Tumor necrosis factor (TNF) is a proinflammatory cytokine involved in a wide range of important physiologic processes and has a pathologic role in some diseases. TNF antagonists (infliximab, adalimumab, etanercept) are effective in treating inflammatory conditions. Antilymphocyte biological agents (rituximab, alemtuzumab), integrin antagonists (natalizumab, etrolizumab and vedolizumab), interleukin (IL)-17A blockers (secukinumab, ixekizumab) and IL-2 antagonists (daclizumab, basiliximab) are widely used after transplantation and for gastroenterological, rheumatological, dermatological, neurological and hematological disorders. Given the putative role of these host defense elements against bacterial, viral and fungal agents, the risk of infection during a treatment with these antagonists is a concern. Fungal infections, both opportunistic and endemic, have been associated with these biological therapies, but the causative relationship is unclear, especially among patients with poor control of their underlying disease or who are undergoing steroid therapy. Potential recipients of these drugs should be screened for latent endemic fungal infections. Cotrimoxazole prophylaxis could be useful for preventing Pneumocystis jirovecii infection in patients over 65 years of age who are taking TNF antagonists, antilymphocyte biological agents or who have lymphopenia and are undergoing concomitant steroid therapy. As with other immunosuppressant drugs, TNF antagonists and antilymphocyte antibodies should be discontinued for patients with active infectious disease.
El factor de necrosis tumoral (TNF) es una citocina proinflamatoria involucrada en una amplia gama de procesos fisiológicos importantes y desarrolla un papel en la patogenia de algunas enfermedades. Los antagonistas del TNF (infliximab, adalimumab, etanercept) son efectivos en el tratamiento de afecciones inflamatorias. Los agentes biológicos antilinfocitarios (rituximab, alemtuzumab), los antagonistas de la integrina (natalizumab, etrolizumab y vedolizumab), de la interleucina 17A (secukinumab, ixekizumab) o los antagonistas de la IL-2 (daclizumab, basiliximab) se usan ampliamente después del trasplante y en trastornos gastroenterológicos, reumatológicos, dermatológicos, neurológicos y hematológicos. Dado el papel relevante de estos elementos de defensa del huésped contra agentes bacterianos, virales y fúngicos, el riesgo de infección durante el tratamiento con estos antagonistas genera preocupación. Las infecciones por hongos, tanto oportunistas como endémicos, se han asociado con estas terapias biológicas, pero la relación causal no está clara, especialmente entre los pacientes con un control deficiente de su enfermedad subyacente o que están recibiendo terapia con esteroides. Los pacientes en tratamiento con estos medicamentos deben ser examinados para detectar infecciones micóticas endémicas latentes. La profilaxis con cotrimoxazol podría ser útil para prevenir la infección por Pneumocystis jirovecii en pacientes mayores de 65 años que estén tomando antagonistas de TNF, agentes biológicos antilinfocitarios, o tengan linfopenia y estén en tratamiento concomitante con esteroides. Al igual que con otros fármacos inmunosupresores, deben suspenderse los antagonistas de TNF y los anticuerpos antilinfocitarios en pacientes con enfermedad infecciosa activa hasta su control.
Invasive fungal disease (IFD) is still a relevant problem with high morbidity and mortality, whose incidence increases proportionally as immunomodulatory treatments are performed. IFD encompasses not only patients with hemato-oncological diseases but also those who undergo transplantation or have underlying chronic or recurrent autoimmune inflammatory diseases (especially, rheumatic, dermatological, gastrointestinal and neurological diseases). Table 1 shows some of these biologic therapies authorized by the Food and Drug Administration (FDA) for clinical use43,63 (Fig. 1). This review describes the etiopathogenesis and epidemiology of a special type of severe infection, IFD, in patients who undergo therapies with monoclonal antibodies and other biological immunomodulators, as well as the risk factors that favor its onset.
Main monoclonal antibodies used in clinical practice.
| Biological agent (launch year) | Therapeutic target | Description | Clinical indications |
|---|---|---|---|
| Tumor necrosis factor α (TNF-α) inhibitors: | |||
| Infliximab (1998) | Anti TNF-α | Chimeric mAb | RA, Ps, CD, AS |
| Etanercept (1998) | TNF-α inhibitor | Recombinant soluble TNF-α receptor | RA, Ps, CD, AS |
| Adalimumab (2002) | Anti TNF-α | Human mAb | RA, Ps, CD, AS |
| Certolizumab-P (2008) | Anti TNF-α | Pegylated human mAb | RA, Ps, CD, AS |
| Golimumab (2009) | Anti TNF-α | Human mAb | RA, Ps, CD, AS |
| Antilymphocyte | |||
| Rituximab (2006) | Anti CD-20 | Chimeric mAb | RA, Ps, AD, ITP, NHL, CLL |
| Ofatumumab (2009) | Anti CD-20 | Human mAb | CLL |
| Obinutuzumab (2013) | Anti CD-20 | Humanized mAb | CLL |
| Alemtuzumab (2001) | Anti CD-52 | Humanized mAb | ME, CLL |
| Interleukin inhibitors | |||
| Anakinra (2003) | Anti IL-1-R | Recombinant nonglycosylated human IL 1 | RA, Ps, AS |
| Rilonacept (2008) | IL-1 inhibitor | Trimeric fusion protein | RA, Ps, CD, AS |
| Tocilizumab (2010) | Anti IL-6-R | Humanized mAb | RA |
| Secukinumab (2014) | Anti IL-17 | Human mAb | Ps |
| Ixekizumab (2016) | Anti IL-17 | Humanized mAb | Ps |
| Lymphocyte activation/migration inhibitors | |||
| Daclizumab (1997) | Anti CD25 | Humanized mAb | ME, transplant rejection |
| Basiliximab (1998) | Anti CD25 | Humanized mAb | Transplant rejection |
| Natalizumab (2004) | Anti-integrin α4 | Humanized mAb | EM |
| Vedolizumab (2014) | Anti-integrin α4-β7 | Humanized mAb | CD |
| Ustekinumab (2009) | Anti p40 (IL2, IL23) | Humanized mAb | Ps |
| Lymphocyte blockage inhibitors | |||
| Pembrolizumab (2016) | Anti PD1 | Humanized mAb | MELA, Uc, HDG, NSCLS |
| Nivolumab (2017) | Anti PD1 | Humanized mAb | MELA, Uc, HDG, NSCLC |
| Atezolizumab (2017) | Anti PDL-1 | Humanized mAb | Uc,NSCLC |
AS: ankylosing spondylitis; CD: Crohn's disease; CLL: chronic lymphocytic leukemia; ITP: idiopathic thrombocytopenic purpura; MAb: monoclonal antibody; ME: multiple sclerosis; PML: progressive multifocal leukoencephalopathy; Ps: psoriasis; RA: rheumatoid arthritis; MELA: melanoma; Uc: urothelial carcinoma; HDG: Hodgkin lymphoma; NSCLC: non-small cell lung cancer; year of approval (41, 59, 61).
