Dear Editor:
In 1888, the German psychiatrist and internist Moritz Jastrowitz described the case of a patient named Panjas, who presented euphoria and inappropriate laughter and crying. The post-mortem examination revealed an extensive tumour located in the frontal lobe. The physician continued his research after the case, observing that the same signs and symptoms appeared in other patients with frontal lobe involvement due to various conditions. Thanks to his research, the syndrome bears his eponym, Moria syndrome, and refers to inappropriate behaviour associated with frontal lobe damage.1
The syndrome is characterised by disinhibition, irritability, lability, echopraxia, echolalia, and aggressiveness.2
Analytical studies showed involvement of orbitofrontal circuits and their connection with the caudate nucleus, the dorsomedial part of the globus pallidus, and the thalamic ventral anterior and dorsomedial nuclei.3 However, extensive projections are observed between these structures and the anterior cingulate gyrus, amygdala, insula, hypothalamus, and temporal lobe, which would reveal involvement beyond the frontal lobe in this syndrome.4–6
The contribution of Moritz Jastrowitz was decisive in 19th-century neurosurgery.7
Current diagnostic techniques using stereo-electroencephalography in epilepsy, as well as therapeutic cerebral stimulation in Parkinson's disease, offer first-hand insight into the correlates of these structures.8
We present the case of a 47-year-old man without relevant history, who was referred to our demyelinating diseases unit by the psychiatry department, where he was under follow-up due to affective symptoms. We detected cognitive alterations including forgetfulness, poor coordination performing everyday activities, and loss of verbal fluency. Uncontrollable, unexplained laughter was surprising, as it was not congruent with his mood. We suspected organicity. The patient reported apathy and lack of enthusiasm in the first visit. He also described episodes of sadness and loss of memory of recent events.
The neurological examination revealed paratonia in the upper limbs, with no reflex or oculomotor alterations. He showed no focal motor or sensory symptoms or dysmetria.
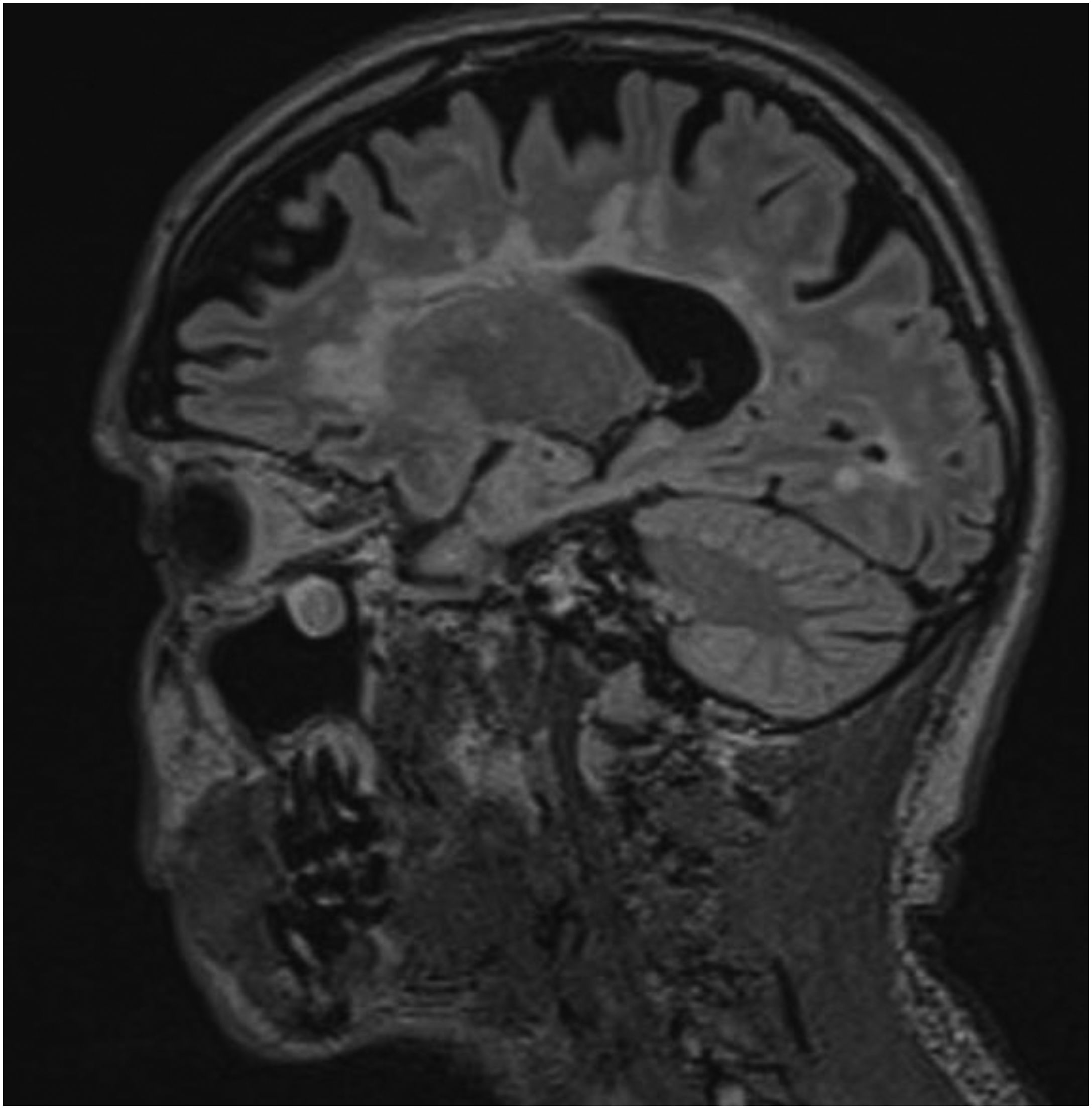
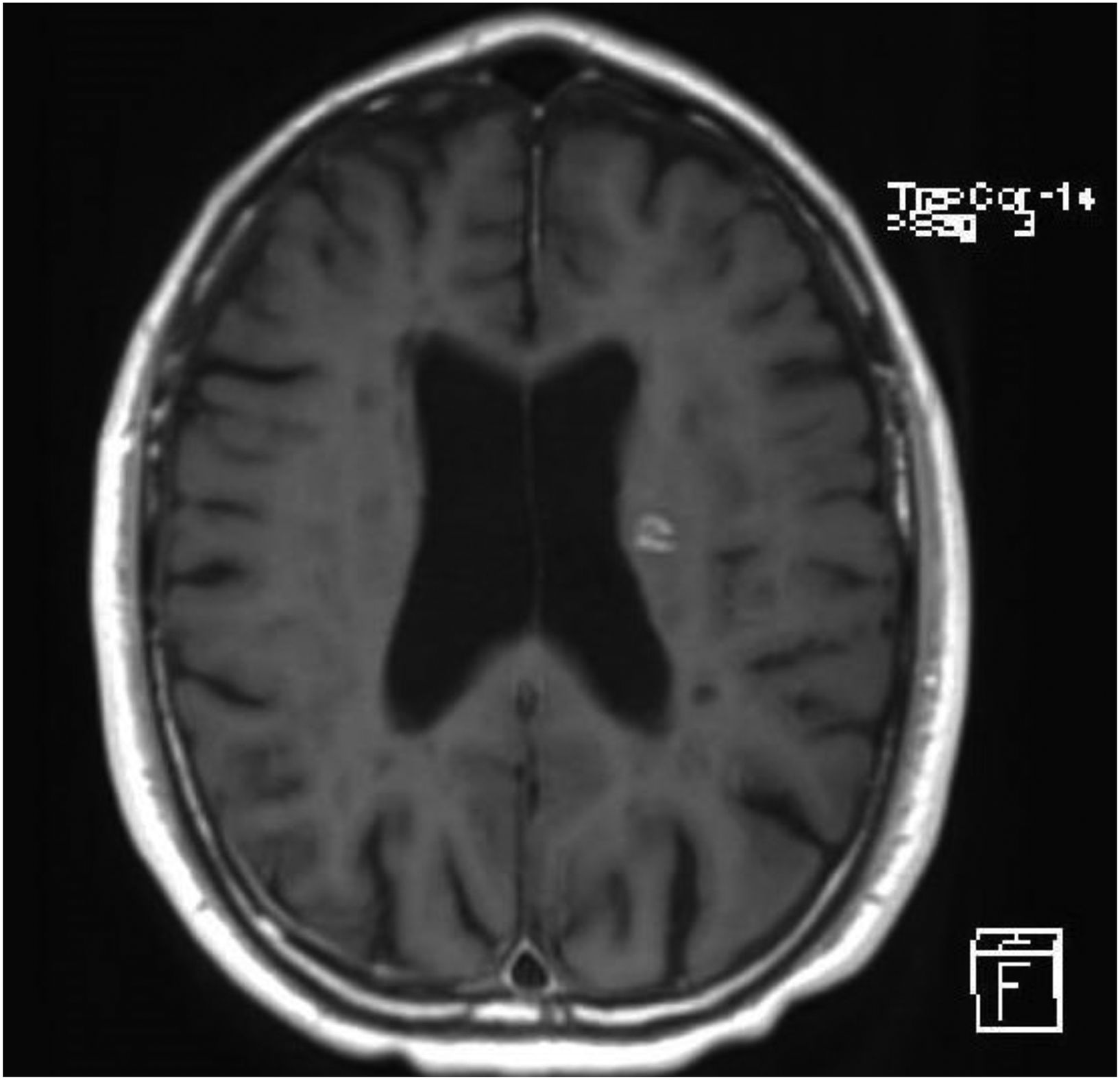
A brain MRI study showed multiple hyperintense lesions in T2-weighted and FLAIR sequences, located in both infra- and supratentorial regions, affecting the brainstem, cerebellum, and all white matter areas (periventricular, juxtaventricular, and subcortical) (Fig. 1). After contrast administration, we observed uptake in 2 lesions located in the left hemisphere that caused inflammatory activity at the time of examination (Fig. 2). In summary, the findings were compatible with advanced multiple sclerosis (MS), meeting the McDonald criteria for dissemination in space and time. A cerebrospinal fluid analysis showed presence of oligoclonal bands.
