Neuropsychological symptoms and cortical atrophy patterns show similarities between typical Alzheimer’s Disease (AD) and its variants. Thus, correct diagnosis is difficult, leading to errors in the therapeutic process. Indeed, the challenge in cognitive neuroscience focuses on identifying key features of cognitive-linguistic profiles and improving the knowledge of neural correlates for accurate differential diagnosis between the heterogeneous profiles of typical and atypical AD.
AimThis systematic review aims to describe different AD profiles, considering their neuropsychological symptoms and neural correlates.
MethodsThe present study followed the PRISMA guidelines and included studies from the PubMed, ScienceDirect, Scopus, and Web of Science databases, published between 2011 and 2021.
ResultsThirty-one articles were included in this systematic review for critical analysis. Results suggest significant declines in episodic and working memory and executive function. Likewise, in all groups, verbal fluency and visuospatial/visuoconstructive skills declined. However, these symptoms overlap between typical AD, logopenic variant primary progressive aphasia, posterior cortical atrophy, behavioural/dysexecutive or frontal variant AD, and corticobasal syndrome. On the other hand, the neural correlate showed a pattern of atrophy in frontal, temporal, parietal, and occipital areas, even compromising the cuneus and precuneus.
ConclusionSpontaneous language and semantic and phonological verbal fluency could be an important biomarker for differential diagnosis between typical AD and its atypical variants. Likewise, clinical assessment should consider using advanced neuroimaging techniques to establish early associations between brain dysfunction and neuropsychological performance, with particular attention to brain areas such as the cuneus and precuneus.
En la Enfermedad de Alzheimer (EA) la sintomatología neuropsicológica y el patrón de atrofia muestran similitudes entre sus variantes atípicas, conduciendo a errores diagnósticos e intervenciones no atingentes a los déficits en sus perfiles típico y atípicos. Así, el desafío en neurociencia cognitiva se centra en poder identificar los elementos neuropsicológicos y las bases neurales para un diagnóstico diferencial certero entre esta heterogeneidad de perfiles.
ObjetivoCaracterizar los distintos perfiles de la EA considerando sus manifestaciones neuropsicológicas y correlato neural.
MetodologíaLa revisión sistemática cualitativa se realizó bajo los criterios de la declaración PRISMA, considerando estudios de las bases de datos PubMed, ScienceDirect, Scopus y Web of Science, entre los años 2011 y 2021.
ResultadosLa búsqueda dio como resultado 31 artículos finales. Los hallazgos sugieren declives significativos en memoria episódica y de trabajo, función ejecutiva, lenguaje y habilidades visuoespaciales/visuoconstructivas, síntomas que se superponen en EA típica, Afasia Progresía Primaria variante logopénica, Atrofia Cortical Posterior, EA variante frontal y Síndrome Corticobasal. Por otra parte, el correlato neural en estos perfiles sugiere un patrón de atrofia frontal, témporoparietal y occipital, comprometiendo adicionalmente el cuneus y precuneus.
ConclusiónEl lenguaje espontáneo y la fluidez verbal semántica y fonológica se proponen como claves en el diagnóstico diferencial. Asimismo, la valoración clínica debe considerar el uso de técnicas de neuroimagen avanzadas para establecer asociaciones en etapas tempranas entre la función cerebral afectada y el desempeño neuropsicológico, dando especial atención a áreas como el cuneus y precuneus.
Alzheimer disease (AD) is a progressive, irreversible neurodegenerative disease with an impact on the functional status of elderly patients, primarily affecting activities of daily living.1 AD is the most frequent cause of major neurocognitive disorder and one of the most frequent causes of morbidity and mortality in elderly individuals.2,3
Classically, AD initially manifests with short-term memory impairment and deficiencies in the coding and storage of new memories, problem-solving, judgement, and executive function, in addition to lack of motivation and poor organisation, leading to problems with multitasking and abstract thinking.4
Studies show that AD prevalence increases during the third and fourth age; it currently affects over 44.3 million people worldwide, a figure expected to treble to 135.5 million by 2050.5 In Europe, AD is more prevalent in women than in men (7.13% vs 3.31%), with an incidence rate of 11.08 cases per 1000 person-years.6
Several studies7–9 describe amnestic AD as the typical form (AD-typ), with 4 atypical forms characterised by specific biomarkers, as follows: logopenic variant primary progressive aphasia (lvPPA), posterior cortical atrophy (PCA), behavioural/dysexecutive or frontal variant AD (bvAD), and corticobasal syndrome (CBS).
Despite the available definitions of atypical variants of AD, it remains challenging in clinical practice to reach a consensus on the presentation of the initial symptoms and the behavioural and neuropsychological profiles of each phenotype. Recent studies10,11 have focused on the differential diagnosis of AD-typ and Lewy body dementia, highlighting the pattern of cortical atrophy as an early marker, as well as the overlap of neuropsychological symptoms (e.g., episodic memory, visuospatial/visuoconstructive skills, and language). Toloza-Ramírez et al.12 report that AD-typ and bvAD present similar characteristics to those observed in frontotemporal dementia (FTD), stressing that the deficits in episodic memory, executive function, and verbal fluency (both semantic and phonological) are essential to the detection of atypical profiles of AD, leading to correct differential diagnosis. While the available evidence demonstrates the importance of neuroanatomical and neuropsychological understanding of typical and atypical variants of AD, further study is needed to establish detailed profiles of lvPPA, PCA, and CBS.
There is a fundamental need for a synthesis of the available information on the neuropsychological characteristics and neural correlates of the different AD profiles to enable the classification and determination of the associated neuropsychological and neuroanatomical profiles. It is also essential to analyse the key elements to be considered in the accurate, timely differential diagnosis of this heterogeneous clinical entity. The general objective of this qualitative systematic review is to characterise the different AD profiles, taking into account their neural correlates and neuropsychological profiles.
MethodsThis qualitative systematic review was conducted in accordance with the criteria and flow diagram established in the PRISMA statement,13 and was registered on the PROSPERO database (project code CRD42021270183). The PRISMA checklist is included in the supplementary material (Appendix 1).