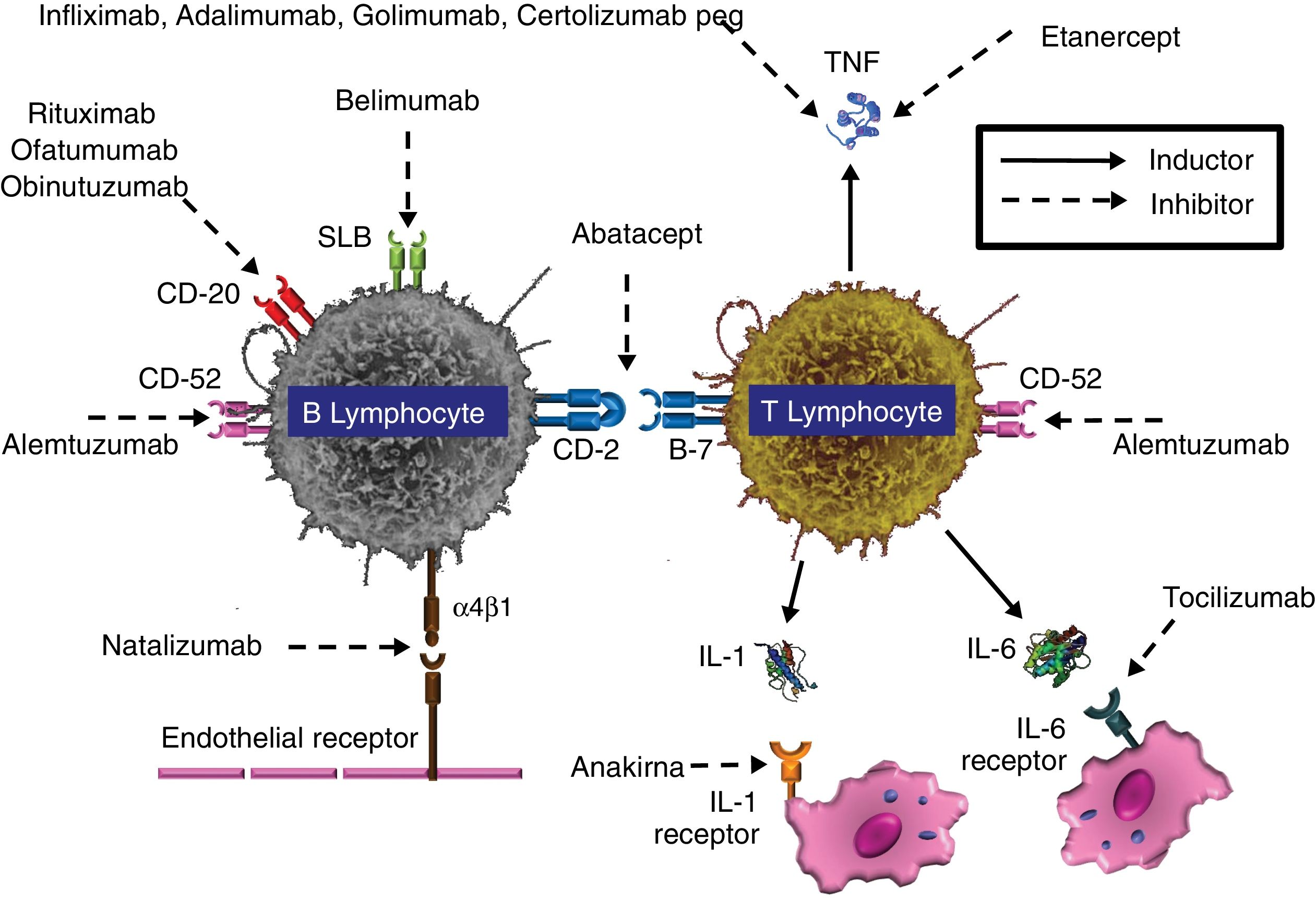
Accepted biological therapies in the treatment of inflammatory and lymphoproliferative diseases. SLB: B lymphocyte stimulator; IL-1: interleukin 1; LI-6: interleukin 6; TNF: tumor necrosis factor.
Modified from Bodro et al.11.
The clinical syndromes caused by opportunistic fungi include (due to their severity and associated mortality rate) invasive pulmonary aspergillosis (both in its disseminated form and the form with central nervous system involvement), candidemia and/or invasive candidiasis, neurocryptococcosis (with poorer prognosis in these patients when compared with patients with HIV infection) and pneumocystosis. Depending on the geographical location, infections have also been reported in association with primary pathogenic fungi (endemic mycoses), which include disseminated histoplasmosis in the extensive pulmonary, hematogenous and neurological forms, and in the forms associated with severe hemophagocytic phenomena. Most of these IFDs that occur in patients who undergo immunomodulatory biological therapies have been associated with the use of TNF antagonists, especially infliximab. However, other drugs also seem to be involved, including CD52 antagonists (alemtuzumab) and, although with insufficient evidence and many uncertainties, CD20 receptor blockers (rituximab) and a number of cytokine inhibitors. Although it is likely that these drugs do not show a clear profile of IFD induction, a number of these agents have been related to an increase in Pneumocystis jirovecii infections; however, it is sometimes difficult to determine the risk due to the use of these drugs and the risk due to concomitant therapies (mainly corticosteroids but also other agents such as cyclosporine, azathioprine, methotrexate and mercaptopurine).
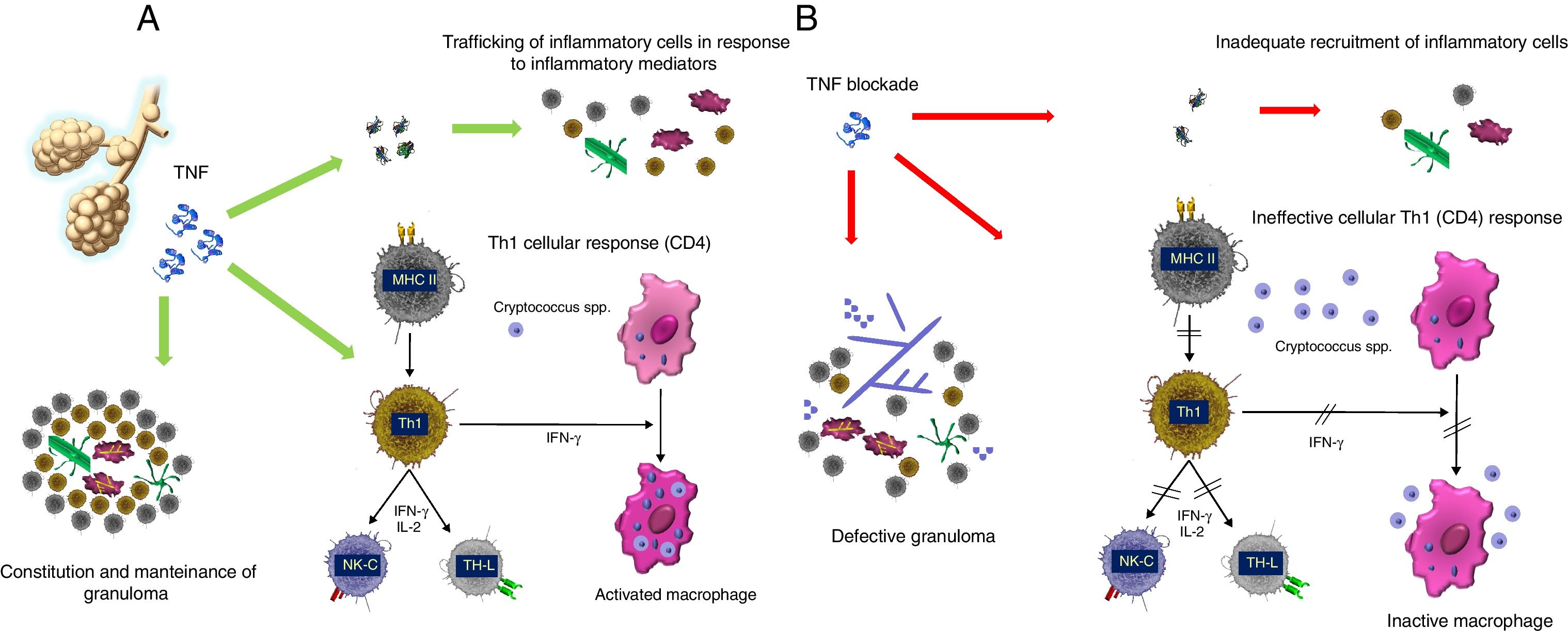
TNF antagonists-biological therapiesTumor necrosis factor (TNF) is an acute-phase proinflammatory cytokine produced and released by various immune cells, including macrophages, monocytes and T lymphocytes. TNF is released in response to various pathogens including viruses, bacteria (mainly intracellular), fungi and parasites. TNF can bind to the cell membrane or be released into the extracellular environment (soluble TNF). This cytokine promotes the activation of the vascular endothelium, facilitating leukocyte extravasation and recruitment, and promotes the production of other proinflammatory cytokines such as IL-1, IL-6 and IL-8. Likewise, TNF is necessary for the correct formation and maintenance of granuloma from the structural and functional standpoint, facilitating the migration of monocytes, dendritic cells, effector T lymphocytes and B lymphocytes.6 TNF is essential for the effective response against infection by mycobacteria and fungi (Fig. 2). The physiological response to TNF is mediated by two receptors, TNFRc-55 and TNFRc-75, present in the membrane of neutrophils, endothelial cells and fibroblasts, and as soluble receptors in blood and synovial fluid.
