Pneumococcal meningitis (PM) has a high morbidity and mortality. The aim of the study was to evaluate what factors are related to a poor PM prognosis.
MethodsProspective observational study conducted on patients admitted to the Pediatric Intensive Care Unit in a tertiary hospital with a diagnosis of PM (January 2000 to December 2013). Clinical, biochemical and microbiological data were recorded. Variable outcome was classified into good or poor (neurological handicap or death). A multivariate logistic regression was performed based on the univariate analysis of significant data.
ResultsA total of 88 patients were included. Clinical variables statistically significant for a poor outcome were younger age (p=.008), lengthy fever (p=.016), sepsis (p=.010), lower Glasgow Score (p<.001), higher score on Pediatric Risk Mortality Score (p=0.010) and Sequential Organ Failure Assessment (SOFA) (p<.001), longer mechanical ventilation (p=.004), and inotropic support (p=.008) requirements. Statistically significant biochemical variables were higher level of C-reactive protein (p<.001) and procalcitonin (p=.014) at admission, low cerebrospinal (CSF) pleocytosis (p=.003), higher level of protein in CSF (p=.031), and severe hypoglycorrhachia (p=.002). In multivariate analysis, independent indicators of poor outcome were age less than 2 years (p=.011), high score on SOFA (p=.030), low Glasgow Score (p=.042), and severe hypoglycorrhachia (p=.009).
ConclusionsPatients younger than 2 years of age, with depressed consciousness at admission, especially when longer mechanical ventilation is required, are at high risk of a poor outcome.
Las meningitis neumocócicas (MN) se relacionan con una elevada morbimortalidad. El objetivo del estudio es evaluar qué factores se relacionan con un peor pronóstico.
MétodosEstudio prospectivo observacional con pacientes diagnosticados de MN ingresados en la Unidad de Cuidados Intensivos Pediátricos de un hospital de tercer nivel (enero 2000-diciembre 2013). El pronóstico fue clasificado en buena o mala evolución (secuelas neurológicas o muerte). Se realizó un análisis multivariante de los resultados significativos obtenidos en el análisis univariante.
ResultadosSe reclutaron 88 pacientes. Las variables clínicas relacionadas de forma estadísticamente significativa con una peor evolución fueron: menor edad (p=0,008), mayor duración de la fiebre (p=0,016), sepsis (p=0,010), menor puntuación en la Escala de Glasgow (p<0,001), mayor puntuación en Pediatric Risk Mortality Score (p=0,010) y Sequential Organ Failure Assessment (SOFA) (p<0,001), ventilación mecánica (p=0,004) y soporte inotrópico (p=0,008) prolongados. Las bioquímicas fueron: mayor elevación de proteína C reactiva (p<0,001) y de procalcitonina (p=0,014) al ingreso, menor pleocitosis en líquido cefalorraquídeo (p=0,003), intensas proteinorraquia (p=0,013) e hipoglucorraquia (p=0,002). En el análisis multivariante, los factores independientes relacionados con una peor evolución fueron: edad inferior a 2años (p=0,011), elevada puntuación en SOFA (p=0,030), menor puntuación en la Escala de Glasgow (p=0,042) e hipoglucorraquia intensa (p=0,009).
ConclusionesLos menores de 2años con mayor depresión del sensorio al ingreso, especialmente cuando requieren soporte ventilatorio prolongado, tienen un mayor riesgo de mala evolución.
Implementation of universal vaccination against Haemophilus influenzae type b (Hib) has changed the epidemiology and decreased the incidence of bacterial meningitis (BM) in developed countries. Nowadays, Streptococcus (S.) pneumoniae and Neisseria meningitidis are the most prevalent causes of BM beyond neonatal period.1,2
Pneumococcal meningitis (PM) has been related with great morbidity and mortality. In spite of diagnosis and therapeutic progress, the mortality rate persists at around 8% with an incidence of neurological handicaps of 20–30%.3–8 Furthermore, cognitive and behavioral sequelae that affect quality of life (academic limitations, language delay, low verbal fluency, psychomotor retardation) have been reported in up to 7–10% of patients with pneumococcal meningitis.9,10
Recognizing the prognostic factors would be very useful in indicating early and individualized treatment in these patients with PM. The contributions of clinical, microbiological and biochemical, and therapeutic approaches, as well as neuroimaging findings, have been analyzed in recent years in PM patients. The idea has been identify patients at greater major risk. The most important prognosis factors in relation to neurological handicaps seem to be hypoglycorrhachia,11 mechanical ventilation requirement,12 late diagnosis, ataxia, and not receiving dexamethasone treatment.13–15 The final prognosis probably depends on a combination of different factors but few studies have undertaken an integral analysis of them. The aim of the present study was to determine what clinical, biochemical, and microbiological factors are related to the prognosis of S. pneumoniae meningitis patients.
MethodsThis was a prospective, observational, non-interventional study of patients admitted to the Pediatric Intensive Care Unit (PICU) at Hospital Sant Joan de Déu, with a diagnosis of PM. In 1999 a database was created in order to prospectively recruit all data of patients with BN. The study period was from January 2000 to December 2013. In Catalonia, with a population of around 7 million and 1.2 million people aged 18 years or younger, this hospital with 345 beds (18 PICU beds) captured around 17% of all pediatric hospital admissions during the study period.