Search strategy for identifying studiesThe literature search was conducted on the PubMed, ScienceDirect, Scopus, and Web of Science databases. The search was limited to articles published between January 2011 and December 2021. The search strategy was as follows: [Alzheimer’s disease] AND [atypical Alzheimer’s disease] OR [Alzheimer variants] OR [amnestic Alzheimer disease] OR [logopenic variant primary progressive aphasia] OR [posterior cortical atrophy] OR [frontal variant Alzheimer disease] OR [corticobasal degeneration] OR [corticobasal syndrome] OR [neuropsychological assessment] OR [cognition] OR [cognitive function] OR [language impairment] OR [memory] OR [executive function] OR [biomarkers] OR [neuroimaging biomarkers]. All search terms were adapted to each database. The complete search strategy for each database is included in the supplementary material (Appendix 2).
Study selection and inclusion/exclusion criteriaWe applied the following inclusion criteria for screening by title and abstract: (a) analytical observational studies of diagnostic tests, reporting the neuropsychological assessment and biomarker test results; (b) studies written only in English; and (c) including patients aged at least 60 years with a clinical diagnosis of AD-typ, lvPPA, PCA, bvAD, and/or CBS. The exclusion criteria applied were the following: (a) editorials, experimental studies, systematic reviews (with or without meta-analyses), protocols, and theses; (b) including patients with FTD, Lewy body dementia, vascular dementia, or mixed dementia; and (c) including patients with history of psychiatric disorders (e.g., depression).
Data extractionStudies were imported to the Mendeley software (version 1.19.4) to eliminate duplicate articles. Subsequently, 3 reviewers (CFR, FGB, and CRB) applied the inclusion and exclusion criteria to the titles and abstracts of all articles. In the event that decisions could not be made based solely on the title and abstract, the reviewers recovered the full texts of the articles. A fourth researcher (DTR) participated in selection consensus.
Evaluation of methodological quality and risk of biasTo evaluate the methodological quality and risk of bias of the 31 articles selected, we used the QUADAS-2,14 an instrument designed and validated for the independent evaluation of methodological quality and risk of bias in studies of diagnostic accuracy. This instrument evaluates 4 key domains: patient selection, the index test, the reference standard, and the patient flow and timing of the study (the latter 2 aspects are grouped together into the domain “flow and timing”); given the type of studies included, the item “reference standard” was not applicable. Studies were categorised into one of 3 risk categories: high, low, or unclear.
Data synthesis strategyTables 1 and 2 present a narrative synthesis of our findings. Table 1 summarises the general characteristics of the studies, including year of publication, study population, mean age, and the instruments/measures used. Table 2 presents the neuropsychological and neural profiles of the typical and atypical forms of AD.
General characteristics of the studies reviewed.
| Title | Authors | Year of publication | Population | Instruments/measures |
|---|---|---|---|---|
| Parallel ICA of FDG-PET and PiB-PET in 3 conditions with underlying Alzheimer’s pathology15 | Laforce R, Tosun D, Ghosh P, Lehmann M, Madison C, Weiner M, Miller B, Jagust W, Rabinovici G | 2014 | 46 patients with AD-typ (amnestic/dysexecutive, linguistic, and visuospatial profiles; mean age: 68, 60.1, and 61 years, respectively) | CDRCDR-SBMRIFDG-PETPiB-PETMMSECVLTBNTDS-FWDS-BWTMT-MStroopROCF-MCATSCA-w |
| Examining prefrontal contributions to past- and future-oriented memory disturbances in daily life in dementia16 | Liu L, Roquet D, Ahmed R, Hodges J, Piguet O, Irish M | 2021 | 154 subjects: patients with AD-typ, behavioural variant FTD, semantic dementia, non-fluent/agrammatic variant PPA, and lvPPA, and controls (mean age: 66.1, 64.6, 64.5, 67.4, 68.9, and 66.4 years, respectively) | ACE-IIIPRM-QMRIFRS |
| Profiling sentence repetition deficits in primary progressive aphasia and Alzheimer’s disease: error patterns and association with digit span17 | Beales A, Whitworth A, Cartwright J, Panegyres P, Kane R | 2019 | 12 patients with semantic variant PPA, lvPPA, and AD-typ (mean age: 65.3, 63.3, and 68 years, respectively) | MRIFDG-PETACE-IIIStroopTMTPPTNNB |
| Common and divergent neural correlates of anomia in amnestic and logopenic presentations of Alzheimer’s disease18 | Leyton C, Hodges J, Piguet O, Ballard K | 2017 | 74 subjects: patients with lvPPA and AD-typ, and controls (mean age: 66.1, 67.6, and 65.7 years, respectively) | SPECTACE-RWAIS-III-DSFASMAE-SFRSSYDBAT |