The importance of granuloma constitution. (A) Mechanism of action of TNF. TNF: tumor necrosis factor; MHC II: major histocompatibility complex molecules class II; Th1: type 1 T helper (Th1) cells; NK-C: natural killer cells; IL-2: interleucin 2; IFN-γ: interferon γ. (B) Effect of TNF antagonists.
Abnormal activity of this cytokine is involved in the pathogenesis of several inflammatory diseases including rheumatoid arthritis, inflammatory bowel disease, ankylosing spondylitis, psoriasis, psoriatic arthritis, hidradenitis suppurativa and graft-versus-host disease (GVHD). TNF antagonists (etanercept [ETA], infliximab [INF], adalimumab [ADA], certolizumab and golimumab) have represented an important advance in treating these diseases.5,39,60,61 ETA is a completely human and dimeric recombinant protein consisting of the fusion of a natural TNF receptor (TNFRc-75) and an IgG constant fraction, which provides stability. ETA mimics the effect of the natural TNF receptor. The rest are mAbs that bind to one or two molecules among the TNF superfamily, preventing their binding to the receptor.
Although each of these molecules blocks TNF activity differently, they are separated into three groups based on the risk of infection the patient is exposed to: (1) ETA, (2) INF, ADA and golimumab, and (3) certolizumab. ETA15 has a structure similar to that of the receptor and does not act as an opsonin (does not subsequently activate the complement), neither causes antibody-dependent cytotoxicity (ADCC) nor induces apoptosis. INF, ADA and golimumab, conversely, promote antibody-mediated cytotoxicity and apoptosis, causing the depletion of the cells to which they bind through the TNF in the cell membrane. INF, ADA and golimumab also bind with greater affinity, strength and stability than ETA.61 Therefore, while ETA causes qualitative immunosuppression, INF and ADA cause both qualitative and quantitative immunosuppression, which explains why the risk of developing an opportunistic infection is greater with INF and ADA and why its onset is earlier. Concerning the risk of infection associated with certolizumab, there is insufficient data; although certolizumab does not activate the complement fixation or induce apoptosis, it does interfere with the granuloma's integrity.67
Despite the difficulty in establishing a direct relationship between the biological drug and the resulting infection, two recent meta-analyses including patients with rheumatoid arthritis showed that those patients treated with these biological agents were more susceptible to developing an opportunistic infection than the untreated patients (odds ratio (OR), 1.79 per 1000 patient-years; 95% CI, 1.17–2.74; this ratio could increase to 17 per 1000 patient-year in those exposed to high doses).31,58 In other studies where patients concomitantly took corticosteroids, the risk of infection was 2-fold greater (OR, 2.03; p<.001). The studies observed a greater prevalence of infection by Mycobacterium tuberculosis (OR, 3.73; 95% CI, 1.72–8.13), P. jirovecii and varicella zoster virus.20,53
Assessing the confounding factors related to the underlying disease, the mAb dosage and the concomitant use of corticosteroids, the published incidence rate of IFD in patients treated with TNF antagonists varied significantly, from 6/100 to 6/1000.5,58,60 In addition to facilitating fungal dissemination by hindering the development of a granulomatous response, TNF antagonists block the Th1-type cell response, facilitating infection by intracellular fungi such as Cryptococcus spp. and Histoplasma spp. Table 2 groups the published cases of IFD in patients who underwent TNF antagonist treatment.
Invasive fungal disease (IFD) in patients who undergo TNF antagonist treatment.
| IFD | n | INF | ETA | ADA | Indication | Involvement | Diagnosis | Treatment | Mortality |
|---|---|---|---|---|---|---|---|---|---|
| Histoplasmosis | 240 | 86% | 8% | 6% | RA/IBD | Pneumonia, disseminated disease | Serology, culture, BAL | Amphotericin B, itraconazole | 20% |
| Coccidioidomycosis | 30 | 93% | 7% | 0% | RA (associated with corticosteroids and MTX) | Pneumonia >80%Asymptomatic 15–20% | Serology, biopsy (lung, skin), culture | Amphotericin B, itraconazole, fluconazole | 12% |
| Aspergillosis | 65 | 73% | 23% | 3% | GVHD in HPT, RA, IBD | Pneumonia | ASPAG (blood-BAL), culture, biopsy | Amphotericin B or voriconazole | 82% hematologic |
| Mucormycosis | 4 | 75% | 0% | 25% | GVHD in HPT, RA | Disseminated disease, orbital cellulitis | Culture, biopsy | Amphotericin B and posaconazole | 100% |
| Candidiasis | 64 | 84% | 14% | 1% | GVHD, RA, IBD | Candidemia with/without endovascular infection, abscess, esophagitis | Culture | Azoles, echinocandins, polyenes | 50% s/t coinfected with P. jirovecii |
| Cryptococcosis | 28 | 60% | 35% | 3% | GVHD, RA, IBD | Pneumonia, fungemia, CNS involvement | Staining, serology, culture | Amphotericin B, fluconazole | 0% |
| Blastomycosis | 7 | 100% | 0% | 0% | GVHD, RA | Pneumonia | Culture | ND | ND |
| PJP | 125 | 89% | 9% | 1% | RA | Pneumonia | Staining, NAAT | Cotrimoxazole | 18% |
ADA: adalimumab; BAL: bronchoalveolar lavage; ETA: etanercept; GVHD: graft-versus-host disease; IBD: inflammatory bowel disease; HPT: hematopoietic stem cell transplantation; NF: infliximab; MTX: methotrexate; NAAT: nucleic acid amplification techniques; ND: no data; PJP: Pneumocystis jirovecii pneumonia; RA: rheumatoid arthritis. Modified from Refs. 5,10,57,58,71.
As a general rule, and especially in the case of IFD risk by filamentous fungi, it is recommended discontinuing the immunosuppressive therapy until the infection has been completely controlled.6 A number of experts, however, support maintaining the immunomodulatory biological treatment in certain individual cases while changing the family or drug for another with lower immunosuppressive power and less dysfunction or imbalance in the immune response. The objective of these changes is to prevent a severe and disabling outbreak or flare-up of the underlying disease, which can entail other complications and sequelae.
Opportunistic mycoses in patients treated with a TNF antagonistCases of IFD due to several species of Candida associated with the use of mAbs have been reported. Tsiodras et al.66 observed in 2008 281 cases of IFD (23% by Candida) in patients treated with TNF antagonist: INF (80%), ETA (16%) or ADA (4%). In 2010, an approximate incidence rates of 4.3 cases per 100,000 patients treated with INF and 0.9 per 100,000 patients treated with ETA were estimated.61 Regardless of the differences in activity between the two drugs, there are other differences in activity related to the indication and the posology that could promote the onset of invasive candidiasis in patients treated with INF. First, INF is administered intravenously (while ETA is not), which facilitates catheter-related infection. INF is indicated for treating Crohn's disease, which is associated with a deep disruption of the intestinal mucosal barrier, with a higher possibility of fungal translocation, while ETA does not have this indication.61 Likewise, combined treatment with corticosteroids is associated with a greater risk of colonization of the mucous membranes and translocation of Candida species from the gastrointestinal tract to the blood.20
The incidence rate of opportunistic infection associated with Aspergillus in patients undergoing treatment with INF has been estimated at 12.4/100,000 patients.40,61 Forty-one percent of these patients were also undergoing corticosteroid treatment, and most were treated with INF for chronic GVHD after allogeneic hematopoietic progenitor cell transplantation (allo-HPT). In these patients, the mortality was greater than 80% and was typically caused by Aspergillus fumigatus and Aspergillus flavus. There are no data on the diagnostic usefulness of detecting the Aspergillus galactomannan antigen and the fungal gene sequences using polymerase chain reaction (PCR) techniques in nonhematologic patients who undergo biological therapy with INF; however, their detection seems appropriate in the diagnosis and monitoring of these infectious processes.
