Several neurological pathologies affect visual functions (transitorily or permanently). However, the magnitude and epidemiological behavior of these as potential causes of low vision and blindness are unknown. This study aims to characterize patients clinically and epidemiologically with visual impairment secondary to neuro-ophthalmologic alterations treated in neuro-ophthalmologic consultation in a reference center in Medellín.
MethodologyA retrospective cross-disciplinary study that assesses the patients with visual impairment secondary to neuro-ophthalmologic alterations treated in neuro-ophthalmologic consultation between 2015 and 2018.
ResultsOf 3313 clinical records reviewed, 140 patients met the eligibility criteria. Median age of diagnosis was 47 years of age (RI: 37.5–45.7) due to its heterogeneous distribution it was determined to group them into those under 18 years of age (35/140; 25%) and adults (105/140; 75%). 108/140 (77.1%) patients had non-ophthalmologic comorbidities, being the cardiovascular disease the most frequent. The main and more prevalent neuro-ophthalmologic diagnosis was optic atrophy (112/140; 80%). According to the visual deficiency category, the majority (61/140; 43.6%) presented a moderate visual deficiency. In adults, moderate visual deficiency was the most frequent, while in the group under 18 years of age, it was blindness. 41/140 (29.3%) were on a visual rehabilitation process and only 3/140 (2.1%) of them had disability certificates.
ConclusionIn this cohort, it is observed that neurological alterations are etiologies of permanent visual deficiencies leading to visual impairment, being optic atrophy, the main neuro-ophthalmologic cause identified, causing in most cases, moderate visual deficiency in adults and blindness in individuals under 18 years of age.
caracterizar clínica y epidemiológicamente pacientes con discapacidad visual secundaria a alteraciones neuro-oftalmológicas atendidos en la consulta de neuro-oftalmología en un centro de referencia de la ciudad de Medellín.
Metodologíaestudio transversal retrospectivo que evaluó los pacientes con discapacidad visual secundaria a alteraciones neuro-oftalmológicas atendidos en consulta de neuro-oftalmología entre 2015 y 2018.
Resultadosse evaluaron 3.313 historias clínicas, de las cuales 140 pacientes cumplieron los criterios de elegibilidad. La mediana de edad al diagnóstico fue 47 años (RI: 37,5 – 45,7), debido a su distribución heterogénea se decidió agrupar en menores de 18 años (35/140; 25%) y adultos (105/140; 75%). 108/140 (77,1%) pacientes tenían comorbilidades no oftalmológicas, siendo la enfermedad cardiovascular la más frecuente. El diagnóstico neuro-oftalmológico principal más prevalente fue la atrofia óptica presentado en 112/140 (80%) de los pacientes, de las cuales 74/112 (66%) era atrofia óptica no especificada. Según la categoría de la deficiencia visual, la mayoría (61/140; 43,6%) presentaban deficiencia visual moderada, seguido de ceguera (43/140; 30,7%) y deficiencia visual grave (36/140; 25,7%). En los adultos, la deficiencia visual moderada fue la más frecuente, mientras que, en los menores de 18 años, fue la ceguera. Respecto a las ayudas ópticas y no ópticas, la mayoría usaban gafas especiales (36/140; 25,7%), 41/140 (29,3%) se encontraban en rehabilitación visual, y sólo 3/140 (2,1%) tenían certificados de invalidez.
Discusiónen esta cohorte de pacientes se observa que las alteraciones neurológicas constituyen una importante etiología de discapacidad visual, siendo la atrofia óptica la principal causa neuro-oftalmológica generando en su mayoría deficiencia visual moderada en adultos y ceguera en menores de 18 años. Finalmente, se evidenció que menos de la mitad de los pacientes con este diagnóstico asisten a programas de rehabilitación visual.