| Subtypes of progressive aphasia: application of the International Consensus Criteria and validation using β-amyloid imaging19 | Leyton C, Villemagne V, Savage S, Pike K, Ballard K, Piguet O, Burrell J, Rowe C, Hodges J | 2011 | 99 subjects: patients with semantic variant PPA, non-fluent/agrammatic variant PPA, and lvPPA; and controls (mean age: 64, 67, 67, and 69 years, respectively) | PETPiBACE-RMMSEPALSNA |
| FDG-PET and MRI in logopenic primary progressive aphasia versus dementia of the Alzheimer’s type20 | Madhavan A, Whitwell J, Weigand S, Duffy J, Strand E, Machulda M, Tosakulwong N, Senjem M, Gunter J, Lowe V, Petersen R, Jack C Jr, Josephs K | 2013 | 81 subjects: patients with AD-typ and lvPPA, and controls (mean age: 71, 65, and 72 years, respectively) | FDG-PETMRIMMSEBNTTMT-BAVLTROCF |
| Early alteration of the locus coeruleus in phenotypic variants of Alzheimer’s disease21 | Olivieri P, Lagarde J, Lehericy S, Valabrègue R, Michel A, Macé P, Caillé F, Gervais P, Bottlaender M, Sarazin M | 2019 | 54 subjects: patients with AD-typ and lvPPA (or focal visuospatial deficits), and controls (mean age: 68.5, 68.9, and 66.3 years, respectively) | CSFPETCDRMADRSMMSEFCSRTROCF-DRMattis DRSWAISAChEISSRIsSSNIsGCIPiB-PET |
| Evidence for a pervasive autobiographical memory impairment in logopenic progressive aphasia22 | Ramanan S, Foxe D, El-Omar H, Ahmed RM, Hodges JR, Piguet O, Irish M | 2021 | 44 subjects: patients with lvPPA and AD-typ, and controls (mean age: 71.8, 73, and 74.4 years, respectively) | MRICDR-FTLD SoBCBI-RACE-RACE-IIIMAESYDBATDS-BWDSTTMT-ATMT-BROCFRAVLTFASAIP |
| Longitudinal grey matter contraction in 3 variants of primary progressive aphasia: a tenser-based morphometry study23 | Brambati SM, Amici S, Racine CA, Neuhaus J, Miller Z, Ogar J, Dronkers N, Miller BL, Rosen H, Gorno-Tempini ML | 2015 | 28 patients with non-fluent/agrammatic variant PPA, semantic variant PPA, and lvPPA (mean age: 67.9, 62.6, and 64.3 years, respectively) | MRIMMSECVLT-SROCF-MTMT-MDKEFSStroopCalculationsPraxisLT (‘D’ words in 1 minute)AFBNT-15CRLSWABMSE AoSRMSE DRCYCLE-R |
| Heterogeneous language profiles in patients with primary progressive aphasia due to Alzheimer’s disease24 | Louwersheimer E, Keulen M, Steenwijk M, Wattjes M, Jiskoot L, Vrenken H, Teunissen C, van Berckel B, van der Flier W, Scheltens P, van Swieten J, Pijnenburg Y | 2016 | 22 patients with lvPPA, lvPPA extended, and PPA unclassifiable (mean age: 65.2, 69.6, and 64.9 years, respectively) | MRIPETPiBCSFEEGMMSECDRAATBNTPALS |
| Understanding the neural basis of episodic amnesia in logopenic progressive aphasia: a multimodal neuroimaging study25 | Ramanan S, Marstaller L, Hodges JR, Piguet O, Irish M | 2020 | 72 subjects: patients with lvPPA and AD-typ, and controls (mean age: 69.6, 69.5, and 71.6 years, respectively) | MRICDR-FTLD SoBCBI-RACE-RSYDBATTMT-ATMT-BDSFDSBFASROCF |
| Connected speech markers of amyloid burden in primary progressive aphasia26 | Slegers A, Chafouleas G, Montembeault M, Bedetti C, Welch A, Rabinovici G, Langlais P, Gorno-Tempini ML, Brambati S | 2021 | 117 patients with PPA (logopenic, semantic, and non-fluent/agrammatic variants), classed as PiB(+) and PiB(–) (mean age: 63.71 and 65.83 years, respectively) | WAB-DCDRCDR-SBPiB-PETMRI |
| Clinical and neuroimaging biomarkers of amyloid-negative logopenic primary progressive aphasia27 | Whitwell J, Duffy J, Strand E, Machulda M, Senjem M, Schwarz C, Reid R, Baker M, Perkenson R, Lowe V, Rademakers R, Jack C Jr, Josephs K | 2015 | 26 patients with lvPPA, classed as PiB(+) and PiB (–) (mean age: 66 and 63 years, respectively) | PiB-PETFDG-PETMRIVBMAPOE genotypingWABBNTPPTMMSETMT-ADKEFSWMS-IIIVOSPMOANSAFT |
| Automated hippocampal subfield volumetric analyses in atypical Alzheimer’s disease28 | Gabere M, Thu Pham N, Graff-Radford J, Machulda M, Duffy J, Josephs K, Whitwell J | 2020 | 273 subjects: patients with lvPPA, PCA, and AD-typ, and controls (mean age: 68 and 62 years, respectively, for the first 2 groups; not reported for AD-typ and controls) | SPECTMMSEPETMRIPiBMoCACDRBDAE-rBNTNPI-QMDS-UPDRS IIIWMS-IIIAVLTROCFVOSP |
| White matter atrophy in Alzheimer’s disease variants29 | Migliaccio R, Agosta F, Possin K, Rabinovici G, Miller B, Gorno-Tempini ML | 2012 | 125 subjects: patients with PCA, lvPPA, AD-typ (early onset), and AD-typ (late onset), and controls (mean age: 61, 63.5, 60.7, 78.3, and 62.3 years, respectively) | MRIMMSECVLT-SFBF-CBF-RDS-BWTMT-MVOSP-NLAFBNT |