Patients from 7 days to 18 years of age diagnosed with PM were included. PM was defined by characteristic clinical signs and symptoms (stiff or painful neck, vomiting, headache, persistent fever, bulging fontanelles) and compatible cerebrospinal fluid (CSF) alterations (CSF cell count up to 10cells/mm3) along with isolation of S. pneumoniae and/or DNA detection of pneumococcal genes by Real-Time PCR16 in blood or cerebrospinal fluid. We excluded patients with known primary immunodeficiency (humoral, cellular, phagocytic, complement alteration, congenital asplenia) or known secondary immunodeficiency (human immunodeficiency virus, nephrotic syndrome, cardiopulmonary chronic disease). The sepsis diagnostic criteria published in 200817 were used.
DNA detection of S. pneumoniae was carried out using published procedures that included the study of pneumolysin (ply) and wzg genes (both had to be simultaneously positive to confirm any case as a positive pneumococcal infection), and subsequent direct capsular typing of S. pneumoniae DNA positive samples.16,18
Pneumococcal strains were identified with standard microbiological methods that included the optochin sensitivity test and an antigenic test targeting the capsular polysaccharide (Slidex pneumo-kit, BioMérieux, Marcy-l’Etolie, France). In addition, strains were also sent to the National Pneumococcus Reference Center at Majadahonda, Madrid, Spain, to complete serotype study with Quellung reaction and to determine the minimum inhibitory concentrations (MICs) of penicillin and other antibiotics with Agar dilution technique. Antibiotic susceptibilities were defined according to the breakpoints of the European Committee on Antimicrobial Susceptibility Testing (EUCAST).19
Serotypes were classified into high invasive disease potential serotypes (1, 4, 5, 7F, 9V, 14, 18C and 19A) and lower invasive disease potential serotypes (all others) according to the classification of Brueggemann20 and Sleeman.21
At our hospital, all patients with a diagnosis of PM are admitted to the PICU for the first 24h of the disease. The protocol treatment included cefotaxime (300mg/kg/day, maximum 12g/day, for 10 days) plus vancomicyn (40mg/kg/day for 3 days, or longer if cefotaxime resistant S. pneumoniae is isolated) and dexamethasone (0.6mg/kg/day; maximum 16mg/day, for 3 days), in all cases.
Variables registered were (a) demographic: age, gender, previous pneumococcal vaccination with 7-valent conjugate pneumococcal vaccine (PCV7), 10-valent conjugate pneumococcal vaccine or 13-valent conjugate pneumococcal vaccine (PCV13); (b) PM risk factors: acute otitis media (AOM), recent cranial surgery, cranial trauma antecedent; (c) clinical: fever hours’ duration previous to PM diagnosis, previous antibiotic treatment; Pediatric Risk Mortality Score (PRISM) III, Glasgow Score and Sequential Organ Failure Assessment (SOFA) score at admission, focal neurological signs (neurological deficit, seizures), mechanical ventilation (MV), and inotrope requirement; (d) biochemistry at admission: lactate, CSF leukocyte count and CSF levels of protein and glucose, Boyer Score punctuation, C-reactive protein (CRP) and procalcitonin (PCT) levels at diagnosis and 24–48h after admission; (e) microbiological: S. pneumoniae serotype, and penicillin, erythromicyn, and cefotaxime susceptibility.
Outcome was classified into two groups: good evolution or poor evolution. Last group included death and neurological handicap: seizures, hemiparesis, ataxia, hydrocephalus, psychomotor retardation, or deafness. Neurosensory hearing loss diagnosis is performed by our pediatric otolaryngology team; patients are evaluated at 1, 2, 6, and 12 months by auditory evoked potentials of brainstem (newborns and infants) or audiometry. Other neurological handicaps are evaluated by our pediatric neurological team during the first 6 months (after this patients are referred to local Neurological and physical therapy teams).
The SPSS statistical software package 19.0 (SPSS, Inc., Chicago, Ill) was used for all statistical analyses. Sample characteristics were expressed as counts and percentages for categorical variables, as mean±standard deviations for normal continuous variables, or as a median and interquartile range (p25–75) when variables were not normally distributed. Statistical differences in mean or median values were assessed through Student's t test or Mann–Whitney U test depending on the normality of the variable distribution. Categorical variables were analyzed by means of the χ2 test. A multivariate logistic regression was performed with the significant data from univariate analysis, in order to determine the prognosis factors associated with a poor outcome. Odds ratio (OR) was analyzed for each of these significant variables. The discriminatory power of the variable in determining poor outcome was evaluated by the area under the receiver operating characteristics (ROC) curve (AUC) and the 95% confidence interval (95% CI). Sensitivity and specificity were determined for each cut-off value of the variable. Probability values less than 0.05 were considered significant.
The research and ethical institutional review board of the Sant Joan de Déu Hospital Foundation approved the study and the patients remained anonymous.
