The fibula osteocutaneous free flap has been a workhorse for mandibular reconstruction since Hidalgo's original description for its use for this purpose. The objective of this manuscript is to review the use of the fibula flap in mandibular reconstruction and to answer some of the commonly held misconceptions as to why some surgeons view it as an inferior reconstructive option to the vascularized iliac crest flap.
Materials/methodsReview of the literature as it relates to the use of the fibula free flap in mandibular reconstruction and the senior author's experience.
ResultsThe bicortical nature of the fibula provides a stable platform for endosseous implant placement, thus allowing for comprehensive oral rehabilitation and improving the quality of life in reconstructed patients. The fibula osteocutaneous free flap, however, is the longest vascularized bone flap available, allowing for reconstruction of the entire mandible. Given these attributes, it would seem unlikely that doubts regarding the adequacy of the fibula free flap in mandibular reconstruction exists. However, the principal arguments challenging the use of the fibula osteocutaneous free flap are the lack of height of the reconstructed fibula compared to the native mandible, unreliable skin perforators to support a skin paddle, insufficient soft tissue coverage, and the presence of vascular anomalies which may preclude its use. These presumed shortcomings are circumvented with various proven techniques to increase fibular height and anatomic studies demonstrating the cutaneous perforator patterns.
ConclusionsWith these simple solutions in mind, the fibula osteocutaneous free flap will likely remain a workhorse for mandibular reconstruction, allowing for the reconstruction of virtually any oromandibular defect. It allows for both aesthetic, as well as, functional reconstruction of the mandible.
Desde la descripción original de Hidalgo sobre su uso con este objetivo, el colgajo libre osteocutáneo de peroné ha sido el factótum para la reconstrucción mandibular. El objetivo de este artículo es revisar el uso del colgajo de peroné en la reconstrucción mandibular y aclarar los conceptos erróneos sostenidos con frecuencia como razón de que algunos cirujanos lo consideren una opción reconstructiva inferior al colgajo vascularizado de cresta ilíaca.
Materiales y métodosRevisión de los estudios publicados sobre el uso del colgajo de peroné en la reconstrucción mandibular y experiencia del primer autor.
ResultadosLa naturaleza bicortical del peroné ofrece una estructura estable para la colocación de un implante endoóseo, lo que permite una rehabilitación oral integral y mejora la calidad de vida de los pacientes sometidos a reconstrucción. El colgajo libre osteocutáneo de peroné es el colgajo óseo vascularizado de mayor longitud disponible, que permite la reconstrucción de toda la mandíbula. Dadas estas propiedades, parece improbable que suscite dudas su idoneidad en la reconstrucción mandibular. No obstante, la razón principal que pone en duda su uso es la falta de altura del peroné reconstruido, comparado con la mandíbula natural, el número tan variable de perforantes septocutáneos que irrigan la paleta cutánea, una cobertura insuficiente de las partes blandas, y la presencia de anomalías vasculares que pueden impedir su uso. Estas supuestas desventajas se evitan con diversas técnicas de eficacia demostrada para aumentar la altura del peroné al igual que con estudios anatómicos que demuestren los patrones de los perforantes septocutáneos.
ConclusionesSi se tienen en cuenta estas soluciones simples, el colgajo libre osteocutáneo de peroné probablemente seguirá siendo el factótum de la reconstrucción mandibular, ya que permite la reconstrucción de casi cualquier defecto oromandibular. Favorece la reconstrucción tanto estética como funcional de la mandíbula.
The fibula free flap was first described by Taylor et al. in 1975 as an osseous flap for reconstruction of tibial defects. However, it was Chen et al. who described the fibula osteocutanous free flap in 1983.1,2 The inclusion of a skin paddle with the fibula greatly enhanced the reconstructive capabilities of the flap as composite defects can be reconstructed simultaneously. The first report of the fibula free flap in head and neck reconstruction was by Hidalgo in 1989 for mandibular reconstruction.3 These early accounts led the way for the fibula osteocutaneous free flap to become a workhorse in mandibular reconstruction. Prior to its introduction, the available vascularized bone flaps for head and neck reconstruction were from the iliac crest, scapula, radius, and rib. The fibula osteocutaneous free flap quickly gained popularity over these vascularized bone flaps for several reasons. In terms of bone quality, only the vascularized iliac crest flap contains more bone stock than the fibula. The abundant cortical thickness, as well as, bicortical nature in which the fibula is harvested allows for comprehensive and successful oromandibular reconstruction with osseointegrated implants.4 It is by far the longest vascularized bone flap available, as approximately 26cm can be harvested, allowing for reconstruction of the entire mandible. The fibula can be harvested as a pure osseous flap or together with muscle and skin, thereby, permitting great flexibility for the reconstruction of virtually any mandibular and soft tissue defects. Flap harvest is relatively straightforward and allows the ablative and reconstructive surgeons to work simultaneously. Donor site morbidity is also relatively minimal as most patients are able to return to their preoperative level of function.