To date, there have been only seven reported cases of mucormycosis in patients treated with TNF antagonists. Despite combined treatment with amphotericin B and posaconazole, and surgical debridement, the mortality rate was greater than 80%.16,18,28,33,45,70,74
According to certain studies, P. jirovecii pneumonia (PJP) has an incidence rate of 0.4% per 5000 patients treated with INF, 0.2% per 7091 patients treated with ETA and 0.3% per 3000 patients treated with ADA, and its associated mortality varies between 10% and 20%.11,38 Although there are few studies, it is suspected that the incidence of PJP is high, especially among people older than 65 years and/or undergoing corticosteroid treatment. Determining whether the infection is acute or latent and reactivated is complex too. This is because we do not know the percentage of a relevant number of oropharyngeal or bronchial carriers among patients undergoing corticosteroid treatment or who have chronic structural lung disease.73 Additionally, P. jirovecii can persist for months after the infectious condition has been resolved. Furthermore, patients with colonization or active infection, and asymptomatic medical personnel with respiratory colonization can act as transmission vectors of this yeast-like fungus to other patients more susceptible to PJP. As with other models of functional lymphopenia (HIV, some hemopathies, transplantation and the effects of drugs such as temozolomide combined with radiation therapy), some authors have proposed prophylaxis with cotrimoxazole for this patient group when the CD4 count is <300cells/μl and when the patient is undergoing extended corticosteroid treatment (≥3 weeks) or at high doses (>25–30mg/day of prednisone or equivalent).2,11,27,34 A recent study reported that patients with 2 or 3 risk factors for developing PJP (age ≥65 years, coexisting pulmonary disease and use of corticosteroids) benefitted from prophylaxis with cotrimoxazole before the treatment with TNF antagonists or IL-6 receptor inhibitors (tocilizumab), as treatment for rheumatoid arthritis, started. Among the 214 patients treated with prophylaxis no cases of PJP were diagnosed (the incidence rate decreased from 0.93 to 0.00 per 100 person-years).27 New diagnostic tools such as the detection of (1→3)-β-d-glucan could be useful for discriminating a true infection from colonization, and thereby could also be useful for monitoring the treatment.
Tsiodras et al. collected 28 cases of cryptococcosis, 60% of which were linked to INF treatment, 40% to ETA treatment and an isolated case undergoing ADA treatment.66 The main risk factors in this patient group for developing cryptococcosis were the presence of hematologic disease of more than 12 months of progression and chronic lymphopenia. The reported cases of cryptococcosis were mainly respiratory and only rarely progressed with neurological involvement or fungemia.5,61 The infection is usually acquired by the pulmonary pathway and, after a latency period, is disseminated by the lymphocytic or hematogenous pathway. In most of the patients treated with INF the infection-related symptoms appeared after the third-fifth dosage, suggesting the reactivation of a latent infection,4,13,22,34,57,64 although in the rare reported cases of cryptococcal meningitis its onset started after the sixth dose and was considered a probable de novo infection. The therapeutic recommendation is to administer amphotericin B followed by fluconazole or fluconazole in monotherapy, leaving the combination of amphotericin B and flucytosine for severe cases, neurological forms or patients with added risk factors. Fluconazole treatment should continue until 8–10 weeks are completed, followed by a chronic suppression phase lasting a variable amount of time with the objective of preventing relapses.42 Serological tests are not useful for detecting latent infection, and primary prophylaxis is not recommended. Given the link established between Cryptococcus neoformans and bird feces (mainly those of pigeons), it is recommended that patients who follow biological therapies avoid any contact with birds and areas that accumulate bird droppings to reduce fungal exposure.50
Mycoses by endemic primary pathogens in patients treated with TNF antagonistsHistoplasmosis is the endemic mycosis most related to TNF antagonists treatment due, in part, to its greater geographical distribution and to the intracellular growth of Histoplasma capsulatum, which is mostly neutralized by macrophages and T lymphocytes, unlike Blastomyces and Coccidioides species that are also controlled by neutrophils. The approximate incidence rate of histoplasmosis is 18 per 100,000 patients treated with INF and 2.6 per 100,000 patients treated with ETA.5,61 The symptoms usually appear between the first and twentieth week of treatment. Histoplasmosis sometimes presents with pustular ulcerated or nodular skin lesions, which facilitate its diagnosis. Chest radiography and computed tomography usually show signs of pneumonitis without infiltrates, although a reticulum-nodular pattern or frank nodules are sometimes detected. The diagnosis is typically established by detecting the Histoplasma antigen in urine, although this is not easily available in some countries. Amphotericin B is recommended for treating the severe acute infection, subsequently de-escalating to itraconazole until 12 months have been completed. A recent series of 98 patients with histoplasmosis observed that the two independent risk factors that differentiated disseminated disease from localized pulmonary disease were positive antigenuria (13.76ng/ml vs. 7.69ng/ml, p=0.008, respectively) and simultaneous therapy with corticosteroids (23 vs. 10, p=0.04, respectively).69 Also in this series, 9.2% of the patients presented IRIS at 6 weeks of discontinuing the TNF antagonist treatment, and all were treated with corticosteroids with good outcomes. However, three episodes of relapse were reported in the following 3 years.69
Blastomycosis occur less frequently, except in patients with HIV infection and transplanted patients.60,61 The clinical manifestations are characterized by dissemination to the skin, bowel and even central nervous system, and respiratory distress frequently develops. The diagnosis is performed through biopsy and mycological cultures or by urinary antigen detection, which can present cross-reactivity with other primary pathogens that cause IFD. The treatment is similar to that of histoplasmosis, starting with amphotericin B in severe and acute conditions, and continuing with itraconazole for 12 months. For patients in whom the immunosuppression cannot be reversed, the antifungal treatment or secondary prophylaxis should be administered for life.39,69
The estimated incidence rate of coccidioidomycosis (Coccidioides immitis or Coccidioides posadasii) is 5.58/100,000 in patients treated with INF and 0.88/100,000 in patients treated with ETA.61 Practically all patients undergoing the TNF antagonist treatment diagnosed with coccidioidomycosis were also treated with corticosteroids and methotrexate. Although 20% of patients can be asymptomatic, respiratory symptoms (cough, dyspnea) and asthenia predominate. Cases of dissemination to skin, joints or meninges have also been documented. The diagnosis is based on lung or skin biopsy, but cultures are dangerous due to aerosolization of the arthroconidia and should always be performed under proper biosecurity conditions. Urinary antigen detection is useful and has fewer cross-reactions with other primary pathogens (Histoplasma or Blastomyces). The Infectious Diseases Society of America (IDSA) guidelines recommend starting treatment with amphotericin B for immunosuppressed patients, including those with TNF antagonists, and moving to an azole (fluconazole, itraconazole, voriconazole) when a clinical response is observed. As with all IFDs, it is also recommended that any TNF antagonist treatment is discontinued until the infectious process has been resolved.7,19
When diagnosing endemic mycoses in a patient undergoing TNF antagonists, it is important to determine whether we are facing a recent infection or a reactivation of a previous exposure. Several authors have postulated that endemic mycoses are recent infections, according to incidence data. The general incidence rate of histoplasmosis is 1–2 cases/100,000 treated patients, a much lower number than expected given that, in endemic areas, the prevalence of pathogen exposure is 40–50% of treated patients. How quickly after following the treatment symptoms appear could help us to establish the difference (1–24 weeks from the first dose).
Given the ease with which Histoplasma, Coccidioides and Blastomyces species disseminate, and the potential severity of these infections in patients undergoing TNF antagonists treatment, screening is recommended for possible exposure through a case history review, searching for typical symptoms, occupational activities and possible exposure during travels or stays in endemic regions. Determining antibody titers in blood and urine for endemic fungi is recommended before starting TNF antagonist treatment if these risk factors are found in the directed anamnesis. Once the immunosuppressive therapy has started, the value of the serological test will be much lower. Antigenuria can be useful for detecting a recent episode during the TNF antagonist treatment; its measurement every 2–3 months in endemic areas is therefore recommended. Antigenic detection in a patient with previously negative titers or a 2–3-fold increase in their initial titer requires an active search for the pathogen and even the start of empiric antifungal treatment.5,61
Although several authors support prophylaxis with itraconazole for patients who undergo solid organ transplantation (SOT) or who have HIV infection in endemic areas,8,9 there are no data on the utility of this strategy for patients with TNF antagonist treatment. The IDSA guidelines suggest considering the use of itraconazole for patients with a history of active histoplasmosis in the past two years and who started treatment with TNF antagonists35,71; however, the guidelines do not recommend itraconazole for coccidioidomycosis. Lifelong treatment with itraconazole is recommended for patients with immunosuppression who develop blastomycosis or coccidioidomycosis and who have no chance of recovering their immune status. This includes patients who, due to their disease progression, cannot withdraw the TNF antagonist agent. Patients should also be advised to avoid environments and activities where dust can be stirred and/or inhaled (Table 3).