ResultsThere were 88 patients recruited. The median age was 18.5 months (range: 1 months–17 years) and 59 patients (67%) were male. The proportion of children who had received pneumococcal vaccination, according to age, was 23.9% (21 patients). Predisposing conditions to pneumococcal meningitis were recognized in 23 children (26.1%): concomitant ear infection was identified in 13 children (14.8%), 9 patients (10.2%) were diagnosed with CSF fistula, and 1 patient (1.1%) had undergone neurosurgery. The duration of illness symptoms before consultation in the emergency room ranged between 1 and 336h, with a median of 36h, and 20 patients (22.7%) had received previous antibiotic treatment. On admission, 74 patients (84.1%) had focal neurologic signs and 59 children (67%) met sepsis criteria. The median length of stay in PICU was 4 days (range 1–58), during which 43 children (48.9%) required MV and 21 (23.9%) required inotropic support.
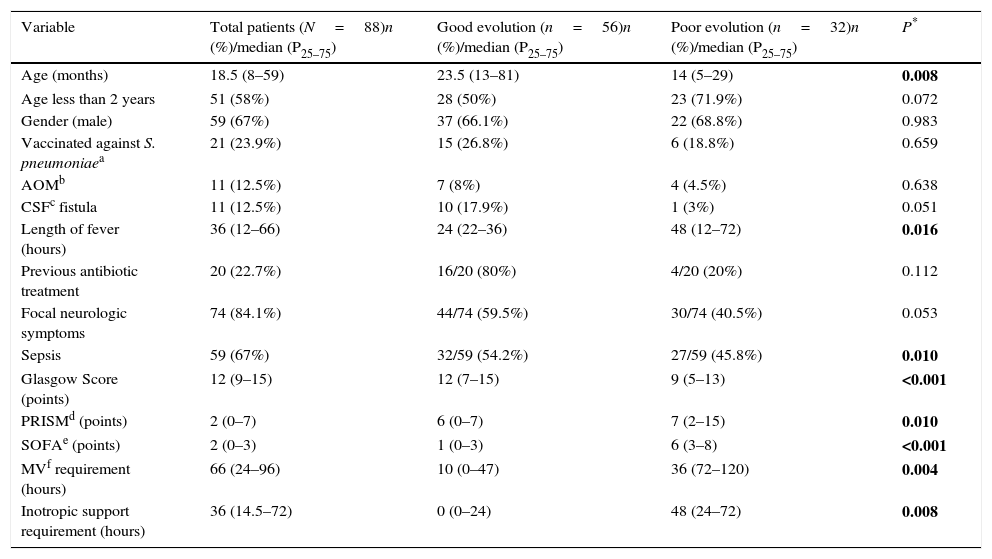
Table 1 shows demographic and clinical baseline findings and the differences obtained according to outcome, assessed by univariate analysis. Variables that were statistically significant related to a poor evolution were younger age (p 0.008), lengthy fever (p 0.016), sepsis associated with PM (p 0.010), lower Glasgow Score (p<0.001), higher score on PRISM (p 0.010) and SOFA (p<0.001), and longer MV (p 0.004) and inotropic support (0.008) requirements.
Demographic and clinical baseline findings and differences obtained according to outcome, assessed by univariate analysis.
| Variable | Total patients (N=88)n (%)/median (P25–75) | Good evolution (n=56)n (%)/median (P25–75) | Poor evolution (n=32)n (%)/median (P25–75) | P* |
|---|---|---|---|---|
| Age (months) | 18.5 (8–59) | 23.5 (13–81) | 14 (5–29) | 0.008 |
| Age less than 2 years | 51 (58%) | 28 (50%) | 23 (71.9%) | 0.072 |
| Gender (male) | 59 (67%) | 37 (66.1%) | 22 (68.8%) | 0.983 |
| Vaccinated against S. pneumoniaea | 21 (23.9%) | 15 (26.8%) | 6 (18.8%) | 0.659 |
| AOMb | 11 (12.5%) | 7 (8%) | 4 (4.5%) | 0.638 |
| CSFc fistula | 11 (12.5%) | 10 (17.9%) | 1 (3%) | 0.051 |
| Length of fever (hours) | 36 (12–66) | 24 (22–36) | 48 (12–72) | 0.016 |
| Previous antibiotic treatment | 20 (22.7%) | 16/20 (80%) | 4/20 (20%) | 0.112 |
| Focal neurologic symptoms | 74 (84.1%) | 44/74 (59.5%) | 30/74 (40.5%) | 0.053 |
| Sepsis | 59 (67%) | 32/59 (54.2%) | 27/59 (45.8%) | 0.010 |
| Glasgow Score (points) | 12 (9–15) | 12 (7–15) | 9 (5–13) | <0.001 |
| PRISMd (points) | 2 (0–7) | 6 (0–7) | 7 (2–15) | 0.010 |
| SOFAe (points) | 2 (0–3) | 1 (0–3) | 6 (3–8) | <0.001 |
| MVf requirement (hours) | 66 (24–96) | 10 (0–47) | 36 (72–120) | 0.004 |
| Inotropic support requirement (hours) | 36 (14.5–72) | 0 (0–24) | 48 (24–72) | 0.008 |
Numbers in bold means that are statistically significant.