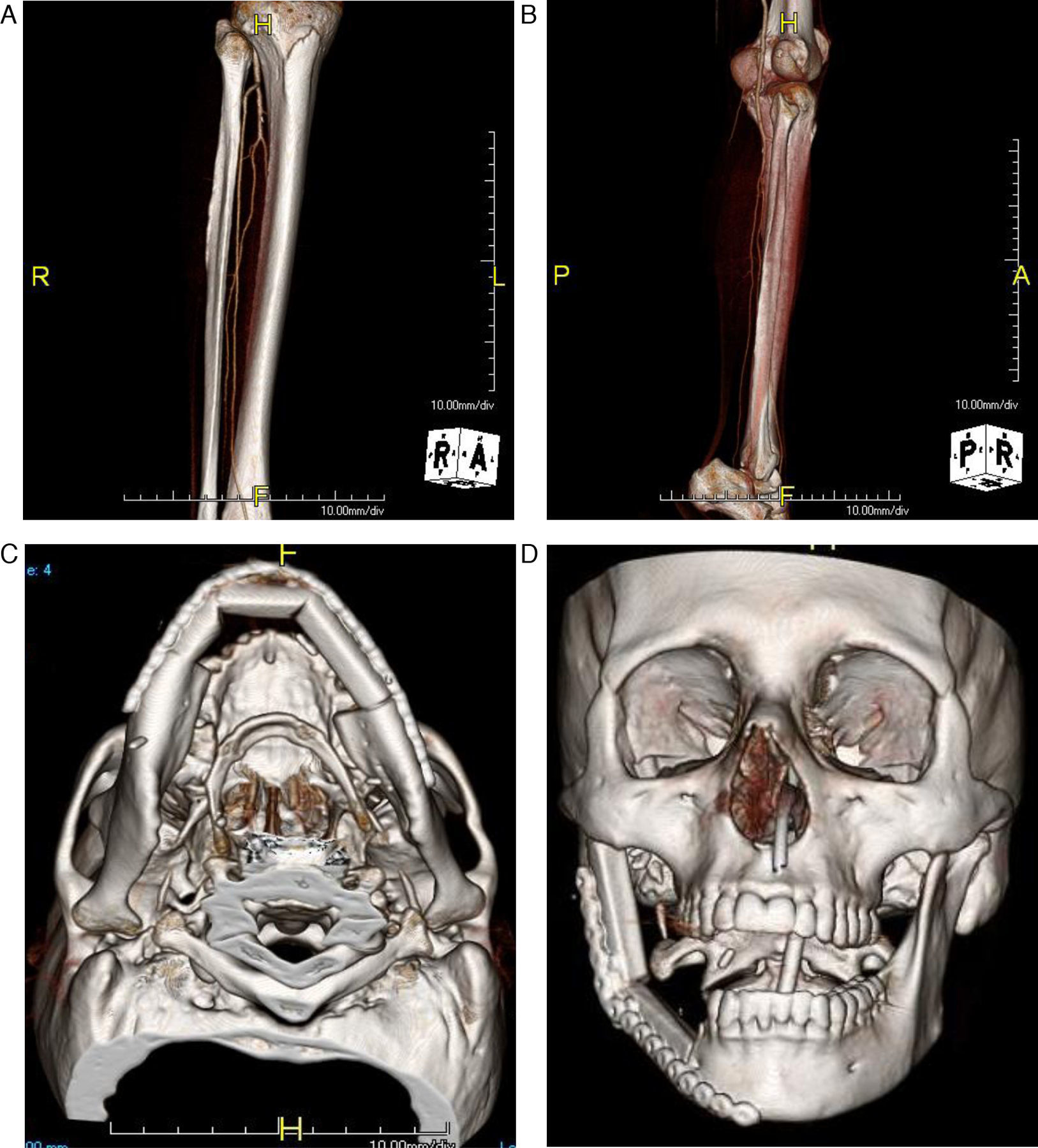
The peroneal artery is the dominant arterial supply to the fibula osteocutaneous free flap while the drainage of the flap is via the 2 venae comitantes. The caliber of the artery on average is 1.5mm while the veins are 3mm.5 These vessel diameters closely match the diameters of most recipient vessels in the neck, thus making microanastamosis straightforward without the need for vessel modifications. The fibula itself has a dual blood supply, receiving blood through both an endosteal as well as a periosteal source. The abundant periosteal supply appears to be more crucial for the survival of the graft than the endosteal supply and permits multiple osteotomies to be made as little as 1cm apart.3 Therefore, the fibula can be easily contoured with a series of closing osteotomies to reconstruct any segmental defect of the mandible (Fig. 1A–D)
(A) Computer tomography angiogram(CTA) of the lower extremity showing the three vessels run off. (B) Lower extremity CTA showing patency of the vessel to the angle. (C) Closing osteotomies of the fibula flap to recreate the anterior mandibular defect with a significant left body defect. (D) Closing osteotomy used to recreate a hemimandibular defect with the proper recreation of the mandibular angle and ramus segment.
