Dear Editor:
Diabetic papillopathy (DP) is a rare complication of diabetes mellitus (DM). It was first described in 1952 by Topilow and Bisland.1,2 DP is characterised by unilateral or bilateral papilloedema in patients with DM1 or DM2 regardless of metabolic control and the severity of diabetic retinopathy.3 While its pathophysiology remains unknown, it may be associated with anterior ischaemic optic neuropathy (AION), although this matter is subject to controversy.3–5 No treatment is usually required, but corticosteroids,6 vascular endothelial growth factor inhibitors (anti-VEFG)7 and even panretinal photocoagulation (PRP) have been used successfully.3 We describe two cases attended at our hospital.
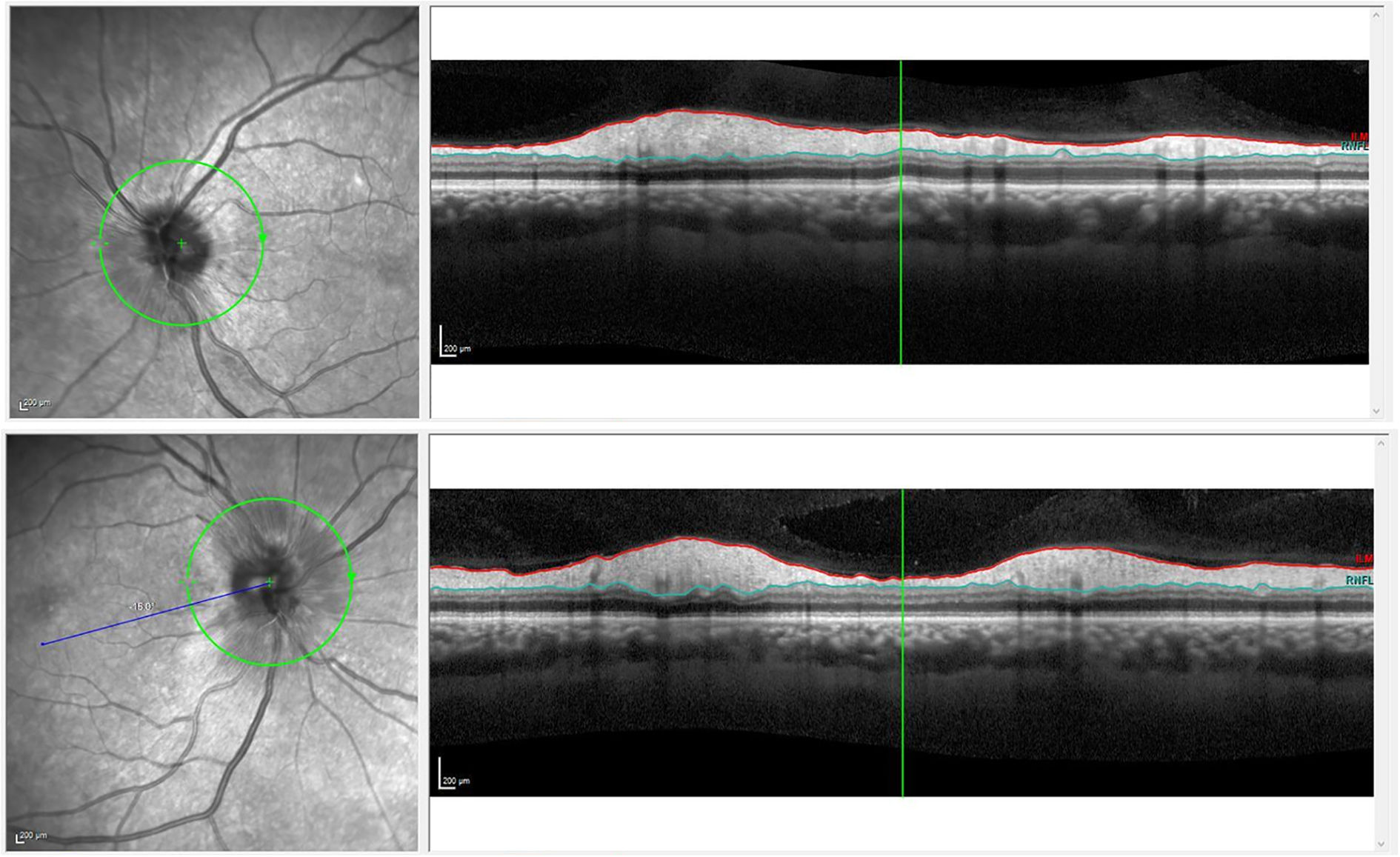
Our first patient was a 59-year-old woman admitted due to gastrointestinal bleeding, who was diagnosed with DM2 during her stay; glycaemic control was achieved rapidly. She reported blurred vision bilaterally. Physical examination revealed normal visual acuity in both eyes, and no relative afferent pupillary defect; eye fundus examination revealed papilloedema, which was confirmed by optical coherence tomography of the retinal nerve fibre layer (OCT-RNFL) (Fig. 1). Brain computed tomography findings were normal. A blood analysis revealed hyperglycaemia and anaemia associated with the gastrointestinal bleeding.
