeting criteria for SPS) that are difficult to view endoscopically could benefit from partial colectomy given the increased risk of synchronous and metachronous advanced neoplasm.45
With the available information, it is difficult to define a vigilance strategy based on evidence, and more studies are necessary to evaluate the appropriate intervals. Nevertheless, the US Multi-Society Task Force on Colorectal Cancer, a group of experts that includes the main American medical societies involved in the management of CRC, has recently published a consensus document proposing a vigilance strategy for serrated polyps depending on size, number, location and histology of the lesions (Table 2).33 Therefore, the highest risk situation is defined by the presence of SSA ≥10mm or with associated dysplasia or the presence of a TSA, in which a follow-up colonoscopy is recommended in 3 years.
Recommendations for the Surveillance of Serrated Polyps.
| Histologic classification | Size | Number | Location | Interval (years) |
| HP | <10mm | Any | Rectum-sigmoid | 10 |
| SSA | <10mm | Any | Any | 5 |
| SSA | ≥10mm | Any | Any | 3 |
| SSA with dysplasia | Any | Any | Any | 3 |
| TSA | Any | Any | Any | 3 |
| SPS | 1 |
SSA: sessile serrated adenoma; TSA: traditional serrated adenoma; HP: hyperplastic polyp; SPS: serrated polyposis syndrome.
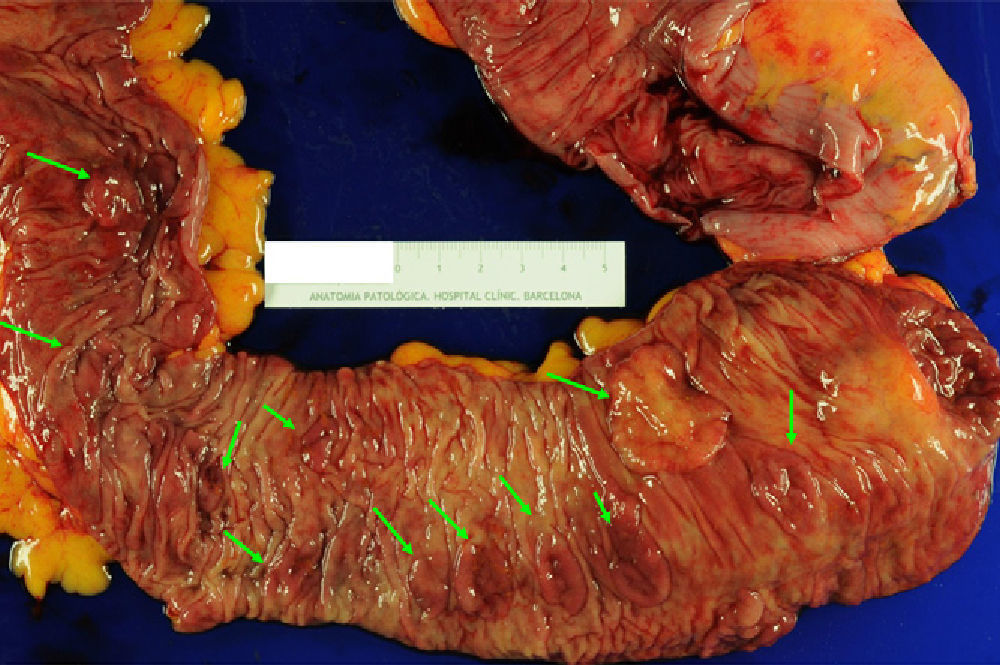
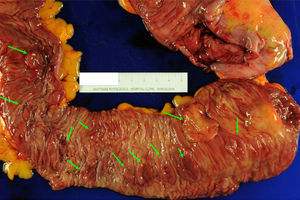
The management of SPS is empirical and contemplates resection of the polyps, endoscopic surveillance and genetic counseling. When a CRC is found, total colectomy is recommended with ileorectal anastomosis in order to eliminate the risk of metachronous lesions6,10,16,26 (Fig. 3). After surgery, control rectoscopy is recommended every 6–12 months to assess the rectal remnant. If the presentation is as multiple polyps, the approach depends on the possibility to complete endoscopic resection. If this is possible, it is recommended to perform follow-up colonoscopies every 1–2 years with the resection of all polyps, or at least those >3–5mm. If, on the contrary, the polyps are not resectable due to their size or number, or proper endoscopic follow-up is not possible, the option of total colectomy with ileorectal anastomosis should be considered.
Colectomy specimen from a 59-year-old patient diagnosed with serrated polyposis syndrome during population-based CRC screening; total colectomy with ileorectal anastomosis was indicated due to polyposis that was not controllable with endoscopy. The presence of multiple flat polyps can be observed (green arrows) predominantly in the proximal colon (ileocecal valve to the right of the image). These polyps showed histology for sessile serrated adenoma, and adenocarcinomas were detected in 2 (T1N1M0 and T2N1M0) (original image by Dr. Cuatrecasas).
As for the risk of CRC in family members, screening colonoscopies are recommended for all first-degree relatives (parents, siblings, children) over the age of 35–40 or 10 years before the age of the youngest case. Follow-up endoscopies are recommended every 5 years, and the intervals should be modified in cases of polyp detection.27
ConclusionsSerrated polyps are a heterogeneous group of lesions (HP, SSA and TSA) that are characterized by a sawtooth histologic appearance. Considered benign until a few years ago, today we know that these polyps can potentially transform into CRC through the so-called serrated pathway to carcinogenesis. Current evidence indicates that serrated polyps require complete resection and endoscopic surveillance based on factors associated with greater risk for neoplastic transformation, such as size ≥10mm, presence of associated dysplasia, multiple lesions and proximal location.
SPS is an entity characterized by the presence of multiple and/or large serrated polyps that are associated with high risk for CRC. The management of patients with SPS and their family members requires multidisciplinary evaluation by endoscopists, gastroenterologists and expert surgeons in units specialized in managing these patients at high-risk for CRC.
Finally, in spite of the advances made in the description of serrated lesions and the progressive understanding of their molecular characteristics and clinical behavior, more prospective studies are necessary in order to establish recommendations and guidelines for evidence-based therapeutic management.
Conflict of InterestsNone to declare.
The authors would like to thank Miriam Cuatrecasas for providing the histologic images.
Please cite this article as: Carballal S, et al. Pólipos serrados y síndrome de poliposis serrada. Cir Esp. 2013;91:141-8.