Perhaps the main advantage for utilizing the fibula osteocutaneous free flap in mandibular reconstruction is the ability to afford a comprehensive oromandibular reconstruction with osseointegrated implants, thereby, restoring masticatory function and improving the patient's quality of life. This characteristic fulfills the fundamental principle of reconstructive surgery of restoring both form and function. Although the vascularized iliac crest flap based on the deep circumflex iliac artery comprises substantially more bone stock, the cortical thickness and bicortical nature in which the fibula is harvested provides a very stable platform suitable for oromandibular reconstruction. This is despite the fact that the average thickness of the fibula is 1.5cm. The fibula is only second to the iliac crest in terms of demonstrating consistent bone quality for osseointegrated implant placement. The scapular free flap based on the subscapular artery system is often inconsistent in terms of the thickness of the lateral border and tip of the scapula. More often, the reconstructed scapular bone quality is unsuitable for osseointegrated implant placement. The other 2 available vascularized bone flaps in mandibular reconstruction, the rib and radius, are mainly used for restoring continuity defects without any intent for additional oromandibular reconstruction.
Comprehensive oromandibular rehabilitation of fibula osteocutaneous free flaps with osseointegrated implants and subsequent prosthetic restoration has consistently been shown to be reliable with high success rates. Kramer et al. followed 51 dental implants placed in 16 consecutive patients over 3.5 years and found the success rate to be 96.1%.6 Resonance frequency analysis also revealed a high stability rate within 12 months of functional loading. They also demonstrated that implants placed in fibulas had success rates similar to those placed in mandibles of healthy individuals. Smolka et al. similarly demonstrated an implant success rate of 92% over a 4.2-year follow-up period.7 They also found that radiation therapy could not be a factor in the success of dental rehabilitation or overall implant survival. This is particularly important given that the majority of patients undergoing fibula osteocutaneous free flap reconstruction will require adjuvant radiotherapy following tumor extirpation. In patients undergoing mandibular resections for benign conditions (benign tumors, osteoradionecrosis, osteomyelitis, or trauma), osseointegrated implants can be placed into the fibula at the time of mandibular reconstruction. Primary implant placement theoretically improves access to the bone, allows better determination of interdental relationships, and shortens oral rehabilitation time.8