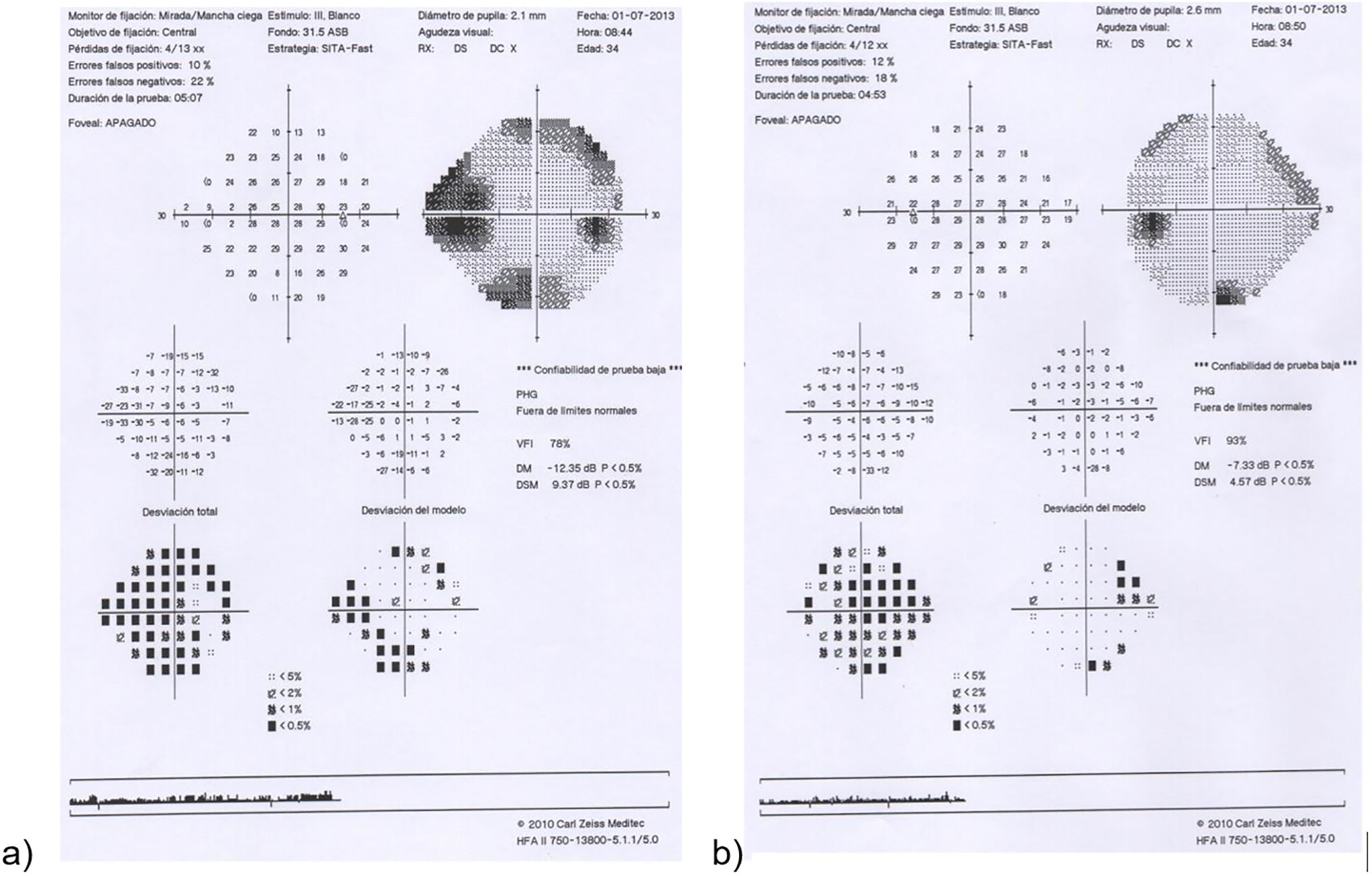
Our second patient was an asymptomatic 30-year-old man diagnosed with DM1 presenting poor metabolic control. He was undergoing sessions of PRP due to proliferative diabetic retinopathy. Visual acuity was 0.4 in the right eye and 0.6 in the left eye. In a follow-up visit, papilloedema was incidentally detected in the left eye. A fluorescein angiography study revealed early papillary hyperfluorescence in the left eye; a visual field test showed enlarged blind spot in the same eye (Fig. 2), and OCT-RNFL detected papilloedema.
The current diagnostic criteria for DP are: confirmed DM1 or DM2, optic disc oedema, no significant optic nerve dysfunction, normal intracranial pressure, and no inflammation, infection, or infiltration.5 In our case, both patients met these criteria.
From an epidemiological perspective, age of onset is variable although DP more frequently affects young patients with DM1.8
Risk factors include small-sized papilla together with a small cup-to-disc ratio (also known as disc at risk), and rapid metabolic control,4,9,10 as in our first case.
Two pathophysiological mechanisms have been described. The first consists of a transient capillary dysfunction that leads to secondary ischaemia, and the second mechanism establishes altered axoplasmic transport in the optic nerve as the cause of oedema.4
Authors supporting the association between DP and AION mention the characteristics common to both entities: diabetes, presence of disc at risk, the possibility of irreversible visual loss, and the presence of both conditions in the same individual. Authors who believe that these entities have different pathogenic mechanisms have observed that oedema persists for longer, age of onset is younger, and bilateral papillary involvement is more frequent in DP.3