| Longitudinal neuroimaging biomarkers differ across Alzheimer’s disease phenotypes30 | Sintini I, Graff-Radford J, Senjem M, Schwarz C, Machulda M, Martin P, Jones D, Boeve B, Knopman D, Kantarci K, Petersen R, Jack C Jr, Lowe V, Josephs K, Whitwell J | 2020 | 57 patients with AD-typ, PCA, and lvPPA (mean age: 75, 65, and 68 years, respectively) | PETPiB-PETVBMAVLTBNTCDRMINTMOANSMoCAROCF |
| Longitudinal tau-PET uptake and atrophy in atypical Alzheimer’s disease31 | Sintini I, Martin P, Graff-Radford J, Senjem M, Schwarz C, Machulda M, Spychalla A, Drubach D, Knopman D, Petersen R, Lowe V, Jack C Jr, Josephs K, Whitwell J | 2019 | 30 patients with lvPPA and PCA (mean age: 64 and 74 years, respectively) | Tau-PETAβ-PETMRIVBMMoCACDRNPI-QMDS-UPDRS III WAB-PWMS-III-VR %BDAEMOANSVOSPROCFCBI |
| Phonological errors in posterior cortical atrophy32 | Tetzloff K, Duffy J, Strand E, Machulda M, Schwarz C, Senjem M, Jack C, Josephs K, Whitwell J | 2021 | 54 patients with PCA and lvPPA (mean age: 64.0 and 68.0 years, respectively) | NASLAPiB-PETMRIAβ-PETWABBNTCFTMoCACDRVOSPNA |
| Regional distribution, asymmetry, and clinical correlates of Tau uptake on [18F]AV-1451 PET in atypical Alzheimer’s disease33 | Tetzloff K, Graff-Radford J, Martin P, Tosakulwong N, Machulda M, Duffy J, Clark H, Senjem M, Schwarz C, Spychalla A, Drubach D, Jack C Jr, Lowe V, Josephs K, Whitwell J | 2018 | 148 subjects: patients with PCA and lvPPA, and controls (mean age: 66 and 69 years, respectively, for the first 2 groups; not reported for controls) | PETPiBMRIMoCACDRNPI-QMDS-UPDRS IIIBNTBDAEENVOSPROCFWMS-III |
| Tau PET imaging predicts cognition in atypical variants of Alzheimer’s disease34 | Phillips J, Das S, McMillan C, Irwin D, Roll E, Da Re F, Nasrallah I, Wolk D, Grossman M | 2017 | 14 patients with lvPPA, PCA, and AD-typ (mean age: 63.4, 57.8, and 62 years, respectively) | MMSEMRIPETGDSPBACBDAE-L1FCSPVLTROCFBNTBDAE-rSFDS-FWVOSP |
| Word retrieval across the biomarker-confirmed Alzheimer’s disease syndromic spectrum35 | Putcha D, Dickerson B, Brickhouse M, Johnson K, Sperling R, Papp K | 2020 | 169 subjects: patients with AD-typ, PCA, and lvPPA, and controls (mean age: 70.4, 63.9, 69.4, and 68.7 years, respectively) | MRIAβ-PETC-PiB PETCSFCDRMMSENACC-UDS2FASSFBNT |
| Neural correlates of cognitive impairment in posterior cortical atrophy36 | Kas A, Cruz de Souza L, Samri D, Bartolomeo P, Lacomblez L, Kalafat M, Migliaccio R, Thiebaut de Schotten M, Cohen L, Dubois B, Habert M, Sarazin M | 2011 | 87 subjects: patients with PCA and AD-typ, and controls (mean age: 61.1, 65.1, and 69 years, respectively) | SPECTVBMMMSEROCFLABBDAE-L1AOcAAOpARDBSADAMCAS |
| Differences in hippocampal subfield volume are seen in phenotypic variants of early onset Alzheimer’s disease37 | Parker T, Slattery C, Yong K, Nicholas J, Paterson R, Foulkes A, Malone I, Thomas D, Cash D, Crutch S, Fox N, Schott J | 2018 | 63 subjects: patients with AD-typ and PCA, and controls (mean age: 61.1, 61.2, and 60.1 years, respectively) | MRIMMSEWASIsRMTNARTGDSTGDAVOSP |
| A pilot study on clinical and neuroimaging characteristics of Chinese posterior cortical atrophy: comparison with typical Alzheimer’s disease38 | Wang X, Lu H, Shi Z, Cai L, Liu S, Liu S, Han T, Wang Y, Zhou Y, Wang X, Gao S, Ji Y | 2015 | 19 subjects: patients with PCA and AD-typ, and controls (mean age: 60.1, 61, and 60 years, respectively) | MMSEMoCAADLCDTPiB-PETFDG-PETMRI |
| Ventral and dorsal visual streams in posterior cortical atrophy: a DT MRI study39 | Migliaccio R, Agosta F, Scola E, Magnani G, Cappa S, Pagani E, Canu E, Comi G, Falini A, Gorno-Tempini ML, Bartolomeo P, Filippi M | 2012 | 20 subjects: patients with PCA and controls (mean age: 60.8 and 62 years, respectively) | MRIVBMMMSERAVLTAMTDSTPSFTTROCFBORBFCTAATBADAVOSPOFTGEREN |
| Executive dysfunction contributes to verbal encoding and retrieval deficits in posterior cortical atrophy40 | Putcha D, McGinnis S, Brickhouse M, Wong B, Sherman J, Dickerson B | 2018 | 90 patients with PCA (mean age: 63.1 years) | MRIMMSECDR-SBNAR |
| Semantic word category processing in semantic dementia and posterior cortical atrophy41 | Shebani Z, Patterson K, Nestor P, Díaz-de-Grenu L, Dawson K, Pulvermuller F | 2017 | 20 patients with semantic dementia and PCA (mean age: 67.7 and 59.2 years, respectively) | MRIACE-RMMSEOHI-rvLS |
| Highly elevated cerebrospinal fluid total Tau level reflects higher likelihood of non-amnestic subtype of Alzheimer’s disease42 | Pillai J, Bonner-Jackson A, Bekris L, Safar J, Bena J, Leverenz J. | 2019 | 54 patients with AD-typ, lvPPA, bvAD, and posterior-biparietal variant AD (mean age: 67.3, 64.4, 66.5, and 68.2 years, respectively) | CSFMRIMoCABNTWMS-IV-LMRAVLTHVLTCVLTPSFWAIS-IV-SJLOWAIS-IV-BD |