Given these advantages of the fibula osteocutaneous free flap, it should come as no surprise why this flap has gained so much popularity over the past 2 decades and become the workhorse flap in mandibular reconstruction. However, there are some who argue that this flap possesses certain limitations that should preclude it from being ideal for oromandibular reconstruction. The most common complaint is the lack of height of the fibula in relation to the native mandible, making dental rehabilitation less than ideal. Additional arguments challenging the use of the fibula osteocutaneous free flap include unreliable skin perforators to support a skin paddle, insufficient soft tissue coverage for large composite mandibular defects, and the presence of vascular anomalies, which may preclude its use. We will address each of these apparent shortcomings and review the techniques and advancements in anatomic studies pertaining to the cutaneous perforator patterns to overcome these proposed limitations. In this way we hope to demonstrate why this invaluable flap will remain a workhorse in mandibular reconstruction for years to come.
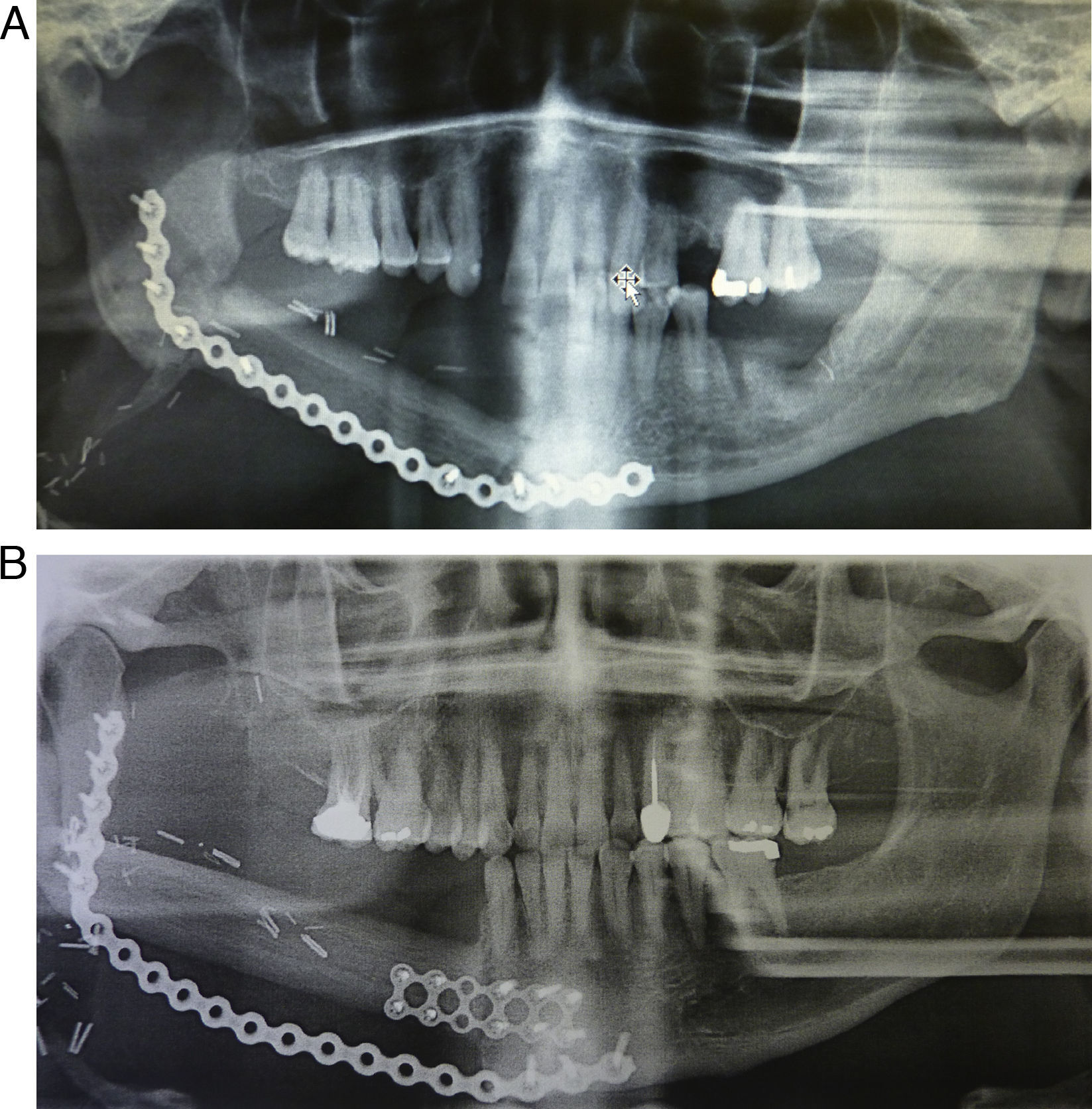
Height discrepancy between the fibula and native mandibleThe average diameter of the fibula is 1.5cm. When used to reconstruct an edentulous mandible, the fibular height closely approximates that of an atrophic mandible, allowing for a relatively straightforward dental rehabilitation. However, when compared to a dentate mandible, there is roughly a twofold height difference between the fibula and the native mandible. This discrepancy is most critical when reconstructing defects in the anterior mandible. The challenge lies in choosing to restore alveolar height to enhance dental rehabilitation versus mandibular height to restore lower facial contour. Placing the fibula at the inferior border of the mandible to improve mandibular contour, however, requires the need for elongated dental prosthesis, which results in unfavorably excessive lever arm forces.9 Three techniques can be employed to overcome this fundamental limitation of the fibula (Fig. 2A–B)
(A) Panoramic radiograph showing placement of the fibula slightly higher on the distal segment to diminish the height difference on the posterior region. (B) Panoramic radiograph showing placement of the fibula in the anterior region about a centimeter above the inferior border. This diminishes the lever arm in implant reconstructions.
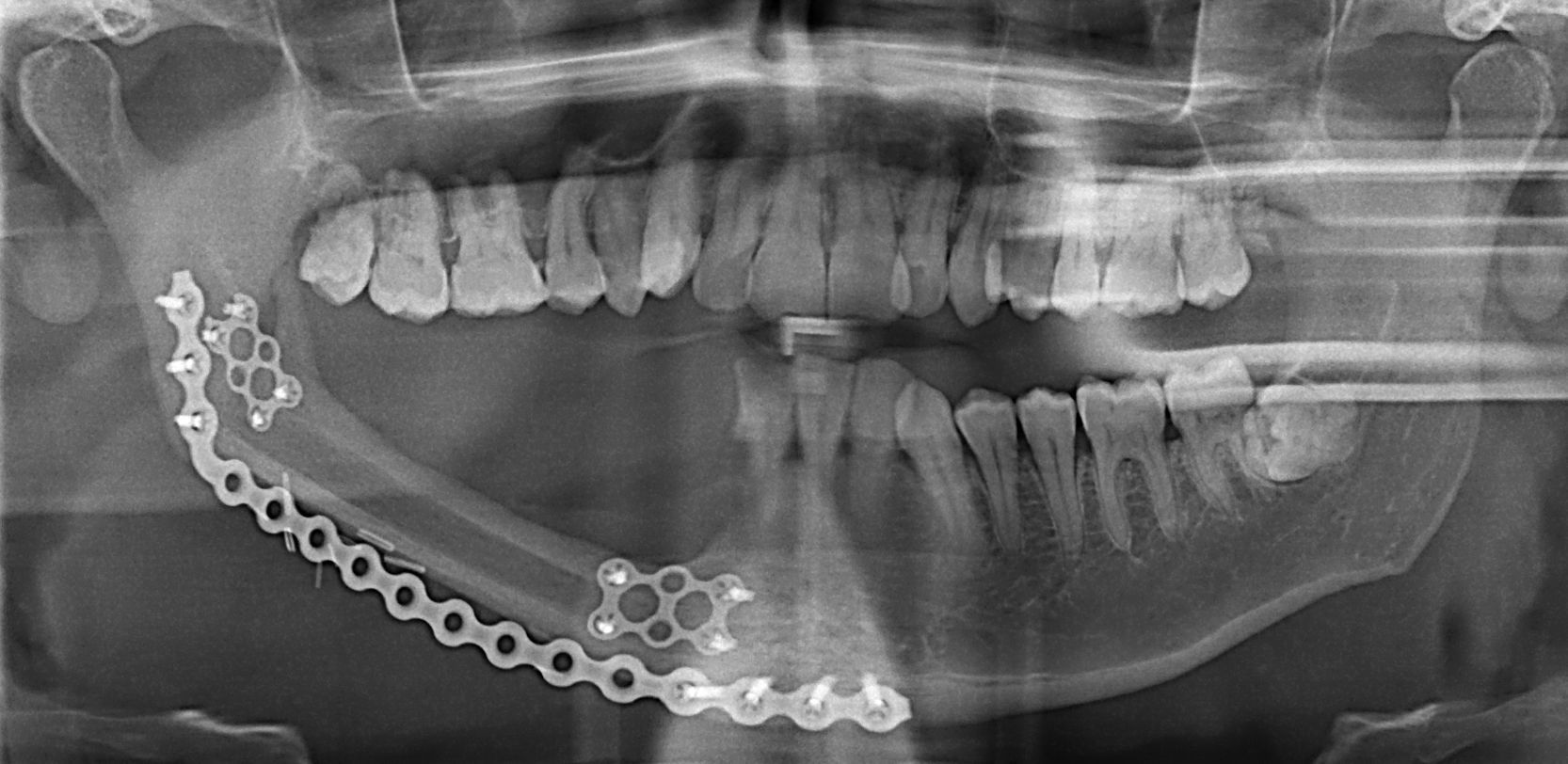
The simplest technique for restoring alveolar and mandibular heights simultaneously is by placing an additional reconstruction plate below the fibula. This inferior reconstruction plate is adapted to the mandible prior to resection and allows the mandibular contour to be preserved. The fibula can then be inset 1cm superior to the inferior mandibular margin, thereby, restoring alveolar height and allowing for improved dental rehabilitation. The contraindication for using this simple method, however, is patients who will undergo radiation therapy as the additional hardware may cause an increased risk of plate exposure9 (Fig. 3).