The visual system, in addition to the eyeball, includes the visual pathway and several brain structures1 that support visual functions such as visual acuity, color vision, stereopsis, contrast sensitivity, and the visual field.2
In the framework of human functioning, disability results from the interaction between deficiencies, in which are included physical and social barriers of each individual, creating limitations in activity and restrictions in participation.3,4 Among the disability categories, visual impairment can be found, including low vision and blindness.3,4 Visual deficiencies are produced when a condition or disease affects a component of the visual system and one or more of the visual functions.2
A prevalence of 43 million blind people and 295 million with low vision was reported in 2020, and it was also reported in the period between 1990 and 2020, the number of blind and low vision people increased by 50.6% and 91.7%, respectively.5 According to global forecasts, due to the population growth of 38% and population aging, it is expected that these figures increase by a factor of 3 by 2050.5–7
Visual impairment can be created due to ophthalmologic or neurological causes, that include injuries in the visual pathway such as optic nerve, chiasm, optic tracts, geniculate body of the visual thalamus, optic radiations, among others.8
In 2020, the main causes of blindness reported were the following: cataracts, uncorrected refractive errors, glaucoma, age-related macular degeneration (AMD), and diabetic retinopathy; the main causes of low vision reported are: uncorrected refractive errors (157 million), cataracts (83.4 million), AMD (6.2 million), glaucoma (4.1 million), and diabetic retinopathy (3.28 million).5 In medium- and low-income countries, cataracts are still the main cause of blindness.9
Despite all above-mentioned etiologies, which are of optical and ophthalmologic nature, studies describing and characterizing the neuro-ophthalmologic causes of visual impairment are limited. Neurological pathologies affect visual functions, including optic neuritis, ischemic, traumatic, and compressive optic neuropathy.10 However, the magnitude and epidemiological behavior of these as potential causes of low vision and blindness are unknown.
The World Health Organization (WHO) reports approximately 1 billion people worldwide with neurological disorders, a figure that corresponds to 15% of the world's population.12 Most neurological disorders may involve ganglion neurons, photoreceptors, or visual pathway, and therefore, have been considered irreparable due to the general belief that nerve tissue does not regenerate or recover, oftentimes unaware that functional rehabilitation programs can be offered based on residual or potentially usable vision.13 Hence, these etiologies transcend ophthalmology and optometry and represent diagnostic, therapeutic, and rehabilitation challenges.
A search carried out in Antioquia (department located in Colombia), reports 110 250 people with disabilities, 32% presented some neurological alteration,11 but without reference to visual impairment of neurological origin. Additionally, a study executed by our research group, indicated that among the causes of visual impairment, 20.9% corresponded to neuro-ophthalmologic causes and 17.2% to neurological causes. This boosted the need to deepen regarding the neuro-ophthalmologic etiology that creates visual deficiencies and visual impairment.
This study aims to characterize patients clinically and epidemiologically with visual impairment secondary to neuro-ophthalmologic alterations treated in neuro-ophthalmologic consultation in a reference center in Medellín between 2015 and 2018.
MethodologyStudy design and populationA retrospective observational study, composed of visually impaired patients secondary to neuro-ophthalmologic alterations, treated in neuro-ophthalmologic consultation of a reference center in Medellín, Colombia between 2015 and 2018.
The inclusion criteria were patients with diagnosis of neuro-ophthalmologic alterations and presence of any permanent visual deficiency in visual acuity or visual field, according to WHO definition. Patients with incomplete clinical records of variables relevant to the study, such as visual acuity and main neuro-ophthalmologic diagnosis, were excluded.
The data contained in the WHO in the International Classification of Diseases-ICD10R and ICD-11 were considered, where low vision and blindness are defined in terms of the classification of visual acuity (VA) ranges: low vision is equivalent to a best-corrected VA worse than 20/60 but equal or better than 20/400 in the better eye, or visual field loss corresponding to less than 20° in the better eye, with the best possible correction; and blindness is a VA worse than 20/400 or visual field loss corresponding to less than 10°.2
Collection processOnce approval was obtained from the participating institution for accessing the clinical records, data collection process began, starting with the review of those clinical records that met the eligibility criteria. A pilot test was conducted with 20 clinical records that allowed modification of the instrument.
The variables assessed were sociodemographic (age at main diagnosis and sex), clinical (main neuro-ophthalmologic diagnosis, category of visual deficit), records (non-ophthalmologic comorbidities, family history of ophthalmologic diseases), and use of optical and or non-optical aids (special glasses, telescopes, magnifying glasses, contact lenses, orientation cane, reading ruler, Braille, software, guide dog, cellphone application, visual rehabilitation).
The information collected was exported to Microsoft Excel (Office 2016 version), where an exploratory analysis was performed to evaluate the quality of the information (missing data, extreme values, among others) and the consistency of the data.