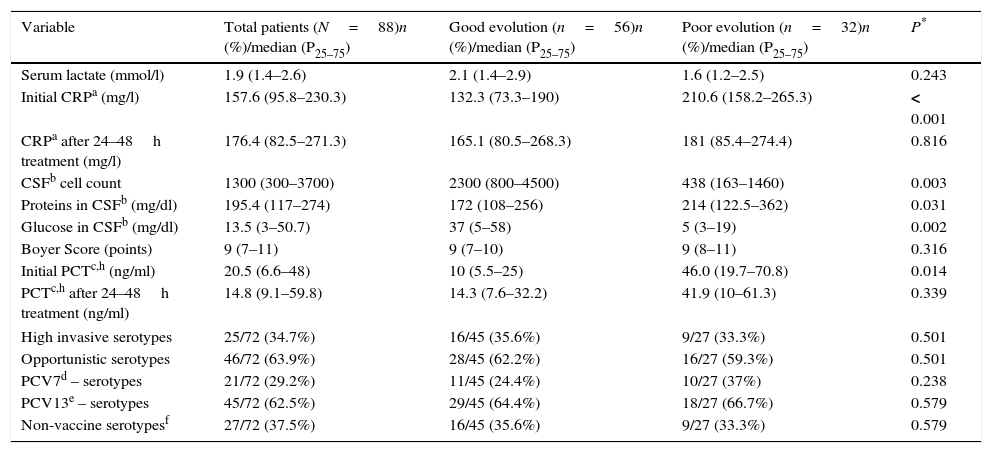
The results assessed by the univariate analysis of the biochemistry variables, as well as baseline findings, are shown in Table 2. Variables that were statistically significant related to a poor evolution were higher level of CRP (p<0.001) and PCT (p 0.014) at admission, relatively low CSF pleocytosis (p 0.003), higher level of protein in CSF (p 0.031), and severe hypoglycorrhachia (p 0.002).
Biochemical and microbiologicalg baseline findings and differences obtained according to outcome, assessed by univariate analysis.
| Variable | Total patients (N=88)n (%)/median (P25–75) | Good evolution (n=56)n (%)/median (P25–75) | Poor evolution (n=32)n (%)/median (P25–75) | P* |
|---|---|---|---|---|
| Serum lactate (mmol/l) | 1.9 (1.4–2.6) | 2.1 (1.4–2.9) | 1.6 (1.2–2.5) | 0.243 |
| Initial CRPa (mg/l) | 157.6 (95.8–230.3) | 132.3 (73.3–190) | 210.6 (158.2–265.3) | < 0.001 |
| CRPa after 24–48h treatment (mg/l) | 176.4 (82.5–271.3) | 165.1 (80.5–268.3) | 181 (85.4–274.4) | 0.816 |
| CSFb cell count | 1300 (300–3700) | 2300 (800–4500) | 438 (163–1460) | 0.003 |
| Proteins in CSFb (mg/dl) | 195.4 (117–274) | 172 (108–256) | 214 (122.5–362) | 0.031 |
| Glucose in CSFb (mg/dl) | 13.5 (3–50.7) | 37 (5–58) | 5 (3–19) | 0.002 |
| Boyer Score (points) | 9 (7–11) | 9 (7–10) | 9 (8–11) | 0.316 |
| Initial PCTc,h (ng/ml) | 20.5 (6.6–48) | 10 (5.5–25) | 46.0 (19.7–70.8) | 0.014 |