Double-barrel fibulaThe double-barrel fibula technique was introduced by Jones et al. in 1988 for increasing the width of the fibula in the reconstruction of segmental defects of the distal femur.10 Bahr et al. adapted this technique in which two vascularized bone struts are folded parallel to each other and connected via the periosteum and muscle cuff to mandibular reconstruction in 1998.11 The harvested graft should be twice the length of the mandibular defect. The two struts are then fixed to each other using plates and screws. This method effectively doubles the height of the mandible, thereby, reducing the vertical distance to the occlusal plane to enhance dental prosthetic rehabilitation while maintaining lower facial contour. Klesper et al. examined 40 cadaveric fibulas and found the double-barrel technique enabled the placement of four 13mm implants with diameters of 3.75mm set 1.5cm apart in 75% of the fibulas. Four 15mm implants could be placed in 52.5% of the fibulas.4 Although the application of the double-barrel fibula technique allows for better implant positioning and angulation, there does not appear to be any improvement in detectable implant stability.6 This again is attributed to the cortical thickness of the single barred fibula which provides a very stable platform suitable for osseointegration, accounting for the high long-term success rate of implant placement. The double-barrel fibula osteocutaneous flap has the additional advantage of permitting primary implant placement in select patients, thereby, reducing the overall time required for oromandibular reconstruction.12
Vertical distraction osteogenesis of the fibulaAnother technique to increase the height of the fibula closer to the occlusal plane of the dentate mandible is vertical distraction osteogenesis of the fibula. This method for gaining fibular height is theoretically simpler than performing the double-barrel technique and does not place the vascular pedicle at risk of possible compression or injury. There have been several case reports over the past decade demonstrating success in increasing the vertical height of the mandible, allowing for improved dental rehabilitation. Nocini et al. reported a case of mandibular reconstruction of a patient who sustained a severe gunshot wound utilizing distraction osteogenesis to increase the vertical height of a free vascularized fibula flap. One year after fibular free flap reconstruction of the mandible, distraction osteogenesis was undertaken 7 days after placement of distractors. The distraction protocol was set at 0.5mm per day for 22 days. After a 3-month consolidation period, orthopantomogram analysis revealed correction of the vertical discrepancy between the fibula and native mandible.13 Eski et al. similarly reported 3 cases of mandibular reconstruction of gunshot wounds where the rate of distraction was 1mm/day after a latency period of 5–7 days. They were able to achieve vertical height increases between 9 and 13mm, which remained stable during the follow-up period of 7–22 months.14 Despite being a relatively simple and proven technique for increasing fibular height, the disadvantage is the additional time needed for distraction prior to osseointegrated implant placement and prosthetic rehabilitation.