A neuropsychological evaluation revealed good dexterity, alertness, and willingness to collaborate. During the examination, the patient presented uncontrollable laughter. He was oriented to time, space, and person, but showed alterations in episodic and delayed memory in recall processes, as well as anomia. The patient presented no alterations in spontaneous language, or repetition or comprehension problems. However, he showed alterations in working memory, inhibition, and cognitive flexibility, as well as bradyphrenia. In conclusion, this patient presents symptoms of cognitive alterations mainly affecting information processing speed and executive functions, together with behavioural alterations compatible with mixed prefrontal syndrome (mainly dorsolateral and orbital), observed with the Free and Cued Selective Reminding Test and the Frontal Assessment Battery.
We indicated corticosteroid treatment with intravenous methylprednisolone (1 g/day for 5 days), followed by disease-modifying therapy with alemtuzumab. He also received quetiapine at 100 mg and escitalopram at 20 mg.
At 3 and 6 months of follow-up, the patient reported feeling better overall, less impatient, less irritable, and more affective. He showed improvements in memory processes, but his motor coordination had worsened and he presented fluency and speech organisation problems. Cognitive alterations affecting episodic memory persisted in recall processes (frontal amnestic syndrome) and prefrontal functions (mixed prefrontal syndrome).
In similar cases, we should perform a differential diagnosis considering psychiatric disorders, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy, inflammatory diseases such as lupus erythematosus, Sjögren syndrome, Behçet disease, polyarteritis nodosa, or paraneoplastic encephalomyelitis; infectious diseases such as neurosyphilis, granulomatous diseases such as neurosarcoidosis, or such demyelinating diseases as MS. We finally diagnosed MS in the light of the imaging findings and progression. Specifically, we diagnosed severe relapsing–remitting MS.9 The patient started treatment with a disease-modifying drug, in this case, alemtuzumab.
This case is unusual due to the initial manifestation with cognitive symptoms, which required close monitoring by the psychiatry and psychology team, with unmotivated laughter leading the psychiatrist to search for an organic cause.
The prevalence of cognitive impairment among patients with MS ranges from 43% to 65%,10 although it has historically been underdiagnosed mainly because it can progress independently of physical impairment.
Currently, the impact of cognitive impairment on patients' quality of life has led to an increased emphasis on its assessment and treatment. However, the available data remain limited and difficult to interpret.
Various studies have shown that the most severely impaired cognitive functions are attention, executive functions, information processing speed, and memory. However, such verbal skills as understanding and naming, as well as simple attention, seem to be less affected.
As our understanding of cognitive impairment in demyelinating disease has improved, impairment of other cognitive functions has been identified, such as learning of new information, sustained attention, visual perception, semantic retrieval, and abstract reasoning.
Cognitive impairment is directly related to the patient's functional status, as it limits professional and social activity and has a significant impact on quality of life.
Among the factors affecting cognitive function, we may highlight those inherent to the disease, such as progression time and the type of MS. Depression is not simply a symptom, as it is derived from a complex series of interacting events whose causal relationships are difficult to determine. Feinstein et al.11 note that patients with MS and depression presented higher numbers of hyperintense lesions in the left inferior medial frontal region and reduced brain volume in the left anterior temporal region. Likewise, fatigue, which is observed in up to 90% of patients, is associated with cognitive impairment.
In the light of all these circumstances, it is essential to address cognitive symptoms from the time of diagnosis of MS; this requires a multidisciplinary approach involving mental health, neuropsychology, and neurology services in the management of these patients.
FundingNo funding was received for this study.
Informed consentYes.
Ethical considerationsThis study was approved by the ethics committee of Hospital Reina Sofía. Córdoba.