| Frontal variant of Alzheimer’s disease and typical Alzheimer’s disease: a comparative study43 | Fernández-Calvo B, Ramos F, de Lucena V | 2013 | 84 subjects: patients with AD-typ and bvAD, and controls (mean age: 75.9, 72.8, and 72.8 years, respectively) | SPECTCDRMMSETMT-ATMT-BWAIS-R-DSSWAIS-R-FDSWAIS-R-BDSA-TestHVLT-RBVRTROCFAFCDTStroop-CFASWAIS-R-SWAIS-R-CROCF-DRNPIIDDDZBI |
| The phenotypical core of Alzheimer’s disease-related and non-related variants of the corticobasal syndrome: a systematic clinical, neuropsychological, imaging, and biomarker study44 | Di Stefano F, Kas A, Habert MO, Decazes P, Lamari F, Lista S, Hampel H, Teichmann M. | 2016 | 69 subjects: patients with AD-related and non–AD-related CBS, and controls (mean age: 68.9, 67, and 69 years, respectively) | MMSEFABVerbal spanFCSRTGSTATROCFBDAEDO80SFPFOACSFSPECT |
| FDG-PET patterns predict amyloid deposition and clinical profile in corticobasal syndrome45 | Parmera J, Coutinho A, Aranha M, Studart-Neto A, de Godoi Carneiro C, de Almeida I, Fontoura Solla D, Ono C, Barbosa E, Nitrini R, Buchpiguel C, Brucki S | 2020 | 45 patients AD-related and non–AD-related SCB (mean age: 62.4 and 63.6 years, respectively) | ACE-RMMSEBCSBFDG-PETPiB-PETNPICDRFAQHYSNISSLAMDS |
Note: A-Test: “A” Random Letter Test of Auditory Vigilance; AAT: Aachener Aphasie Test; Aβ: beta amyloid; ACE-III: Addenbrooke’s Cognitive Examination III; ACE-R: Addenbrooke’s Cognitive Examination-Revised; AChEI: acetylcholinesterase inhibitors; ADAS-Cog: Alzheimer’s Disease Assessment Scale-Cognitive; ADAS-Cog13: Alzheimer’s Disease Assessment Scale-Cognitive Subscale 13; ADL: Barthel Index for Activities of Daily Living; AF: Animal Fluency Test; AFT: Action (Verb) Fluency Test; AIP: Autobiographical Interview Protocol; AMT: Attentive Matrices Test; AOcA: Assessment of Ocular Apraxia; AOpA: Assessment of Optic Apraxia; AS: Agraphia Score; AT: Apraxia Test; AVLT: Auditory Verbal Learning Test; BADA: Batteria per l’analisi dei deficit afasici; BADLS: Bayer Activities of Daily Living Scale; BCSB: Brief Cognitive Screening Battery; BDAE: Boston Diagnostic Aphasia Examination; BDAE-L1: Boston Diagnostic Aphasia Examination-L1; BDAE-r: Boston Diagnostic Aphasia Examination-repetition; BF-C: Benson Figure-Copy; BF-R: Benson Figure-Recall; BFRT: Benton Facial Recognition Test; BORB: Birmingham Object Recognition Battery; BNT: Boston Naming Test; BNT-15: 15-item Boston Naming Test; BSAT: Brixton Spatial Anticipation Test; BVMT-R: Brief Visuospatial Memory Test-Revised; BVRT: Benton Visual Retention Test; BVS: British Vocabulary Scale; CA-w: Calculation (Arithmetic, written); CAM-COG: Cambridge Cognitive Examination; CATS: Comprehensive Affect Testing System; CBI: Cambridge Behavioural Inventory; CBI-R: Cambridge Behavioural Inventory-Revised; CBTT: Corsi Block Tapping Test; CDR: Clinical Dementia Rating scale; CDR-FTLD SoB: Clinical Dementia Rating-Frontotemporal Lobar Degeneration-sum of boxes; CDR-SB: Clinical Dementia Rating scale-sum of boxes; CDT: Clock Drawing Test; CERAD-NAB: Consortium to Establish a Registry for Alzheimer’s Disease-Neuropsychological Assessment Battery; CFT: Category Verbal Fluency Test; CIRS: Cumulative Illness Rating Scale; COWAT: Controlled Oral Word Association Test; CPDR: Constructional Praxis-Delayed Recall; CRLS: Clinician Rating of Language Symptoms; CSF: cerebrospinal fluid; CVLT: California Verbal Learning Test; CVLT-MS: California Verbal Learning Test, Mental Status version; CVLT-SF: California Verbal Learning Test, Short Form; CWT: Colour Word Test; CYCLE: Curtiss-Yamada Comprehensive Language Evaluation; CYCLE-R: Curtiss-Yamada Comprehensive Language Evaluation-Receptive; DA: digital agnosia; DAD: Disability Assessment for Dementia scale; DKEFS: Design Fluency Filled Dots Condition; DO80: Picture Naming Test DO80; DRS: Dementia Rating Scale; DS-BW: Digit Span Backward; DS-FW: Digit Span Forward; DST: Digit Span Test; EEG: electroencephalography; FAB: Frontal Assessment Battery; FAQ: Functional Activities Questionnaire; FAS: Verbal Fluency Test (words beginning with ‘F,’ ‘A,’ and ‘S’); FBI: Frontal Behavioural Inventory; FCSRT: Free and Cued Selective Reminding Test; FCT: Figure Completion Test; FDG-PET: F-fluorodeoxyglucose positron emission tomography; FRS: Frontotemporal Dementia Rating Scale; FTD: frontotemporal dementia; GCI: Global Cortical Index; GDA: Graded Difficulty Arithmetic Test; GDS: Geriatric Depression Scale; GDST: Graded Difficulty Spelling Test; GEREN: GEREN standardised paper-and-pencil battery of tests; GNGT: Go/No Go Task; GNT: Graded Naming Test; GST: Gerstmann Syndrome Test; HATA: hippocampus-amygdala transition area; HSCT: Hayling Sentence Completion Test; HT: Hayling Test; HVLT: Hopkins Verbal Learning Test; HVLT-R: Hopkins Verbal Learning