Unreliable skin perforators to support a skin paddleThe fibula osteocutaneous free flap has had a reputation for having unreliable skin perforators early on in its clinical application as early experiences were stricken with high rates of skin paddle loss. In fact, Hidalgo early on reported the use of a skin island based on septocutaneous perforators might be problematic when used in composite mandibular reconstructions. He attributed this to shorter segments of bone used in mandibular reconstruction, which may not contain cutaneous perforators or the perforators may have been injured during the osteotomies.3 These early accounts of high rates of skin paddle losses were largely due to paddle designs on the proximal and middle thirds of the fibula. Winters et al. studied the vascularization pattern of 20 fibula osteocutaneous free flaps with skin paddles designed over the proximal and middle third of the fibula. They found an axial musculocutaneous perforator originating high from the peroneal artery in half the cases. In five of these 10 cases, no other skin perforators were found within the boundary of the skin paddle. For this reason, they recommended dissection of the musculocutaneous perforator up to the peroneal artery unless one or more septocutaneous perforators within the boundaries of the skin paddle are found in order to minimize the risk of skin paddle loss.15 Another early solution for improving skin paddle reliability was to include the soleus or flexor hallicus longus underneath the skin paddle to take advantage of musculocutaneous perforators.16
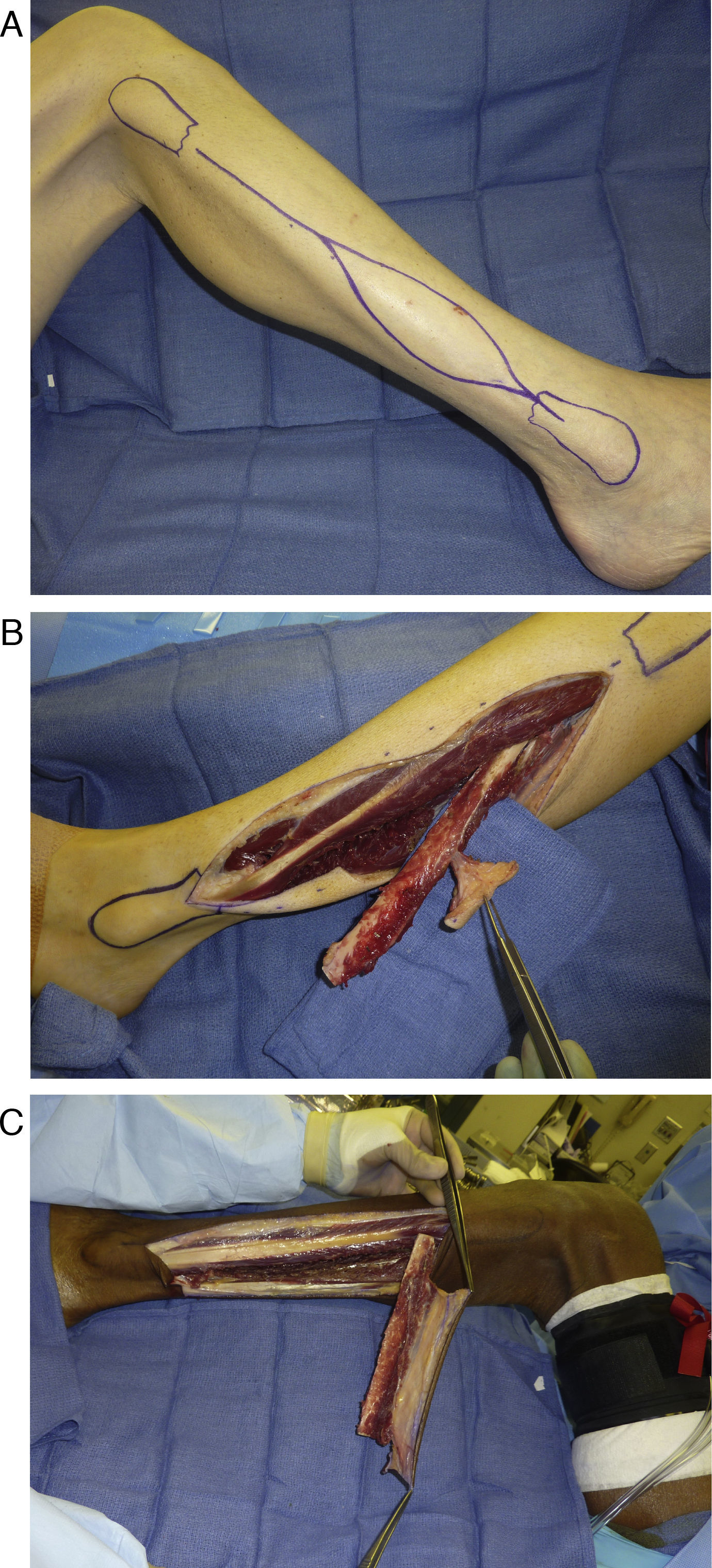
Anatomic studies of cutaneous perforators redirected attention to the distal third of the fibula for skin paddle design. Wei et al. in 1986 demonstrated in an anatomic study of 20 cadaver legs and 15 clinical cases that adequate perfusion to the lateral skin of the lower leg was provided by septocutaneous perforators of the peroneal artery alone.17 The distal fibula was shown in several other studies to consistently possess more cutaneous perforators than the proximal fibula. Yu et al. recently reexamined the perforator anatomy in 80 patients undergoing fibula free flap reconstruction and reaffirmed the distal third of the fibula contained more perforators to support a skin paddle, with one to three perforators consistently present.18 The majority of these perforators in the distal fibula were septocutaneous in nature. With the evidence provided by these anatomic studies demonstrating the presence of abundant cutaneous perforators in the distal third of the fibula, the reliability of the skin paddle should no longer be in question as long as it is designed on the distal third of the fibula, incorporating at least one of these perforators (Fig. 4A–C).