| PCTc,h after 24–48h treatment (ng/ml) | 14.8 (9.1–59.8) | 14.3 (7.6–32.2) | 41.9 (10–61.3) | 0.339 |
| High invasive serotypes | 25/72 (34.7%) | 16/45 (35.6%) | 9/27 (33.3%) | 0.501 |
| Opportunistic serotypes | 46/72 (63.9%) | 28/45 (62.2%) | 16/27 (59.3%) | 0.501 |
| PCV7d – serotypes | 21/72 (29.2%) | 11/45 (24.4%) | 10/27 (37%) | 0.238 |
| PCV13e – serotypes | 45/72 (62.5%) | 29/45 (64.4%) | 18/27 (66.7%) | 0.579 |
| Non-vaccine serotypesf | 27/72 (37.5%) | 16/45 (35.6%) | 9/27 (33.3%) | 0.579 |
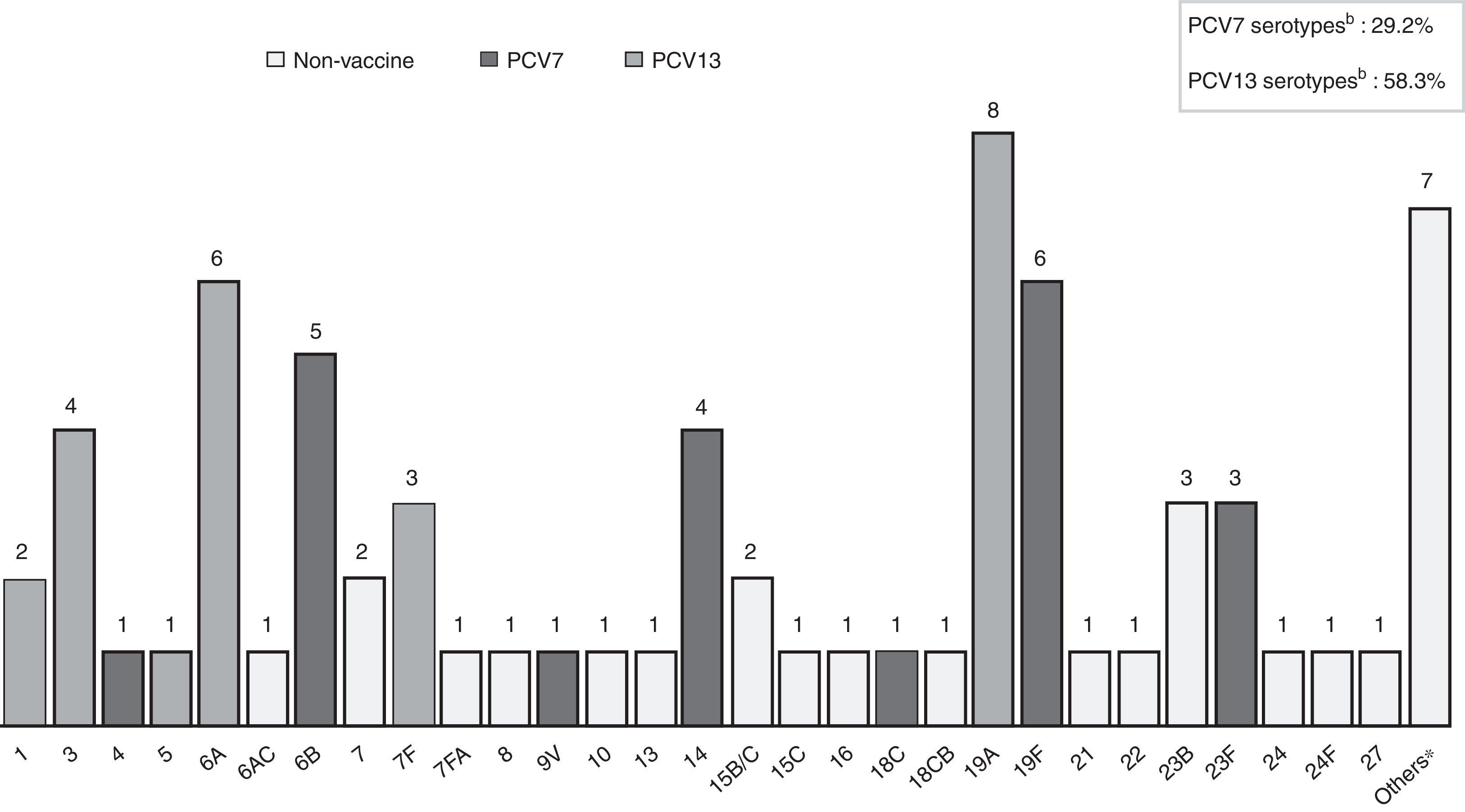
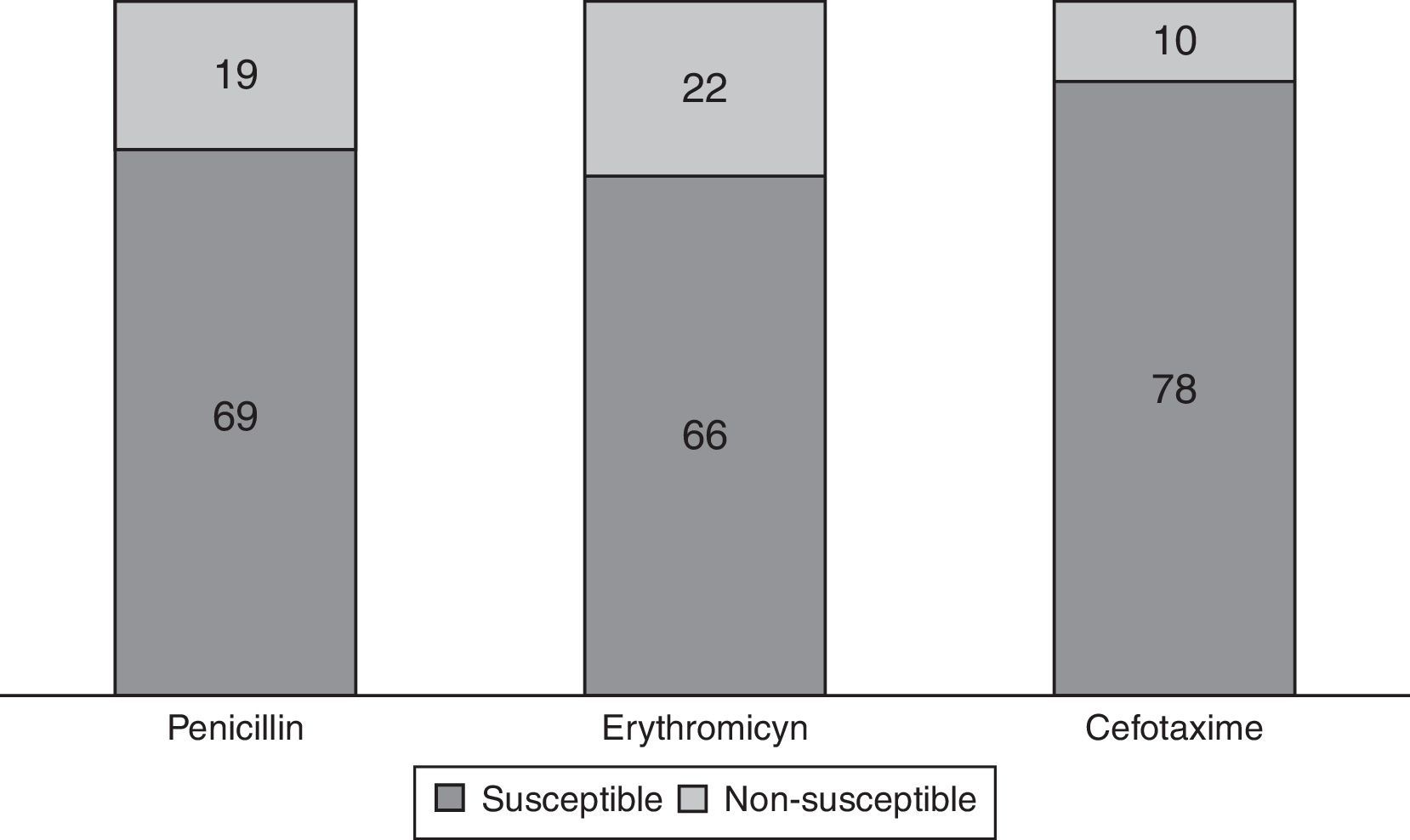
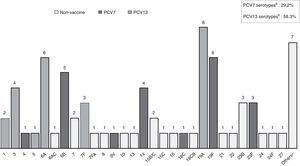
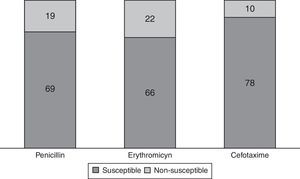
S. pneumoniae serotypes were identified in 72 patients (81.8%), whose distribution is shown in Fig. 1. Some 29.2% of them are included in PCV7 and 62.5% in PCV13. Table 2 presents baseline microbiological findings and the differences obtained according to outcome, assessed by univariate analysis. There was no statistically significant relation between the serotype that caused meningitis and outcome, whether or not it was included in the vaccine. There was also no statistically significant relation between outcome and the serotype's invasive capacity. Figure 2 shows antibiotic susceptibility, a test that was carried out on all strains. There were 19 strains fully resistant to penicillin (MIC