Test-Revised; HYS: Hoehn and Yahr Scale; IDDD: Interview for Deterioration in Daily Living Activities in Dementia; IGT: Iowa Gambling Task; JLO: Judgement of Line Orientation; LAB: Limb Apraxia Battery; LM: logical memory; LS: lexical stimuli; LT: letter fluency; MADRS: Montgomery-Asberg Depression Rating Scale; MAE: Multilingual Aphasia Examination; MAE-S: sentence repetition subtest of the Multilingual Aphasia Examination; Mattis DRS: Mattis Dementia Rating Scale; MC: mental calculation; MCST: Modified Card Sorting Test; MDS: Movement Disorders Society Criteria; MDS-UPDRS III: Movement Disorders Society sponsored revision of the Unified Parkinson’s Disease Rating Scale part III; MINT: Multilingual Naming Test; MMSE: Mini–Mental State Examination; MOANS: Mayo Older American Normative Studies; MoCA: Montreal Cognitive Assessment; MRI: magnetic resonance imaging; MSE AoSR: Motor Speech Evaluation-Apraxia of Speech; MSE DR: Motor Speech Evaluation-Dysarthria Rating; MWMNT: Manchester Word-Picture Matching and Naming Tests; NA: neuropsychological and/or neurological assessment; NACC-UDS2: National Alzheimer’s Coordinating Center (NACC) Uniform Data Set (UDS) version 2.0 or 3.0 battery; NAR: Neuropsychological Assessment Rating; NART: National Adult Reading Test; NAT: Northwestern Anagram Test; NIS: Neuropsychiatric Inventory Scale; NNB: Northwestern Naming Battery; NPI: Neuropsychiatric Inventory; NPI-Q: Neuropsychiatric Inventory, Brief Questionnaire version; OA: orobuccal apraxia; OFT: Overlapping Figures Test; OHI-rv: Oldfield Handedness Inventory, reduced version; PALPA: Psycholinguistic Assessment of Language Processing in Aphasia; PALS: Progressive Aphasia Language Scale; PBAC: Philadelphia Brief Assessment of Cognition; PET: positron emission tomography; PF: phonemic fluency; PiB: Pittsburgh Compound-B; PPVT: Peabody Picture Vocabulary Test; PPT: Pyramid and Palm Trees; PRM-Q: Prospective and Retrospective Memory Questionnaire; PSF: Phonemic and Semantic Fluency; PVLT: Philadelphia Verbal Learning Test; RAVLT: Rey Auditory Verbal Learning Test; RCPM: Raven Coloured Progressive Matrices; RDBSA: Right-Left Distinction Body Schema Assessment; RMT: Recognition Memory Test; ROCF: Rey-Osterrieth Complex Figure; ROCF-DR: Rey-Osterrieth Complex Figure-Delayed Recall; ROCF-M: Rey-Osterrieth Complex Figure-Modified; RWT: Regensburg Word Fluency; SEA: Social Cognition and Emotional Assessment; SF: semantic fluency; SLA: Speech-Language Assessment; SMAF: Functional Autonomy Measurement System; SNSB-D: Dementia version of the Seoul Neuropsychological Screening Battery; sRMT: Short Recognition Memory Test; SS: Spatial Span; Stroop: Stroop test; SSNI: serotonin-norepinephrine reuptake inhibitors; SSRI: selective serotonin reuptake inhibitors; STROOP-C: Colour part of the Stroop test; SVLT-DR: Seoul Verbal Learning Test-Delayed Recall; SYDBAT: Sydney Language Battery; TASIT: Awareness of Social Inference Test; TDBC: Three-Dimensional Block Construction Test; TMT: Trail Making Test; TMT-A: Trail Making Test, Part A; TMT-B: Trail Making Test, Part B; TMT-M: Trail Making Test-Modified; TROG: Test for the Repetition of Grammar; TT: Token Test; VBM: voxel-based morphometry; VOSP: Visual Object and Space Perception battery; VOSP-NL: number location subtest of the Visual Object and Space Perception battery; VST: Verbal Similarity Task; WAB: Western Aphasia Battery; WAB-D: drawing subtest of the Western Aphasia Battery; WAB-P: praxis subtest of the Western Aphasia Battery; WAIS: Wechsler Adult Intelligence Scale; WAIS-III-DS: auditory-verbal digit span subtest of the Wechsler Adult Intelligence Scale-III; WMS-III-VR %: % visual reproduction retention subtest of the Wechsler Memory Scale III; WAIS-IV: Wechsler Adult Intelligence Scale, fourth edition; WAIS-IV-BD: block design subtest of the Wechsler Adult Intelligence Scale, fourth edition; WAIS-IV-S: similarities, subtest of the Wechsler Adult Intelligence Scale, fourth edition; WAIS-R-BDS: backward digit span subtest of the Wechsler Adult Intelligence Scale-Revised; WAIS-R-C: comprehension subtest of the Wechsler Adult Intelligence Scale-Revised; WAIS-R-DSS: digit symbol substitution subtest of the Wechsler Adult Intelligence Scale-Revised; WAIS-R-FDS: forward digit span subtest of the Wechsler Adult Intelligence Scale-Revised; WAIS-R-S: similarities subtest of the Wechsler Adult Intelligence Scale-Revised; WASI: Wechsler Abbreviated Scale of Intelligence; WMS-III: Wechsler Memory Scale-III; WMS-IV: Wechsler Memory Scale-IV; WMS-IV-LM: logical memory subtest of the Wechsler Memory Scale IV; ZBI: Zarit Burden Interview.
Summary of the neuropsychological profiles and neural correlates described.