Proposal for the preventive approach and management of fungal infections after immunotherapy.
| Preventive issues | Recommendation category* |
|---|---|
| High index of clinical suspicion | C III |
| Assess possible relationship with geographical areas of endemic mycoses | C III |
| Rapid access to health care | C III |
| Do not start TNF antagonist or other biologics with deep lymphocyte depletion in patients with filamentous fungi infection or colonization | C III |
| Avoid exposure to aerosols with a high potential of contamination by fungal spores (vacuum cleaners, fans, carpets, ornamental plants and demolition or excavation zones) | C III |
| Avoid contact with birds and areas with bird excrement | C III |
| Avoid starting TNF antagonist in patients with previous IFD as much as possible | C III |
| Prophylaxis against P. jirovecii with cotrimoxazole (or other appropriate treatment) in patients with high-dose corticosteroids who require TNF antagonist or other immunosuppressants | C III |
| Early empiric treatment of IFD | C III |
| Early empiric diagnosis and treatment of Pneumocystis jiroveci pneumonia | C III |
| Discontinue TNF antagonist during the fungal infection | C III |
| Assess the restarting of TNF antagonist once the infection has been controlled | C III |
IFD, invasive fungal disease.
MAbs are also used for treating malignant lymphoid diseases, such as chronic lymphocytic leukemia (CLL) and non-Hodgkin's lymphoma (NHL). These diseases have, by nature, an underlying impairment of humoral immunity but include significant impairment of cell immunity as the disease progress, refractoriness or a lack of immune response, and the succession of various treatment schemes. In these processes, which can progress with normal T lymphocyte counts, an increase in T lymphocyte suppressors and inhibitory cytokines is detected, as well as a reduction in CD4+ lymphocytes and functional impairment of natural killer (NK) cells.44 Although IFD is conventionally attributed to a state of prolonged neutropenia, both the functional and quantitative abnormalities in T and B lymphocytes (and thereby in the secreted cytokines) increase the risk of opportunistic infection by impeding the setting of an effective immune response. The antilymphocyte mAbs include the anti-CD20 rituximab (RIT), ofatumumab (OFA) and obinutuzumab (OBI), and the anti-CD52 alemtuzumab (ALE).
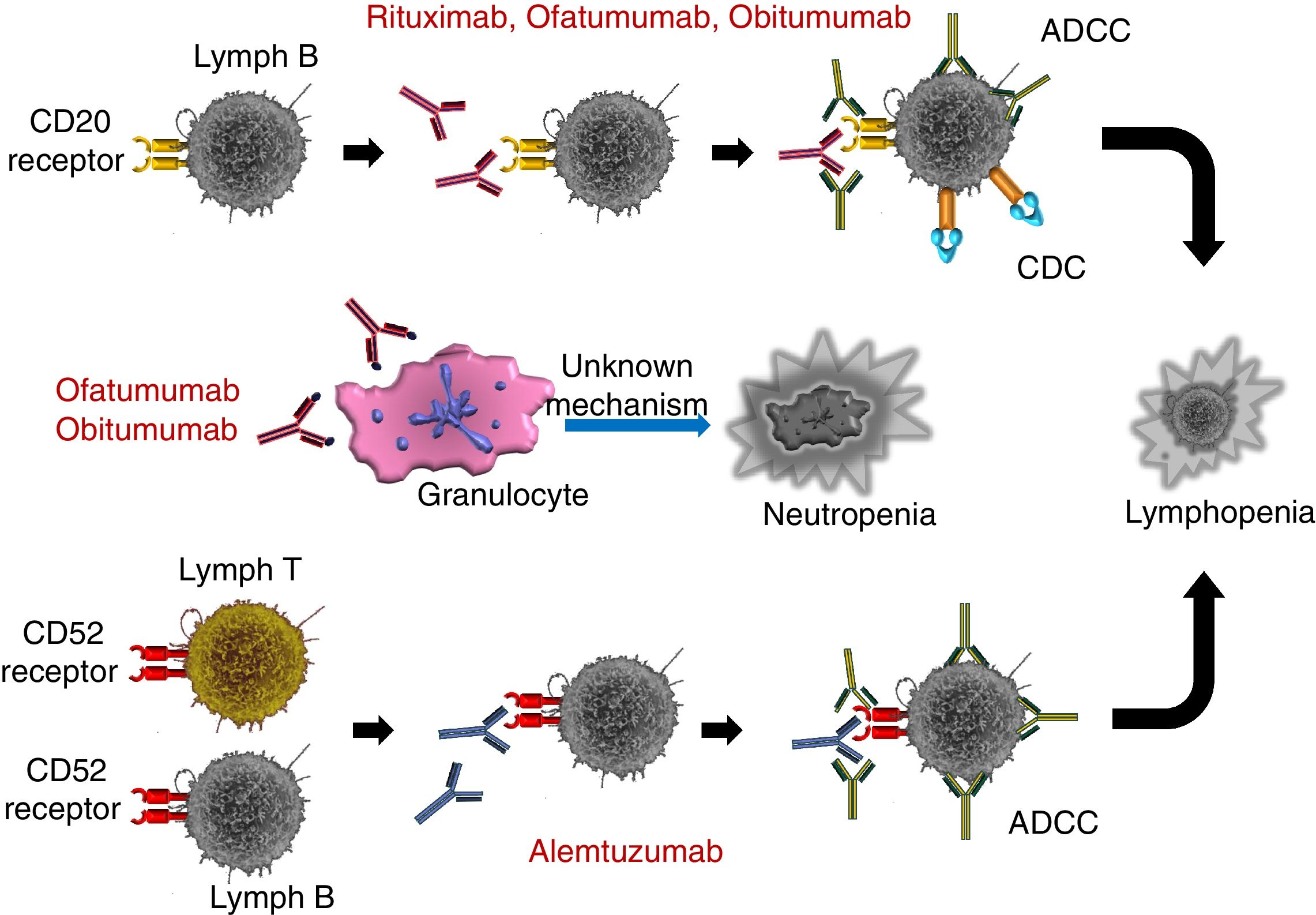
RIT (3,4) is a chimeric mAb consisting of constant region sequences from a class I IgG human and variable region sequences from light and heavy-chain murine antibodies. RIT binds specifically to the CD20 antigen, which is expressed in pre-B and mature B lymphocytes. This antigen is expressed in more than 95% of non-Hodgkin's lymphomas of B lymphocytes. The Fab domain of RIT binds to CD20 on the surface of the B lymphocyte, while the Fc domain can recruit immune response effectors to mediate the lysis of the B lymphocytes. The possible effector-mediated cell lysis mechanisms include complement-dependent cytotoxicity (CDC), as the result of C1q binding, and antibody-dependent cell-mediated cytotoxicity (ADCC) mediated by one or more Fc region receptors present on the surface of the granulocytes, macrophages and NK cells. It has also been shown that RIT binding to B-lymphocyte antigen CD20 induces cell death by apoptosis. The most common risk factors for infection in patients treated with RIT depend on the type of blood disease and its treatment (such as corticosteroids combined with polychemotherapy schemes, as well as hematopoietic stem cell transplantation), the time from transplantation to RIT infusion, IgM levels, total lymphocytic count, CD4+ lymphocyte count and the CD4/CD8 ratio.26
OFA is a fully human mAb that binds to a different CD20 epitope than the one RIT binds to. OFA causes greater CDC and ADCC in its target (B lymphocytes) than RIT, even in cases of low CD20 expression. OFA has shown greater potency in vitro than RIT and efficacy in cases of CLL refractory to RIT. Phase 3 and phase 2 studies62,68 have shown that OFA causes a dose-independent increase in the incidence and duration of prolonged and late-onset neutropenia,44 which is greater than that linked to RIT treatment, although this increase has not been accompanied by an increase in infection rates.65
OBI is a new generation humanized mAb that binds to CD20 through different epitopes than those of RIT and OFA. OBI has been modified by glycoengineering, achieving greater affinity by immune effector cells for the Fc receptor fraction (NK, macrophage and monocytes) compared with RIT and OFA. OBI causes ADCC and phagocytosis in B lymphocytes and, to a lesser extent, CDC. Given that OBI is a type 2 antibody, it also induces more direct cell death. As with OFA, OBI causes severe and prolonged neutropenia that requires antifungal prophylaxis (datasheet recommendation). However, several cases of IFD in patients who were treated with cotrimoxazole prophylaxis have been published.46 The fungal infections include PJP, although cases of candidemia and IFD by ubiquitous fungi such as Penicillium have also been reported. This compels us to maintain the clinical suspicion of IFD in patients undergoing OBI treatment and who have persistent fever despite prophylaxis.