equal or greater than 0.12mg/L) and 10 strains (11.4%) fully resistant to cefotaxime (MIC greater than 2mg/L); 6 of them were identified during the years 2000–2006 and the remaining 4 cases were detected during the last 3 years of the study (3 strains matched with multiresistant S. pneumoniae serotype 19A). There was no statistically significant relation between antibiotic sensitivity and outcome (p 0.143 for cefotaxime, p 0.795 for erythromicyn, p 0.760 for penicillin).
Streptococcus pneumoniae serotype distribution causing bacterial meningitis in 72 available patients. a7-valent conjugate pneumococcal vaccine (PVC7) includes serotypes 4, 6B, 9V, 14, 18C, 19F AND 23B. b13-valent conjugate pneumococcal vaccine (PVC13) contains the seven serotypes included in PCV7 and the six additional serotypes 1, 3, 5, 6A, 7F, 19A. *Others: serotypes non-included in PVC7, PVC13 or 23-valent pneumococcal polysaccharide vaccine (PPV23).
Streptococcus pneumoniae antibiotic sensitivity distribution in 88 available patients. Breakpoint for interpretation of minimum inhibitory concentrations (MICs) established by European Committee on Antimicrobial Susceptibility Testing (EUCAST): MIC equal or greater than 0.12mg/l for penicillin implies non-susceptibility; MIC greater than 0.25mg/l for erythromicyn implies non-susceptibility; MIC greater than 0.5mg/l for cefotaxime implies non-susceptibility.