| Ref. | Neuropsychological profile | Neural correlate |
|---|---|---|
| 15 | Patients with AD-typ (amnestic/dysexecutive profile) scored poorly in visual and verbal memory tasks; patients with AD-typ (visuospatial profile) performed worse on visuospatial tasks; and patients with AD-typ (linguistic profile) performed considerably worse in sentence repetition and phonological verbal fluency tasks. | AD-typ (linguistic profile) was associated with temporoparietal and left inferior frontal atrophy. In contrast, patients with AD-typ (visuospatial profile) displayed involvement of bilateral occipitoparietotemporal regions, the right posterior cingulate cortex, precuneus, and lateral right parietal cortex. In AD-typ (amnestic/dysexecutive profile), the atrophy pattern involves the bilateral inferior frontal lobe, cuneus, and inferior temporal lobe, with minimal right inferior parietal hypometabolism. |
| 16 | Retrospective and prospective memory was affected in patients with AD-typ and behavioural variant FTD. Episodic amnesia and memory disorders similar to those occurring in AD-typ were also observed in patients with behavioural variant FTD. Patients with lvPPA showed no significant problems with retrospective or prospective memory. | The orbitofrontal cortex was affected in patients with AD-typ and with behavioural-variant FTD. This cortex appears to be a neural substrate of memory impairment in both groups. Furthermore, the precuneus and posterior cingulate cortex seem to be involved in retrograde and anterograde memory function. Patients with AD-typ displayed atrophy in prefrontal and in lateral and medial temporal regions, including the hippocampus. This demonstrates the role of prefrontal regions in supporting complex aspects of memory in everyday life. |
| 17 | The authors propose that phonological errors constitute a linguistic biomarker for differentiating between lvPPA and AD-typ, with the former group showing marked deficits. Patients with lvPPA also present verbal deficits in short-term memory. In contrast, patients with AD-typ present deficits in repetition tasks, with ending omissions and unrelated word substitutions. Furthermore, an associated attentional alteration was found to be the cause of these errors. | Patients with lvPPA displayed hypometabolism in parietal, temporal, and occipital regions, predominantly in the left hemisphere. Similarly, the authors observed asymmetrical loss of volume of the left superior temporal gyrus, with mild predominance in the extra-axial sulcal spaces around the convexities bilaterally. This was related with reduced activity in the parietal and temporal lobes, which was more severe on the left side. Patients with AD-typ displayed symmetrical mild-to-moderate brain atrophy, with early widening of sulci, in addition to reduced cortical metabolism in the frontal and parietal lobes, involving the precuneus and anterior cingulate. |
| 18 | The AD-typ group presented linguistic symptoms of mild anomia and circumlocutions. In contrast, the lvPPA group displayed severe anomia and large numbers of phonological and paraphasic errors, as well as deficits in word repetition tasks, a key characteristic in differential diagnosis. Furthermore, both groups presented deficits in single-word comprehension. | Patients with AD-typ presented symmetrical, bilateral cortical thinning in the parietal and temporal lobes, which was of moderate severity, potentially explaining the later onset of phonological impairment in this patient group. The macroscopic atrophic changes in the posterior superior temporal gyrus were of greater magnitude in the lvPPA group; this structure may also present microscopic neuroanatomical changes that cannot be detected with MRI. Furthermore, tau inclusions in the posterior superior temporal gyrus, associated with the risk of cognitive impairment, are more abundant in lvPPA, as are white matter changes contributing to linguistic disconnection in this group. |
| 19 | Patients with lvPPA present phonological errors in comprehension and repetition tasks, indicating impairment of phonological integration, which is closely linked to the integrity of such cognitive domains as attention and working memory. | The pattern of cortical beta amyloid deposition was comparable in patients with lvPPA and with AD-typ, although the load was lower in the former disease. |
| 20 | Significant differences in performance in cognitive and linguistic tests were observed between AD-typ and lvPPA, with patients in the latter group performing significantly worse in the BNT. Therefore, the object naming subdomain of language is an important element in early neuropsychological evaluation. | Compared to patients with AD-typ, patients with lvPPA displayed more marked hypometabolism and loss of grey matter volume in the left inferior parietal and lateral temporal lobes, posterior cingulate, and precuneus. Patients with AD-typ showed hypometabolism in temporal and lateral parietal regions and the posterior cingulate. They also showed greater involvement of right occipital and parietal regions, particularly the posterior cingulate. |
| 21 | The lack of correlation between locus coeruleus signal intensity and cognition in the atypical AD group may be explained by the more heterogeneous cognitive profiles observed in this group, characterised by lvPPA and focal visuospatial deficits. | Lower locus coeruleus signal intensity showed a significant positive correlation with poor visual and verbal episodic memory performance in AD-typ. The locus coeruleus may be an early target for tauopathy, as neurofibrillary tangles may act as a transmission initiator in AD or a so-called “systemic propagon,” with no direct association with cortical amyloid deposition. |
| 22 | Patients with AD-typ showed significant dysfunction of episodic memory (deficits in semantic association), verbal recognition, working memory, and executive function. Patients with lvPPA presented difficulties in language (naming, comprehension, and sentence and word repetition), attention, and working memory. They also tended to present greater difficulties with autobiographical memory. | Patients with lvPPA presented more significant atrophy in the left parietal lobe and temporal perisylvian cortex, extending to left posterior hippocampal areas, medial prefrontal areas (bilaterally), and the left insula, post-central gyrus, and occipital pole. |
| 23 | Patients with lvPPA displayed difficulties in naming, sentence repetition, and phonological verbal fluency tasks, with syntactic/grammatical errors. They also showed significant deficits in spontaneous speech information content. Impairment of verbal memory (learning) and executive function was mild. | Patients with lvPPA presented bilateral grey matter contraction in the anterior segment of the left superior temporal gyrus, left inferior temporal gyrus, and fusiform gyrus. Therefore, unlike the other groups, patients with lvPPA mainly displayed a contraction of left temporal and hippocampal regions. Regarding white matter involvement, the lvPPA group showed greater progression in the right superior longitudinal fasciculus and left posterior cingulate. To a lesser extent, the left inferior longitudinal fronto-occipital fasciculus also presented white matter contraction. |
| 24 | Patients with lvPPA showed linguistic symptoms related to deficits in lexical retrieval secondary to damage in left inferior parietal and left posterior superior temporal regions, as well as phonemic and semantic paraphasia. They also presented errors in repetition tasks due to impairment of short-term memory and the phonological loop, which depend on the dorsal stream, including the left posterior temporal gyrus. | The linguistic deficits observed in lvPPA were more extensive than those reflected in the current classification criteria, as these patients presented greater atrophy of the left temporal lobe, as well as the language streams, which are anatomically and functionally connected and whose involvement probably becomes more severe over the course of lvPPA progression. |