ALE is a humanized mAb against the CD52 antigen developed for treating malignant lymphoid diseases, including non-Hodgkin's lymphoma, B-cell CLL, prolymphocytic leukemia and T-cell lymphoma.5,61 ALE is currently used to treat multiple sclerosis in its relapsing-remitting form, although with different dosage schedules. After binding with the CD52 antigen, which is expressed by T and B lymphocytes (in addition to monocytes, macrophages and eosinophils), ALE develops ADCC. Cell immunity is the most affected by ALE (Fig. 3), causing deep and prolonged lymphopenia, mainly of CD4+ lymphocytes, although the deficiency is never pure. By affecting B lymphocytes, ALE also decreases the number of circulating antibodies. Therefore, the immune condition resembles that of SOT recipients and immunosuppressed patients with HIV infection/AIDS and no antiretroviral treatment.
The most common fungal infections associated with the use of antilymphocyte mAbs are caused by P. jirovecii and Cryptococcus species, although infections by Aspergillus, Candida, Alternaria, Histoplasma and Mucorales have also been reported.26 There is little information on the incidence of cryptococcal infection, which is probably due to the difficulty in establishing a link with the drug in hematologic patients who are immunosuppressed for other reasons. Nevertheless, this type of infection is still considered a fairly exceptional event.46 Cryptococcosis can progress in the same way, in terms of clinical behavior, as in other situations of cellular immunosuppression that predispose patients to be infected by this pathogen (HIV, SOT). There are also no recommendations regarding the time of treatment for cryptococcosis in patients undergoing antilymphocyte mAbs treatment. However, in other similar immunosuppression models, a 6–12-month treatment has been suggested. For SOT recipients, the recommendation is to reduce the threshold of immunosuppression and perhaps discontinue treatment with anti-CD20 or ALE when faced with an episode of IFD59 or to look for alternative drugs with lower involvement of the T-cell immune response.
PJP is very common among patients undergoing treatment with these mAbs, although the incidence rate associated with the drug is difficult to establish, often because the patients are undergoing corticosteroid therapy. A recent meta-analysis showed that the incidence rate of PJP in patients with lymphoma undergoing regimens that included RIT was higher than in those patients whose regimen did not include RIT (28/942 vs. 5/977), showing a greater risk of PJP (relative risk (RR), 3.65; 95% CI 1.65–8.07, p=0.001), with no statistically significant differences between the biweekly and triweekly regimens.25 PJP usually presents between the fourth and sixth week when CD4+ lymphocytes count is below 200cells/mm3. The diagnosis is usually based on clinical parameters and is confirmed by the observation of cysts through indirect immunofluorescence or molecular studies. The treatment of choice is cotrimoxazole, although there are alternative regimens with pentamidine, clindamycin or atovaquone. Due to PJP's high incidence rate, primary prophylaxis is recommended until the CD4 counts are above 200lymphocytes/mm3.27,30,36
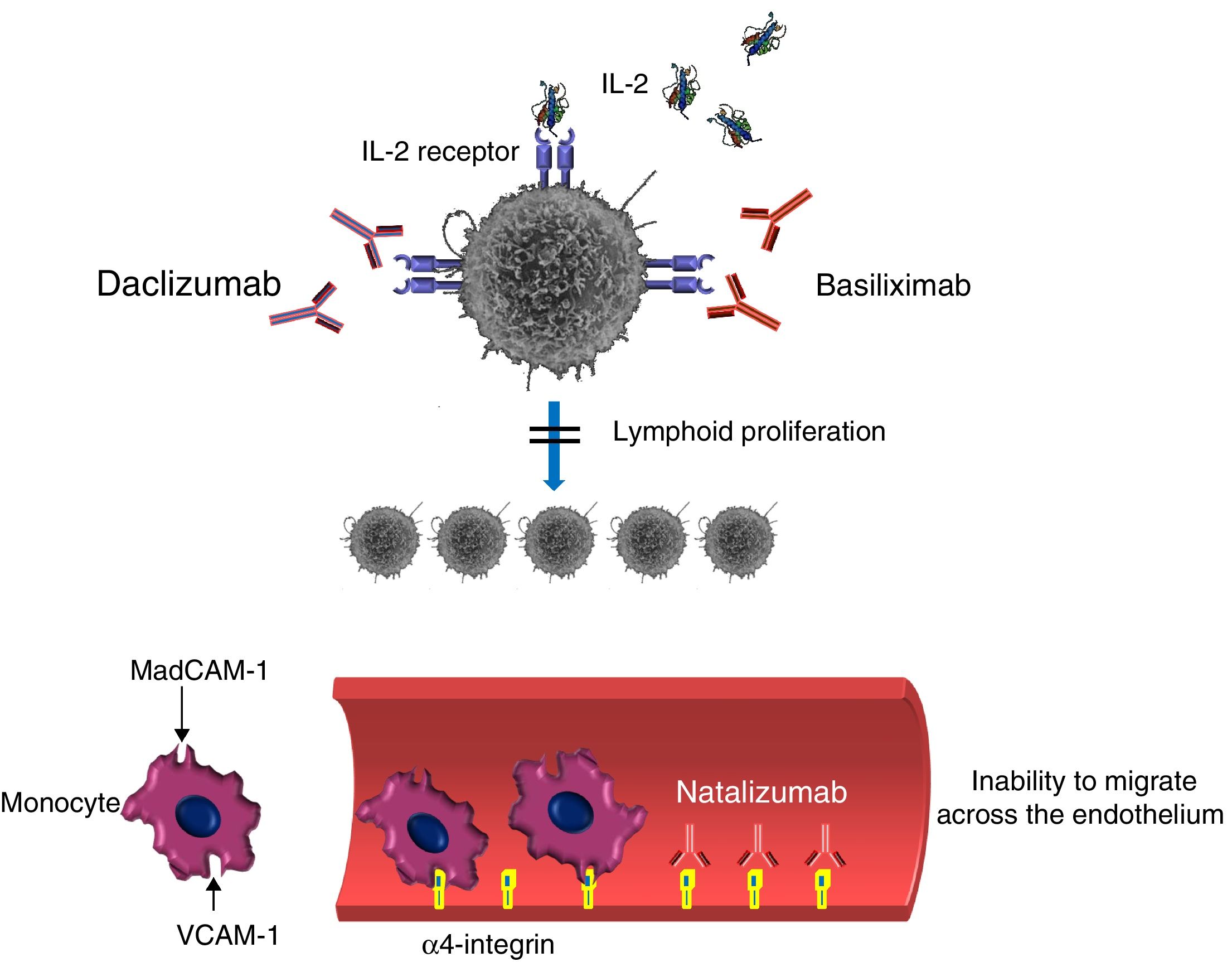
Monoclonal antibody inhibitors of lymphocyte activation, proliferation or migrationDaclizumab (DAC) is a humanized IgG mAb (90% human and 10% murine) that specifically binds to the alpha subunit or Tac (also known as CD25) of the IL-2 receptor expressed by the activated lymphocyte, preventing IL-2 from acting as a ligand of this receptor (Fig. 4).41,47 Bound DAC is highly specific when the lymphocyte is activated but not when it rests. The administration of DAC blocks, among other actions, the proliferation of activated lymphocytes and the secretion of cytokines by activated Th1 and Th2 lymphocytes (an important pathway involved in the immune cell response when there is allograft rejection). DAC also decreases CD25 expression in activated CD4+ lymphocytes. DAC is also used as prophylaxis for preventing acute rejection in patients who receive a kidney transplant for the first time. DAC blocks the expansion of autoreactive T lymphocytes and has been recently approved for using in recurrent forms of multiple sclerosis.37
Basiliximab (BAS) is a chimeric mAb that acts as an immunosuppressor by specifically binding to the IL-2 receptor alpha chain (CD25). As with DAC, BAS behaves as an IL-2 receptor antagonist by inhibiting the binding of IL-2 to activated lymphocytes (Fig. 4).25 As with other mAbs, determining the incidence rate of IFD after treatment with DAC or BAS is difficult. Bacterial, fungal and viral infections (or combinations thereof) have been reported. The most common fungal infections include candidiasis, aspergillosis, PJP, scedosporiosis and mucormycosis.47
The incidence of PJP, especially in kidney and lung transplant recipients, has declined since the prophylactic use of cotrimoxazole. The type of transplant and the clinical situation also determine the type of fungal infection. Candida infections are more common in the digestive system and intra-abdominal transplants, and after prolonged hospitalizations. Invasive aspergillosis, in contrast, is more common in lung transplantation, where other filamentous fungi are also involved.