Regarding possible biases and their control, the selection bias of the population was controlled by including the entire population of patients who met the eligibility criteria of the study, based on the clinical history of patients seen by medical subspecialty. Aiming to reduce information bias, a single collection tool was designed, considering the eligibility criteria and the variables required for this research; data that did not match was verified with a new review of the clinical records by one of the researchers.
Statistical analysisQuantitative variables were described by the median and interquartile range (IQR) due to the non-compliance with the assumption of normality using the Kolmogorov–Smirnov normality test. Categorical variables were expressed as absolute frequencies and percentages. Since some of the characteristics assessed may vary depending on the age group, the information is described in the total of the group evaluated and according to age categorized as: under 18 years of age and over 18 years of age (greater than or equal to 18). All analyses were performed in IBM SPSS 24.0.
Ethical considerationsThe research was conducted following the guidelines of the 2013 version of the Declaration of Helsinki and Resolution 008430 of 1993 of the Ministry of Health of the Republic of Colombia for which it was considered risk-free research; it was approved by Health Research Ethics Committee of Pontificia Bolivariana university by record no. 2 of February 2017 and the endorsement of the participating reference center was obtained.
ResultsA total of 3313 clinical records were assessed, of which 140 (4.22%) patients met the eligibility criteria (Fig. 1). Seventy-two (51.4%) were female, the median age at primary diagnosis was 47 years (RIC: 37.5–45.7); 35/140 (25%) were younger than 18 years of age and 105/140 (75%) were adults.
Concerning the presence of non-ophthalmologic comorbidities, 108/140 (77.1%) patients had some comorbidity, the most frequent being a circulatory system disease. In adults, circulatory system diseases were most common in 39/84 (46.4%) patients, followed by endocrinological diseases, 29/84 (34.5%), while in minors, the most frequent was neurological disease in 10/24 (41.6%) patients (Table 1).
Non-ophthalmologic comorbidities according to age groups in the study population.
| Non-ophthalmologic comorbidities | Total | <18 years | Adults |
|---|---|---|---|
| (n = 108) | (n = 24) | (n = 84) | |
| n (%) | n (%) | n (%) | |
| Cardiovascular system disease | 41 (37.9) | 2 (8.3) | 39 (46.4) |
| Endocrine disease | 34 (31.5) | 5 (20.8) | 29 (34.5) |
| Neurological disease | 27 (25) | 10 (41.6) | 17 (20.2) |
| Sensory organ disease | 15 (13.9) | 2 (8.3) | 13 (15.5) |
| Respiratory system disease | 13 (12.03) | 5 (20.8) | 8 (9.5) |
| Mental disease | 11 (10.2) | 1 (4.2) | 10 (11.9) |
| Locomotive system disease | 11 (10.2) | 3 (12.5) | 8 (9.5) |
| Digestive system disease | 8 (7.4) | 2 (8.3) | 6 (7.4) |
| Neoplasm | 6 (5.5) | 2 (8.3) | 4 (4.8) |
| Autoimmune disease | 5 (4.6) | 2 (8.3) | 3 (3.6) |
| Genitourinary system disease | 5 (4.6) | 1 (4.2) | 4 (4.8) |
| Injuries, wounds, and other external factors | 5 (4.6) | 0 | 5 (5.9) |
| Genetic disease | 3 (2.8) | 3 (12.5) | 0 |
Besides, antecedents of family history of ophthalmologic diseases were reported in 16/140 (11.4%) patients, being 7/16 (43.7%) in siblings, followed by 6/16 (37.5%) in parents and 1/16 (6.3%) in aunt, nephew, and son. Optic atrophy was the most frequent family history in 4/16 (25%) relatives, followed by myopia in 3/16 (18.8%), glaucoma in 2/16 (12.5%), and in 1/16 (6.3%) retinal detachment, low vision, blindness, cataract, maculopathy, keratoconus, and retinoblastoma.