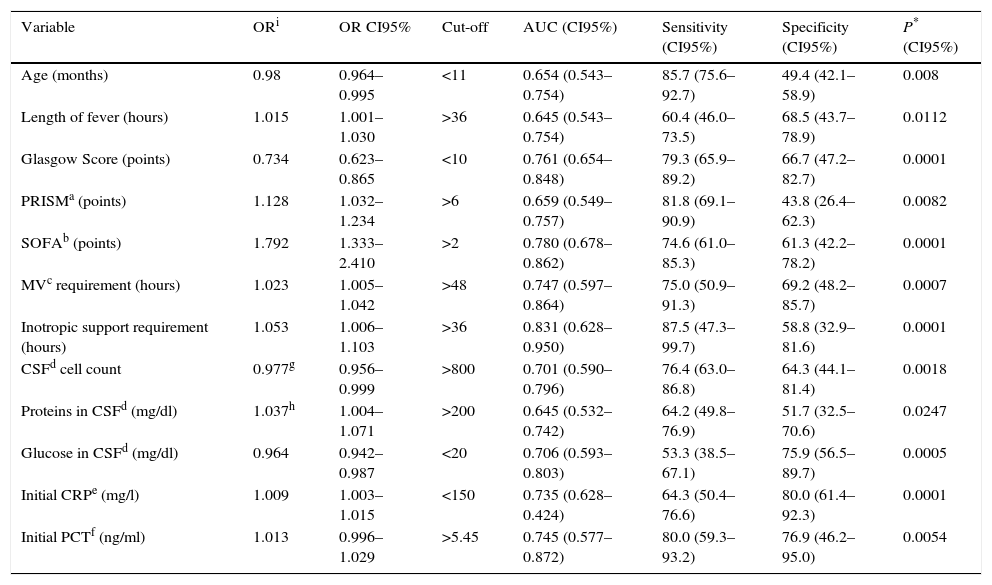
OR, cut-off value, and the sensitivity and specificity for the variables statistically associated with poor outcome, are presented in Table 3. Variables that proved to be independent indicators of poor evolution in the multivariate analysis were being younger than 2 years of age (p 0.046; OR 2.56, CI 1.006–6.489), SOFA higher than 2 points (p 0.001; OR 4.637, CI 1.805–11.913), Glasgow Score lower than 10 points (p 0.001; OR 5.625, CI 1.992–15.885), and hypoglycorrhachia lower than 20mg/dl (p 0.015; OR 3.826, CI 1.388–10.544).
Odds ratio and Sensitivity and specificity for each cut-off value of the significant variable in univariate analysis.
| Variable | ORi | OR CI95% | Cut-off | AUC (CI95%) | Sensitivity (CI95%) | Specificity (CI95%) | P* (CI95%) |
|---|---|---|---|---|---|---|---|
| Age (months) | 0.98 | 0.964–0.995 | <11 | 0.654 (0.543–0.754) | 85.7 (75.6–92.7) | 49.4 (42.1–58.9) | 0.008 |
| Length of fever (hours) | 1.015 | 1.001–1.030 | >36 | 0.645 (0.543–0.754) | 60.4 (46.0–73.5) | 68.5 (43.7–78.9) | 0.0112 |
| Glasgow Score (points) | 0.734 | 0.623–0.865 | <10 | 0.761 (0.654–0.848) | 79.3 (65.9–89.2) | 66.7 (47.2–82.7) | 0.0001 |
| PRISMa (points) | 1.128 | 1.032–1.234 | >6 | 0.659 (0.549–0.757) | 81.8 (69.1–90.9) | 43.8 (26.4–62.3) | 0.0082 |
| SOFAb (points) | 1.792 | 1.333–2.410 | >2 | 0.780 (0.678–0.862) | 74.6 (61.0–85.3) | 61.3 (42.2–78.2) | 0.0001 |
| MVc requirement (hours) | 1.023 | 1.005–1.042 | >48 | 0.747 (0.597–0.864) | 75.0 (50.9–91.3) | 69.2 (48.2–85.7) | 0.0007 |
| Inotropic support requirement (hours) | 1.053 | 1.006–1.103 | >36 | 0.831 (0.628–0.950) | 87.5 (47.3–99.7) | 58.8 (32.9–81.6) | 0.0001 |
| CSFd cell count | 0.977g | 0.956–0.999 | >800 | 0.701 (0.590–0.796) | 76.4 (63.0–86.8) | 64.3 (44.1–81.4) | 0.0018 |
| Proteins in CSFd (mg/dl) | 1.037h | 1.004–1.071 | >200 | 0.645 (0.532–0.742) | 64.2 (49.8–76.9) | 51.7 (32.5–70.6) | 0.0247 |
| Glucose in CSFd (mg/dl) | 0.964 | 0.942–0.987 | <20 | 0.706 (0.593–0.803) | 53.3 (38.5–67.1) | 75.9 (56.5–89.7) | 0.0005 |
| Initial CRPe (mg/l) | 1.009 | 1.003–1.015 | <150 | 0.735 (0.628–0.424) | 64.3 (50.4–76.6) | 80.0 (61.4–92.3) | 0.0001 |
| Initial PCTf (ng/ml) | 1.013 | 0.996–1.029 | >5.45 | 0.745 (0.577–0.872) | 80.0 (59.3–93.2) | 76.9 (46.2–95.0) | 0.0054 |
The mortality rate was 8% (7 patients) and one or more sequelae were reported in 25 patients (28.4%), with outcome classified as poor evolution (32 patients, 36.4%). Neurological handicaps observed were deafness (11 patients), seizures (12 patients), hemiparesis (7 patients), psychomotor retardation (3 patients), cranial nerve palsy (3 patients), hydrocephalus (2 patients), blindness (1 patient), and ataxia (1 patient).