Unlike the situation with RIT or ALE, DAC and BAS do not cause lymphocyte depletion because the latter neither promote ADCC or complement-mediated lysis, nor induce apoptosis. However, DAC and BAS impede lymphocyte proliferation in the immune response and thereby act as facilitators for the development of the infectious process. In pivotal studies, the rate of infection in the DAC group was 2% greater than that of the placebo group.37
Tocilizumab (TOC) is a humanized mAb that acts by binding to IL-6 receptors (both soluble and membrane-bound). IL-6 is produced by T and B lymphocytes, monocytes and fibroblasts and participates in several physiological processes such as T-lymphocyte activation, antibody secretion induction, the induction of the hepatic synthesis of acute-phase proteins and hematopoiesis stimulation. TOC is employed in patients with rheumatoid arthritis with a poor response to methotrexate, either combining methotrexate and TOC or administering the latter in monotherapy. In an adalimumab study (ADACTA), TOC was shown to be more effective than ADA in monotherapy and is therefore also used for the rescue treatment of patients who have shown a poor response to a first biologic treatment, which typically is a TNF antagonist.54 TOC has also been used in some types of large-vessel vasculitis, such as Takayasu's arteritis. TOC reduces neutrophil, platelet and hepatic enzyme levels, which may difficult the diagnosis in cases of infection. The risk of neutropenia could potentially be increased in patients who have been previously treated with TNF antagonists. No clear association between TOC-induced neutropenia and the onset of severe infections has been observed in the clinical trials performed to date, although there are reported cases of invasive candidiasis and pneumonia by P. jirovecii and Cryptococcus in patients who were treated with the highest doses of TOC.12 A study on 1200 patients with rheumatoid arthritis (600 with TOC treatment and the other 600 with nonbiological treatment) showed a 2-fold greater chance of developing a respiratory infection in the group treated with TOC.24,56 The bacterial etiology was predominant, and there were no differences in fungal infection in the study.
Anakinra (ANA) is a recombinant molecule that acts as a competitive IL-1 receptor antagonist. IL-1 is released by monocytes, macrophages and dendritic cells in response to a stimulus caused by TNF. ANA is employed for treating rheumatoid arthritis, both in monotherapy and in combination with methotrexate, because in this disease IL-1 and IL-6 are involved in joint inflammation processes. ANA has also been used in cases of severe or refractory Still's disease. ANA frequently causes neutropenia of varying degrees, which has not been linked to a greater risk of severe or opportunistic infections. To date, there is no published evidence of IFD associated with the use of ANA.
Ustekinumab (UST) is a fully human mAb that specifically binds to the p40 protein subunit present in IL-12 and IL-23. UST only acts on circulating cytokines, preventing their binding to receptors and thereby blocking their modulation of the immune response toward the Th1 and Th17 phenotypes. By not acting on the cytokine bound to its receptors, UST does not cause ADCC. UST is marketed for the treatment of psoriasis and psoriatic arthritis, conditions in which Th1 and Th17 response is involved. UST has recently been tested in refractory cases of inflammatory bowel disease. This lymphocyte signaling inhibition pathway does not appear to increase patient susceptibility to fungal infections.
Secukinumab (SEC) is a fully human IgG1-type mAb that acts selectively against IL-17. IL-17 participates in the pathogenesis of psoriasis, although it is also involved in protection against infections, primarily due to Candida. Cases of candidiasis have been reported in patients undergoing treatment with SEC, as with other anti-IL-17 agents such as brodalumab (BRO) and ixekizumab (IXE). Although the rate of mucocutaneous candidiasis (oropharyngeal and genital) is low, the rate is higher than that associated with TNF antagonists.10 The risk of candidiasis is dose-dependent, and these cases are usually mild to moderate, noninvasive, and do not require suspension of the biological treatment. These infections respond well to topical or oral antifungal treatment. The recommendation for patients who undergo treatment with anti-IL-17 agents is to conduct a follow-up of the risk factors for candidiasis and, if necessary, start an antifungal treatment.55
Natalizumab (NAT) is a humanized anti-α-integrin (IgG4k) mAb that binds to the α-4 subunit of the human integrin, membrane proteins that are present in the membrane of all leukocytes except for neutrophils.21,52 Vedolizumab (VED) is a second-generation anti-α-integrin mAb that, unlike NAT, acts by specifically binding to the α4β7 integrin present in lymphocytes that migrate to the intestine, causing an immunosuppressive effect in this organ. NAT binds particularly to the α-β integrin, blocking the interaction with its analog receptor, the vascular cell adhesion molecule 1 (VCAM-1), and to osteopontin ligands and connection segment 1 (CS-1), an alternatively splices to fibronectin domain (Fig. 4). NAT also blocks the interaction of the α4-β7 integrin with the mucosal addressin cell adhesion molecule 1 (MadCAM-1). The fundamental consequence of the two blocks is the inability of mononuclear leukocytes to migrate through the endothelium toward the inflamed parenchymal tissue. One of NAT's complementary mechanisms of action could be the suppression of ongoing inflammatory reactions in the affected tissues by inhibiting the interaction of leukocytes with α4 expression with their ligands in the extracellular matrix and in the parenchymal cells. Due to the drug's tropism to the central nervous system, NAT is used as therapy in highly active relapsing multiple sclerosis. The use of NAT has been related to severe viral infections, especially by BK and JC polyomaviruses, whose incidence rate has increased from 0.1 per 1000 patients to 2.3 per 1000.23 Serum JC virus antibodies were found in 50–60% of the patients after starting treatment with NAT. Serum titration affects the continuity or termination of treatment.29 The use of NAT has also been related to other viruses (varicella-zoster and herpes simplex) and mycobacteria. In fungal infections, there are only references to infection by Candida.23 However, the use of NAT is not recommended for patients with a history of IFD or immunosuppression factors predisposing to opportunistic infections.75
VED has been approved for use in patients with ulcerative colitis and Crohn's disease refractory to conventional treatment, including TNF antagonists.14,17 As mentioned, VED acts in the intestines by blocking the interaction between α4β7 integrin present in T and B lymphocytes and MadCAM-1. So far, no cases of progressive multifocal leukoencephalopathy have been reported. In terms of the risk of fungal infection, the controlled GEMINI I and II studies recorded isolated cases of vulvovaginal and oral candidiasis. However, considering the disruption of the intestinal barrier linked to the underlying disease of patients treated with VED, the risk of a candidiasis etiology should not be underestimated in cases of sepsis.
Monoclonal antibody inhibitors of lymphocyte blockageThe signaling pathway of programmed cell death protein-1 (PD-1) inhibits the immune effector responses against malignant cells, which block this pathway thanks to the expression of PD-1 ligand (PDL-1). Briefly, the expression of PDL-1 in tumor cells blocks the activation of LT and consequently allows immunological tolerance (reduction in lymphocyte proliferation, cytokine production and cytolytic activity against tumor cells). In vitro, tumors that overexpress the PDL-1 ligand are less susceptible to the cytolytic action of T lymphocytes. Some mAbs have been developed against the PD-1 immune checkpoint (pembrolizumab, nivolumab), which block the PDL-1 binding site, preventing the LT inactivation signal. These mAbs are indicated in the treatment of non-small cell lung cancer (NSCLC), advanced (unresectable or metastatic) melanoma in adults, refractory or relapsed classical Hodgkin lymphoma, advanced or metastatic urothelial cancer (in patients who have not received cisplatin) and head and neck squamous cell carcinoma (HNSCC).48,49 In addition, specific mAbs have been developed against PDL-1 (atezolizumab, avelumab, durvalumab). To date, there is no evidence that treatment with these mAbs leads to increased susceptibility to infections, including fungal infections. According to its mechanism of action, its use is not expected to increase the risk of infection. In fact, there is evidence in murine models that support the theoretical idea that these mAbs collaborate and increase the immune response against chronic fungal infections, such as persistent lung infection by C. neoformans.51
In addition to the biological therapies mentioned in this article, clinical oncohematology routinely uses numerous chemical synthesis molecules of a nonprotein nature (nonbiological) that, due to their activity in inhibiting intracellular signaling pathways, are closely related to infections, including fungal infections. Examples of this include the published cases of PJP after the use of tyrosine kinase inhibitors, such as ibrutinib,3 the indication for prophylaxis against P. jirovecii for patients treated with idelalisib1 and the cases of cryptococcal pneumonia and esophageal candidiasis in patients who underwent treatment with tofacitinib.32,72
Focus and scopeFrom the above, we can suggest a number of recommendations (in addition to those already listed in Table 3) for the screening of IFD in patients who undergo biological therapies.