The most prevalent primary neuro-ophthalmologic diagnosis was optic atrophy presented in 112/140 (80%) of patients (Table 2), of which 74/112 (65.2%) had unspecified optic atrophy, followed by glaucoma in 8% (9/112), secondary to compressive optic neuropathy in 4.5% (5/112), in 3.6% (4/112) ischemic optic neuropathy, hereditary and optic neuritis, neuromyelitis, and post-papilledema in 2.7% (3/112), 2/112 (1.8%) by meningioma and 1/112 (0.9%) by congenital alteration, ependymoma, neuro-lues, and idiopathic. Less frequently, ischemic (non-arteritic) optic neuropathy was evidenced in 5/140 (3.6%) patients, followed by optic neuritis and visual pathway alteration in 4/140 (2.9%) patients each (Table 2).
Main neuro-ophthalmologic diagnosis.
| Main neuro-ophthalmologic diagnosis | Total | <18 years | Adults |
|---|---|---|---|
| (n = 140) | (n = 35) | (n = 105) | |
| n (%) | n (%) | n (%) | |
| Optic atrophy | 112 (80) | 27 (77.1) | 85 (80.9) |
| Ischemic optic neuropathy (non-arteritic) | 5 (3.6) | 0 | 5 (4.8) |
| Optic neuritis | 4 (2.9) | 1 (2.9) | 3 (2.9) |
| Visual pathway alteration | 4 (2.9) | 1 (2.9) | 3 (2.9) |
| Cortical blindness | 3 (2.1) | 1 (2.9) | 2 (1.9) |
| Optic nerve hypoplasia | 3 (2.1) | 3 (8.6) | 0 |
| Optic nerve drusen | 3 (2.1) | 1 (2.9) | 2 (1.9) |
| Macular dystrophy | 2 (1.4) | 0 | 2 (1.9) |
| Othera | 4 (2.9) | 1 (2.9) | 3 (2.9) |
The most frequent visual impairment category was moderate, 61/140 (43.6%). In adults, moderate visual impairment was the most frequent in 47/105 (44.8%), while, in those under 18 years of age, it was blindness in 15/35 (42.9%) (Table 3).
Table 4 describes the category of visual impairment according to the main diagnosis; the most common diagnosis was optic atrophy in the 3 groups. Of the patients with ischemic optic neuropathy (non-arteritic), optic neuritis, and macular dystrophy, none presented blindness (Table 4).
Visual impairment category according to the main diagnosis
| Main neuro-ophthalmologic diagnosis | Moderate visual deficiency | Severe visual deficiency | Blindness |
|---|---|---|---|
| (n = 61) | (n = 36) | (n = 43) | |
| n (%) | n (%) | n (%) | |
| Optic atrophy | 48 (78.7) | 30 (83.3) | 34 (79) |
| Ischemic optic neuropathy (non-arteritic) | 3 (4.9) | 2 (5.5) | 0 |
| Optic neuritis | 2 (3.3) | 2 (5.5) | 0 |
| Visual pathway alteration | 1 (1.6) | 1 (2.7) | 2 (4.6) |
| Cortical blindness | 0 | 0 | 3 (7) |
| Optic nerve hypoplasia | 1 (1.6) | 0 | 2 (4.6) |
| Optic nerve drusen | 2 (3.3) | 0 | 1 (2.3) |
| Macular dystrophy | 2 (3.3) | 0 | 0 |
| Othera | 2 (3.3) | 1 (2.7) | 1 (2.3) |
Moderate visual deficiency: one patient with traumatic optic neuropathy and another with visual loss due to middle cerebral artery aneurysm. Severe visual deficiency: a patient with bilateral optic nerve edema due to Vogt Koyanagi Harada disease. Blindness: a patient with ischemic optic neuropathy (arteritic).