DiscussionThe case-fatality ratio and sequelae reported in this study (8% and 28.4% respectively) are similar to those observed in other series. Arditi et al.3 (USA 1993–1996) reported a mortality rate of 7.7% and neurologic sequelae in 25% of children, and Buckingham et al. (USA 1991–2001) obtained in their study a case-fatality ratio of 9%, a deafness incidence of 55%, and other neurologic sequelae in 13% of cases.4 Our results are also in accordance with a meta-analysis from prospective studies of 4520 children in 1993 (mortality rate of 4.8–8.1% and neurologic handicap in 16–26% of cases).5 In Europe, Kornelisse et al. reported similar results,6 but Wasier (France 1990–2002) observed higher morbidity and mortality rates (case-fatality rate of 49% and neurologic sequelae in 47% of cases).7 Despite the improvement in diagnosis and treatment achieved in recent years, PM remains a severe and life-threatening disease with a high incidence of neurologic handicaps in survivors, as Pagliano8 and Chandran22 showed in more recent studies.
Clinical variables that we related with a poor evolution are lengthy fever (which implies delayed antibiotic treatment), sepsis associated with PM, low level of consciousness at admission, longer inotropic support requirement, higher scores on PRISM and SOFA, and longer MV requirement. When multivariate analysis was performed, clinical factors that proved to be independent predictors of poor evolution were the last two. These results are in accord with the reviewed bibliography although they have not been analyzed together.6–8,23 Younger age was also associated with a poor evolution in univariate analysis, with age under 2 years an independent factor of worse outcome in multivariate analysis. We did not find similar conclusions in the reviewed bibliography; however, Grimwood et al.24,25 observed a poor cognitive outcome in children under 1 year of age in their studies.
CSF variations in bacterial meningitis have been analyzed in adults and children. In our study, patients who had higher levels of protein in CSF and severe hypoglycorrhachia progressed poorly. Moreover, severe low glucose level in CSF was an independent predictor of worse evolution in multivariate analysis. These results are in accord with other studies.4,6,8 An association between relatively low CSF cell count and poor evolution has also been reported in other studies.8,23 Similar findings were reported in the model of pneumococcal meningitis cases made by Brandt, who postulated that an insufficient inflammatory response against pneumococcal antigens during the first hours of the disease was associated with lower CSF and blood cell count, thereby permitting bacterial overgrowth within the CSF. In such cases, when antibiotherapy is administered, the consequent bacterial lysis results in more pronounced inflammatory changes within the meninges and vascular complications are encouraged.26,27
Biological markers, such as CRP and PCT, are useful for discrimination between viral and bacterial etiology,28,29 but there are few studies that demonstrate their prognostic utility or changes in them with effective treatment.30 In our study, patients who had higher initial CRP and PCT values had statistically significant poorer evolution. We think that more studies are needed to determinate the utility of these markers in treatment monitoring.
An increase in incidence of invasive pneumococcal disease (IPD) by serotypes not included in the PCV7 has been widely reported worldwide.1,31–34 We also observed a higher rate of non-PVC7 serotypes causing meningitis (70.8%). In Spain the decrease in the prevalence of PCV7 serotypes after the introduction of the vaccine has not been as marked as in the USA, probably due to lower and irregular coverage (in Catalonia the distribution is mainly via the private market and only 23.9% of children were vaccinated in our study). In any case, recent studies made in our country show that serotype replacement,32–34 despite other factors such the use of new drugs, may also play an important role.34,35 An increase in multiresistant pneumococcal serotypes has also been reported, with serotype 19A the most frequently isolated in the USA and in Europe, especially in patients with meningitis and primary bacteriemia.34,36
The association among pneumococcal serotype, antibiotic sensitivity, and outcome remains a controversial subject. We reported 8 strains that match up with S. pneumoniae serotype 19A (11.1%), 3 of which were isolated during the last 3 years and were fully resistant to cefotaxime (MIC greater than 2mg/L), but we did not find a statistically significant relation between them as other authors have.1,3,37 This is probably due to the empirical double therapy in our treatment protocol. Taking the recent increase in pneumococcal serotypes with decreased sensitivity to multiple antibiotics into account, combined antibiotic treatment (cefotaxime plus vancomicyn) would be advisable in countries with high pneumococcal resistance rates. Moreover, it is well known that the rate of PM decreases with routine pneumococcal conjugate vaccination in children,1,31,33,34,38,39 so this preventive measure against PM in children would be also advisable.
This study has some limitations. One is the relatively low number of patients, but other studies in the literature show similar populations and the patients in our study were quite uniform. Our results may be applicable to patients admitted to PICU in other developed countries, but the situation will be different in developing countries.
ConclusionsIn our study, PM was shown to be a severe infectious disease and that is still closely related to an elevated morbidity and mortality rate. Patients younger than 2 years with depressed consciousness at admission, especially if longer MV is required, are at high risk of poor evolution. Other biochemical data such as severe hypoglycorrhachia and high levels of CRP and PCT at admission may help to identify which patients need to be closely monitored and aggressively treated, in order to try to improve the prognosis. Delayed antibiotic administration was also shown to be related to a poor outcome. Therefore early administration of empirical antibiotic treatment is essential.
Conflict of interestAuthors disclose any potential financial or ethical conflicts of interest regarding the contents of this submission.
FundingThis study has not been financially sponsored.
Ethical approvalThe study was done according to the Helsinky declaration and approved by the Sant Joan de Déu Ethical Assistant Committee.