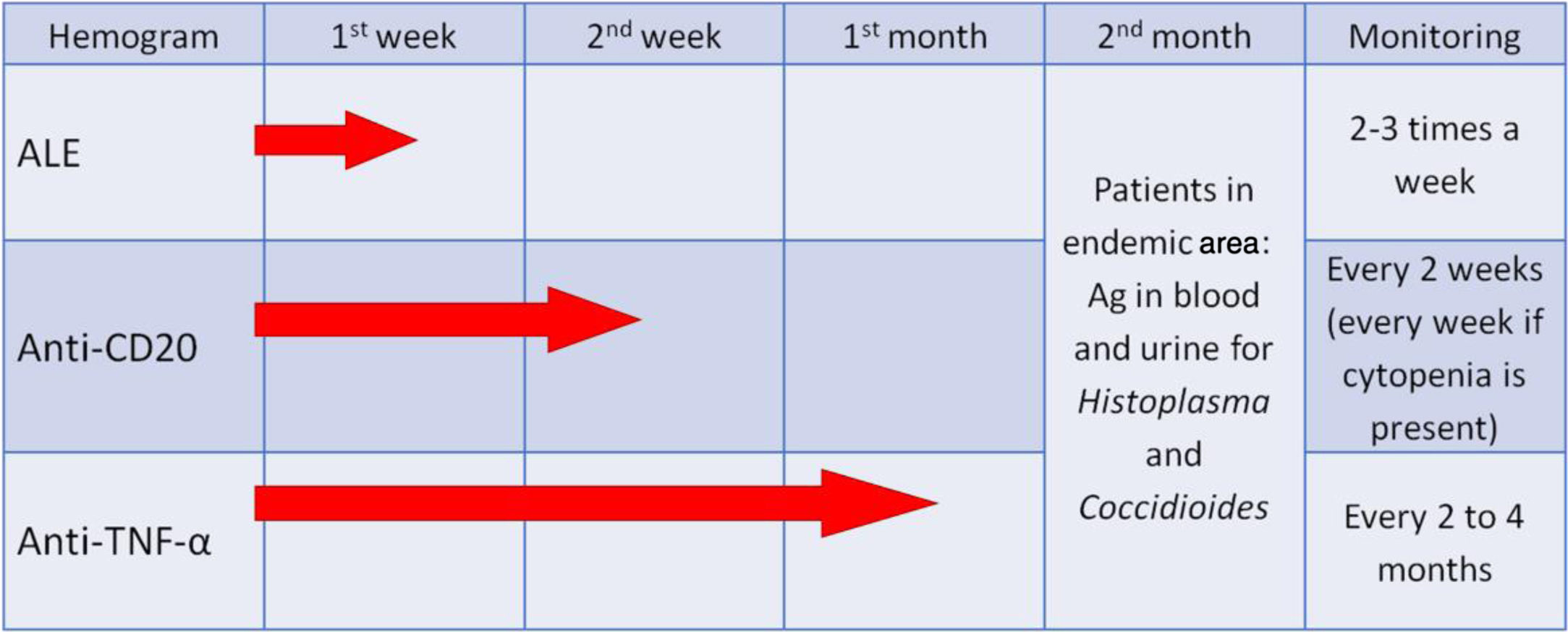
One of the fundamental requirements for patients treated with these innovative drugs is the implementation of an appropriate case history review, analyzing prior fungal exposure and the possible pathogenic determinants of the onset of infection (Table 4). It is important to emphasize that the risk of IFD does not depend exclusively on fungal exposure or the biological treatment but rather on the possible joint effect with other immunomodulators and chemotherapy agents administered simultaneously. It is also important to identify patients from geographic areas with high incidence rates of endemic mycoses to perform an active search for these pathogens through the detection of antigens of Histoplasma and Coccidioides in urine and/or blood. During the treatment, it is essential that leukocyte levels are monitored (Fig. 5) and prophylaxis established based on the laboratory results (e.g., cotrimoxazole for CD4 lymphocytic counts <200cells/mm3 or prolonged neutropenia) or when faced with certain clinical conditions (e.g., itraconazole or another active azole for patients with a history of recent histoplasmosis).
IFD risk factors related to patient and therapy. Keep in mind that the effects on the immune system are never pure or selective.
| IFD risk factors undergoing immunotherapy | |
|---|---|
| Patient related | MAb related |
| NeutropeniaLymphopeniaUnderlying disease statusDuration of the underlying disease | Treatment concomitant with corticosteroidsNeutropenia (ADA, INF, OFA, OBI, OCD)Neutrophil functional defect (ETA)Lymphopenia (ALE)Lymphocytic functional defect (DAC, BAS) |
ADA: adalimumab; ALE: alemtuzumab; BAS: basiliximab; DAC: daclizumab; ETA: etanercept; INF: infliximab; OBI: obinutuzumab; OFA: ofatumumab.
Biological therapies with monoclonal antibodies, anti-receptor anti-target agents and signal transduction pathway inhibitors play an important role in treating numerous underlying autoimmune inflammatory diseases, as well as in the therapeutic immunomodulation of hematological malignancies and of transplant-related phenomena. However, their use can be associated with a small increase in the risk of severe infections, many of which have an opportunistic nature, and of IFD in particular. However, unlike the search criteria for latent infections established for the paradigm of tuberculosis, the utility of any available or previously available screening technique for demonstrating prior fungal infections, before starting the biological therapies, is uncertain. Unlike tuberculosis and leishmaniasis, which are characterized by reactivations of latent or hidden infections, many fungal diseases in this particular patient population could be considered “de novo infections”, in contrast to the classically understood reactivation, although questions still arise about pneumocystosis and cryptococcosis in this regard. Thus, screening for this type of fungal infection, before starting biological therapies, has not shown effectiveness or seemed to be worthwhile. The offering of some type of prophylaxis, especially secondary, should be based on the patient's medical history, whether they have experienced some type of IFD and if they show the high risk that can still be present or could be induced by such therapies. There is scarce data on the use and effectiveness of prophylactic treatments with antifungals among patients who are candidates for or who have undergone biological therapies.
The educational activities for instructing patients treated with biological agents could be more important for preventing situations and activities with a high risk of exposure to fungi, especially in environments with a high density of spores or conidia. Therefore, the entirety of the environment in which we live and travel needs to be considered. Determining the origin or stay in endemic areas of certain mycoses, many of them by dimorphic fungi (e.g., Histoplasma), is another important preventive measure. Lastly, we should instruct patients, relatives and medical personnel on the most prevalent symptoms and signs of IFD and on the highest risk of exposure and development in patients undergoing biological therapies. We should also recommend early contact and consultation with physicians who are experts in this type of infectious diseases and special hosts to reach a diagnosis and treatment as fast as possible. These educational and informational programs should include, from the numerous and varied clinical manifestations, a weighing up and a list of the main risk factors for IFD, which are often misleading and complex. The programs should mention the need for an early recognition that can lead to an early start of the most appropriate antifungal regimen, thereby reducing the high morbidity and mortality of these IFDs.
Author contributionsAll authors have written, read and approved the final manuscript.
FundingAuthors have received funding from Astellas Pharma and Micellium Foundation for supporting the text style corrections.
Competing interestsThe authors declare that they have no competing interests.
To Juliette Siegfried (Senior Health Writer) for the style correction of manuscript.