Clinical presentation is variable, ranging from no symptoms to significantly decreased visual acuity, mainly in association with macular oedema.8
Eye fundus examination reveals oedematous and hyperaemic papilla with capillary dilatation. Haemorrhage, exudates, and cystoid macular oedema are also frequent; visual field alterations are also common, with enlarged blind spot being the most frequent. Fluorescein angiography shows early, diffuse papillary hyperfluorescence, revealing the radial pattern of the dilated capillaries.3,4,8
Diagnosis is established by exclusion; patients should present normal arterial pressure, no signs of intracranial hypertension, normal blood analysis, and no CT results that may better explain the presence of such alteration.4,7
Corticosteroids and anti-VEGF drugs stabilise the blood–retinal barrier, which may explain the successful outcomes in patients receiving this treatment.3,6,7 However, this is an off-label indication.
In conclusion, although diabetic papillopathy is an infrequent optic neuropathy, it is important to include it in the differential diagnosis of papilloedema, as it is a frequently self-limited, underdiagnosed process that generally requires no treatment due to its benign nature. When necessary, pharmacological treatment should be indicated on an individual basis, with coordination between neurologists and ophthalmologists, as no clear recommendations have been established.
Ethical considerationsThe informed consent of the patient has been obtained for this publication, and we have followed the procedures of our centre for the treatment of the patient's data.
Conflict de interesThe authors have no conflict of interest to declare.