Insufficient soft tissue for large composite mandibular defectsAnother misconception of the fibula osteocutaneous free flap is that it possesses insufficient soft tissue for reconstruction of large composite oromandibular defects. The scapula or DCIA flaps with their accompanying skin paddles are often considered first for reconstruction of composite defects with a large soft tissue component. However, the skin paddles of these two flaps, while abundant, are generally thick and inflexible making lining of the oral cavity less than ideal. The pliability of the fibula osteocutaneous free flap skin paddle is second only to that of the forearm and, therefore, lines the oral cavity well. In addition, the thin posterior crural septum containing the septocutaneous perforators provides greater skin mobility, allowing for freedom in design and inset of 3 dimensional composite defects.19
The average dimension of the skin paddle is 6cm by 12cm, while the maximum dimension is 14cm by 32cm.5 These skin paddle sizes allow for reconstruction of most composite oromandibular defects, including the ability to line the floor of mouth and near total glossectomy defects or large skin defects with mandibular reconstruction. If additional soft tissue bulk is required, a cuff of soleus or flexor hallicus longus can be included with the flap. Inclusion of muscle in the flap also has the additional advantage of reducing the risk of skin paddle failure. For through and through defects, two large skin paddles can be harvested with the fibula. The proximal paddle is supported by the musculocutaneous perforator in the proximal third of the fibula while the distal paddle takes advantage of the septocutaneous perforators located in the distal third of the fibula.18
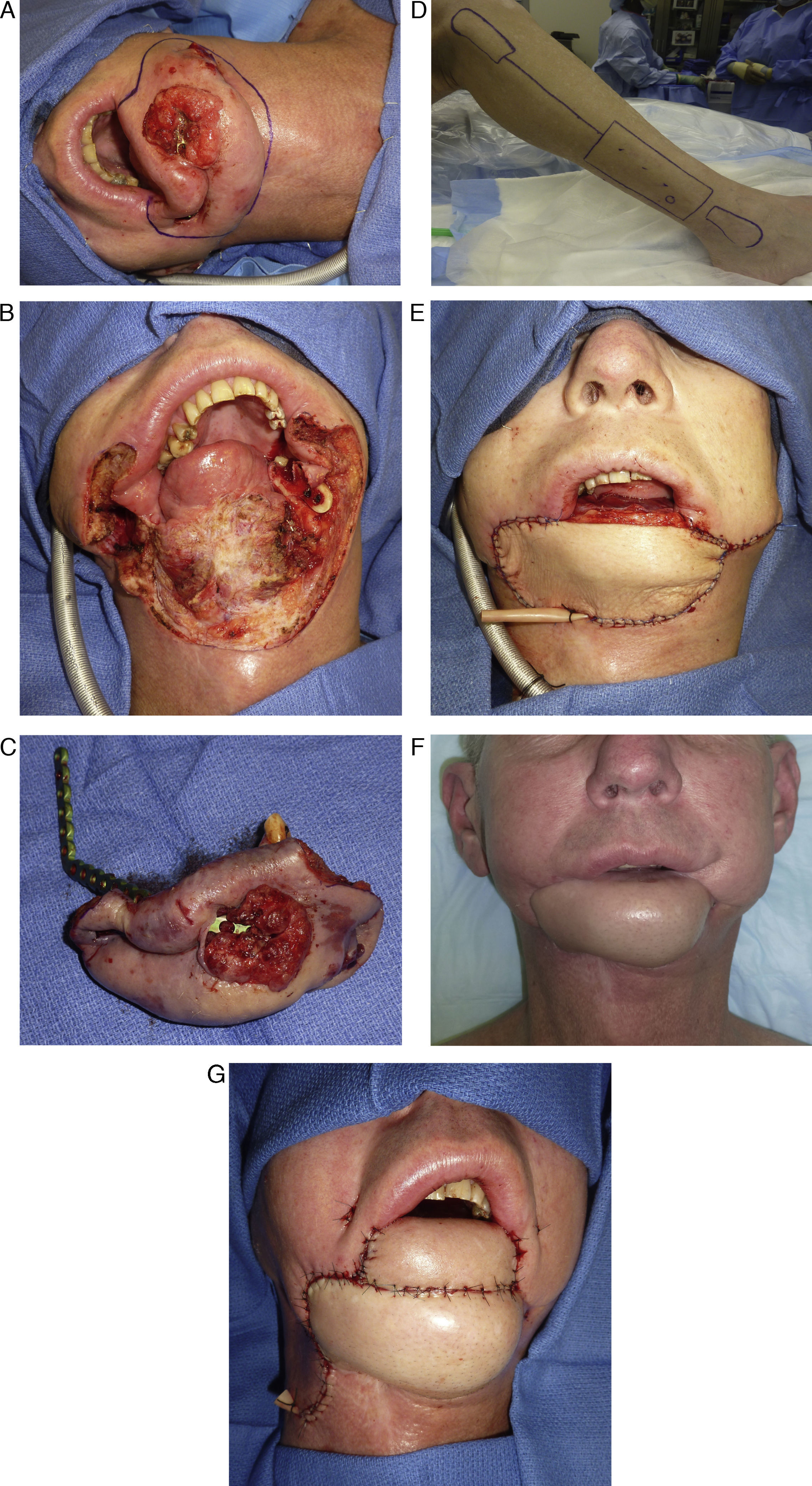
A technique for maximizing the utility of the skin paddle is to design it as a rectangle rather than the common fusiform pattern. This effectively increases the width of the skin paddle at both the superior and inferior ends to that of the middle portion, which corresponds better to the contours of most defects. Since any skin paddle width that is greater than 4–6cm will require a skin graft, designing the skin paddle in a fusiform pattern is unnecessary, as no attempt should be made to close the donor site defect primarily for risk of causing compartment syndrome. Knowing that the donor site defect will be skin grafted allows the skin paddle to be custom designed to the shapes of soft tissue defects. In this way, the skin paddle is utilized more efficiently and capable of lining large oromandibular defects (Fig. 5A–G).