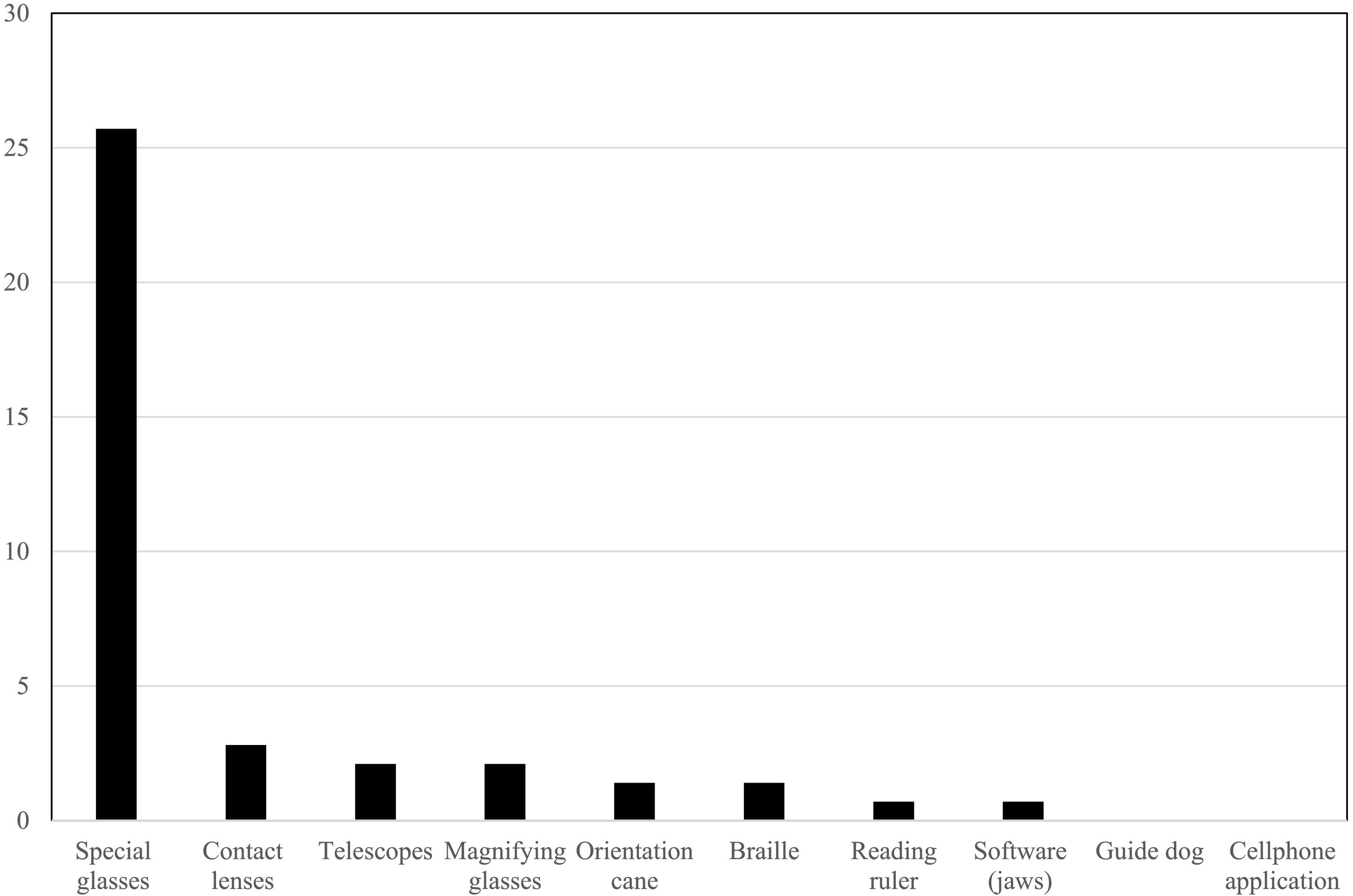
Regarding the optical and non-optical aids reported in the clinical record, about a quarter, 36/140 (25.7%) of the patients, used special glasses, and less frequently the other optical aids. None of them had a guide dog or cellphone application (Fig. 2). 39/140 (27.9%) of the patients had a report of a single functional eye, 41/140 (29.3%) were undergoing vision rehabilitation, and only 3/140 (2.1%) had disability certificates.
DiscussionCurrently, neuro-ophthalmologic pathologies remain less known than other ophthalmologic pathologies, where the literature documenting this group of health conditions as causes of permanent visual deficiencies and visual impairment is limited. In the cohort study that we conducted, optic nerve condition was the most frequent cause of visual deficiency, with optic atrophy being the most prevalent in this group. As far as known, this is the first study to provide local, national, and international information on the matter.