(A) Patient with a very large recurrent mandibular malignancy with the planned area of resection marked. (B) Extensive defects of the mandible, floor of mouth, chin and total lower lip. (C) Resected specimen. (D) Planned fibula osteocutaneous flap, note a large rectangular skin paddle marked for harvest. (E) Inset of the fibula osteocutaneous flap used to recreate the defect with the exception of the lip. (F) Post operative view of the healed reconstruction about two months after the initial reconstruction. (G) Final reconstruction of the defect with a radial forearm free flap used to reconstruct the lip defect and set on top of the fibula flap.
Patients reporting symptoms of claudication or have a known history of peripheral vascular disease should not have fibula osteocutaneous free flaps performed and alternative vascularized bone flaps such as the scapula or iliac crest free flaps should be considered. In practice, the number of individuals with lower extremity vascular abnormalities severe enough to preclude the use of the fibula free flap is low. However, the incidence of vascular anomalies such as peripheral vascular disease will vary with different populations. Although it is infrequent for a planned fibula osteocutaneous free flap to be aborted, the consequences of causing lower extremity ischemia or harvesting a flap with compromised vessels are too great to not warrant careful preoperative evaluation of the vasculature.
There is, however, debate regarding the appropriate method for evaluating the lower extremity vasculature. There are many proponents of performing simply a preoperative examination of the dorsalis pedis pulse. They argue that the incidence of vascular anomalies associated with a normal pulse examination is so low that routine use of angiography is not warranted.20 Only when there is an abnormal pulse examination or evidence of lower extremity trauma is angiography recommended. However, advocates for the routine use of preoperative vascular imaging contend that a dominant peroneal artery supplying the dorsalis pedis artery will also give a normal pulse examination. This is the case in patients with severe atherosclerosis of the anterior and posterior tibial arteries or peronea arteria magna, a congenital condition where both the anterior and posterior tibial arteries are absent. Fibula free flap transfer in this case will result in devastating lower extremity ischemia and is, therefore, contraindicated. Given the possibility of causing severe consequence of removing the major vascular supply to the lower leg or jeopardizing flap viability, it is not unreasonable to obtain vascular imaging preoperatively. Previously, angiography was the gold standard for imaging the lower extremity vasculature, allowing visualization of vessel irregularities and arteriosclerotic vessels. Angiography, however, is rather invasive with potential complications such as bleeding, hematoma formation, thrombosis, and contrast allergy. In most centers, any of the CT angiography, MR angiography, or color flow Doppler imaging has largely supplanted traditional angiography. All three modalities have been shown in several studies to be less invasive while accurately identifying vascular anomalies and significant arterial disease.20–22 Successful fibula free flap transfers were achieved with normal examinations while irregular findings allowed for reconsideration of alternative donor sites. In our center, patients undergoing fibula free flap reconstructions will routinely undergo preoperative CT angiography. This simple, quick, and noninvasive examination insures safe harvest of fibula osteocutaneous free flaps with healthy vascular pedicles.
ConclusionThe fibula osteocutaneous free flap has demonstrated great versatility in the reconstruction of complex mandibular defects since its introduction by Hidalgo for mandibular reconstruction. It allows for comprehensive oromandibular reconstruction with osseointegrated implants and dental prosthesis rehabilitation, thereby, restoring masticatory function. The perceived limitation of insufficient fibular height can be circumvented by placing an inferior reconstruction plate and insetting the fibula more superior, double-barreling, or performing vertical distraction osteogenesis. The concerns of harvesting the fibula in patients with severe peripheral vascular disease or vascular anomalies can be avoided with appropriate preoperative imaging of the vasculature. Other misconceptions regarding the skin paddle reliability or insufficient amount of soft tissue for reconstruction of large composite defects have been refuted by several anatomic studies. Given the reconstructive potential for comprehensive restoration of form and function, the fibula osteocutaneous free flap will remain a workhorse in composite mandibular reconstruction for years to come.
Right to privacy and informed consent. The authors declare that no patient data appears in this article.
Confidentiality of Data. The authors declare that no patient data appears in this article.
Protection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Conflict of interestThe authors declare that they have no conflict of interest.