In the study population, most patients were in the fifth decade of life, the same as reported in other studies on the prevalence of visual impairment.15,16 In them, the condition of low vision is largely age-dependent. For example, one research studies performed in United State of America, concluded that 73% of the patients were over 65 years of age.17
The age distribution found in this study could be due to the impact of non-ophthalmologic comorbidities on the optic nerve. Risk factors have been identified which make it prone to damage by ischemia, such as hypertension, diabetes mellitus, and obstructive sleep apnea; thus, optic atrophy could be the result of an ischemic process.18
In contrast, more neurological comorbidities were identified in children than adult's patients. Although less prevalent of cardiovascular or endocrine comorbidities are also associated with the development of optic atrophy.19,20 Among them, we recorded in the present study the following: hydrocephalus, hypoxic–ischemic encephalopathy, general learning disability, 7 and 8 chromosomopathy, astrocytoma, and ependymoma.
A retrospective study of 241 clinical records of pediatric patients in Sydney, Australia, reported optic atrophy secondary to visual pathway tumors in 25% of patients. These entities contribute to compressive, infiltrative injuries, and altered optic nerve development. It is important to emphasize these comorbidities in low vision rehabilitation programs to optimize self-care, avoid the risk of declines, and progress toward functional independence.21–23
Within the population studied, the diagnosis of visual impairment occurred more frequently in women than men (51.4% women vs. 48.6% men), which is consistent with a study conducted in China that assessed the prevalence of visual impairment among 6725 adults. A higher prevalence of low vision and blindness was found in women than in men.16 Additionally, in a cross-sectional study, including 213 626 people, it was reported blindness in 360 people, 160 were men and 200 were women; and of the 5560 people with visual impairment, 2025 were men and 3535 were women.24
The prevalence of visual impairment in this study was 4.22%, which is consistent with studies in the literature, for example, a cross-sectional study found a 5.7% of prevalence.25 The Canadian study defined visual impairment as binocular acuity worse than 20/40, which may explain why its prevalence is slightly higher than found in our study. Likewise, a systematic review of the literature, found a prevalence of non-refractive visual impairment of 4.0%.26
In adults, the moderate visual deficiency was the most frequent, which is consistent with the global literature and official WHO figures.5,15,16 Optic atrophy was the main neuro-ophthalmologic diagnosis, which is similar to Dineen et al study.27 In others researches, optic atrophy was described as an important cause of visual impairment, which allows inferring that optic atrophy would be one of the main neuro-ophthalmologic determinants of visual deficiency.28–32
In children under 18 years of age, blindness was the most prevalent in this population, with optic atrophy being the main cause; in contrast to our findings, there are 2 studies that even though also found that blindness was the main visual impairment in children, the prevalence of the causes of blindness in these studies, was first cortical blindness and then optic nerve atrophy (Blindness 4%, optic atrophy 1.5%; blindness 31.5%, optic atrophy 16.5%).33,34
There are limited studies about optical and non-optical tools in patients with neuro-ophthalmologic visual deficiency. There are many aids that have advanced with technology35,36; in our study, special glasses continue to be the main optical tool; on the other hand, few patients used software and applications, it could be explained by difficulties in accessing devices in the health system and acquisitive power.
Finally, one-third of patients were attended in a vision rehabilitation program, which differs from other studies,37,38 where the majority of their population did attend such program, taking into account that their data was extracted from the general population with visual deficiency, not discriminating by neuro-ophthalmologic causes, possibly due to the difficulty in our country to access this resource by the patient.
One limitation is the possibility of errors in the storage and recording of data contained in the clinical records due to the fact our methodology is based on a retrospective study. However, many strategies were employed to ensure data quality, such as double-checking clinical records in case of missing or different data. Although only 140 patients were captured, the recruitment period was extensive and the number assessed as the total number of patients seen in the neuro-ophthalmology consultation of one of the professionals in the city, considering that Medellín city only has 2 professionals with expertise in this area.
In conclusion, it was observed that neurological alterations are the cause of permanent visual deficiencies leading to visual impairment, with unspecified optic atrophy being the main neuro-ophthalmologic cause in all categories of visual deficiency. In adults, a moderate visual deficiency was the most common, while in children under 18 years of age it was blindness. Finally, it was found that less than half of the patients with neuro-ophthalmologic diagnosis and visual impairment have a report in their clinical history of attending vision rehabilitation programs, an aspect to be improved in health professionals through training and awareness-raising processes regarding the approach and management of clinical history for the visually impaired population.
Conflicts of interestThe authors report no conflicts of interest.
This research has not received specific support from public sector agencies, the commercial sector, or non-profit entities.