| 25 | Patients with lvPPA presented marked deficits in naming, comprehension, repetition, phonological verbal fluency, executive function, sustained attention, working memory, episodic memory (both verbal and non-verbal), and visuoconstructive skills. | Patients with lvPPA showed marked atrophy of the left hippocampus, bilateral posterior parietal regions (including the angular gyrus), lateral temporal lobe, and medial and lateral prefrontal regions. They also presented severe alterations in the frontal poles, left orbitofrontal cortex, and left precuneus. |
| 26 | PiB(+) patients displayed low idea density due to difficulties producing concrete nouns, laborious speech production, fluency alterations associated with word retrieval, and short sentences, even in individuals without grammatical or verb production alterations, as in the case of patients with lvPPA. Pragmatic difficulties due to problems with semantic processing and grammatical construction were more often observed in patients with semantic or non-fluent/agrammatic variant PPA than in those with lvPPA. | Patients with lvPPA displayed considerable atrophy of the superior and the posterior middle temporal gyri, inferior parietal lobe, posterior and anterior inferior longitudinal fasciculus, uncinate fasciculus, and superior longitudinal fasciculus. The group of patients with lvPPA presented similar levels of atrophy in the left anterior insula, which was correlated with difficulties in the processing of emotional concepts. |
| 27 | Both groups performed similarly in all speech and language tasks, with similar levels of cognitive impairment and mild memory deficits. Furthermore, the PiB(–) lvPPA group performed worse in action/verb fluency tasks and better in the cube analysis task. | Clinical and neuroimaging characteristics varied according to the presence or absence of Aβ deposits in patients with lvPPA; significant hemispheric asymmetry was observed between the PiB(+) and PiB(–) groups. From a neuroanatomical perspective, both groups presented degeneration of parietal and temporal white matter tracts, including the uncinate, inferior longitudinal, superior longitudinal, and inferior fronto-occipital fasciculi, with greater involvement in the left hemisphere. Compared to PiB(+) patients, PiB(–) patients with lvPPA displayed greater atrophy in the medial frontal lobe, left anteromedial temporal regions, left inferior longitudinal fasciculus, uncinate fasciculus, and forceps minor. |
| 28 | Patients with PCA mainly presented progressive visuospatial/perceptual problems, whereas those with lvPPA presented problems with word retrieval, naming, and sentence repetition, as well as phonological errors and deficits in auditory comprehension of single words (knowledge of the words was intact). | Both lvPPA and PCA were associated with loss of volume in hippocampal subfields. While lvPPA was associated with predominantly left CA1 involvement, patients with PCA displayed predominantly right-sided involvement of the presubiculum, parasubiculum, fimbria, HATA, and CA4. |
| 29 | The AD-typ group showed marked deficits in visual memory, executive function, and language tasks. In turn, patients with PCA presented significant visuospatial deficits. Patients with lvPPA presented good overall cognitive performance, despite significant executive and phonological impairment. Significantly, patients with AD-typ performed better than those with PCA in semantic verbal fluency tasks. | In the AD-typ group, atrophy affected medial areas of the parietal and temporal lobes and the posterior part of the corpus callosum, as well as the right lateral temporal lobe, extending to parahippocampal regions and the cingulate (anterior, middle, and posterior gyri) in advanced stages. In the PCA group, atrophy affected temporo-parieto-occipital regions bilaterally, predominantly on the right side, as well as the posterior cingulate cortex and the posterior part of the corpus callosum. Patients with lvPPA presented marked atrophy of the left parietal lobe. |
| 30 | Language deficits were more pronounced in the lvPPA group, whereas patients with PCA presented greater visuospatial impairment. Patients with AD-typ presented pronounced memory deficits. Severity, as quantified by the MoCA test at baseline, was not associated with variability in the different phenotypes observed. | Frontal tau deposition and temporal lobe atrophy are proposed as key findings for differentiating PCA and lvPPA from AD-typ, although they change each year with disease progression. Atrophy of bilateral occipitotemporal regions is considered a biomarker of PCA, whereas left temporal and parietal atrophy is considered to indicate lvPPA. The hippocampus was preserved in both profiles. Patients with lvPPA presented greater frontal and left temporoparietal uptake in tau-PET studies, with atrophy of the left temporal lobe. PCA was associated with elevated uptake on tau-PET and atrophy of the right occipital lobe. In turn, tau-PET studies of patients with AD-typ revealed elevated uptake in the right medial temporal lobe and in frontal regions, in addition to atrophy of the right medial temporal lobe, with the occipital lobes being preserved. |
| 31 | No statistically significant correlation was observed between the pattern of atrophy and overall neuropsychological changes. Relevant changes were only observed in the lvPPA group, with impaired functional status associated with the presence of oculomotor apraxia. | Both groups presented different but overlapping patterns of longitudinal propagation, with more pronounced atrophy in frontal and temporoparietal areas. Patients with lvPPA displayed greater atrophy of the left temporal lobe, extending to right temporo-parieto-occipital areas, as well as the left posterior cingulate and fusiform region. A slower rate of atrophy was observed in these regions, with the exception of the hippocampus, in which atrophy progression was correlated with age. In contrast, patients with PCA displayed greater atrophy of occipital and posterior parietal regions, with tau deposition in anterior frontal areas and in the temporal lobe. Cross-sectional analysis of patients with lvPPA showed greater tau uptake in the left temporal area, extending to the right frontal and temporal lobes and the parietal and occipital lobes. In contrast, PCA was associated with greater cross-sectional tau uptake in the occipital lobe and posterior brain regions, later extending to anterior frontal and temporal regions. |
| 32 | PCA was associated with visuospatial/perceptual deficits, as well as phonological impairment of speech production, difficulties with repetition, and omission/substitution of sounds in speech. Patients with lvPPA presented significant impairment in sentence repetition, naming, and phonological fluency. | In PCA, performance in the fluency subtest of the WAB was also correlated with left lateral temporal lobe volume, which may reflect a subtle lack of fluency due to pauses to find words, as there were no signs of agrammatism. These findings were correlated with loss of volume in the left inferior parietal and lateral temporal lobes. |
| 33 | Tau uptake in the bilateral frontal and temporal lobes and left parietal region was associated with deficits in sentence repetition, probably reflecting an interruption of the language network. Bilateral tau uptake in the temporal lobes was also associated with deficits in naming; this is consistent with the role of this brain region in anomia and semantic processing. | The PCA group displayed bilateral occipital lobe atrophy (inferior, middle, and superior gyri), with greater right predominance than was observed in the lvPPA group. Tau uptake in the bilateral occipital lobes was associated with the severity of simultanagnosia and visuoperceptual dysfunction. Furthermore, patients with PCA displayed greater uptake than those with lvPPA in the calcarine, cuneus, and lingual regions, bilaterally. In contrast, the lvPPA group showed greater atrophy in the left temporal lobe (temporal pole) and medial prefrontal cortex than the PCA group. |
| 34 | Patients with PCA presented deficits in visuospatial function, with preserved language and episodic memory. Patients with AD-typ presented episodic memory deficits in verbal and visual delayed recall tasks. | The lvPPA group presented uptake in the left anterior superior temporal gyrus, which accounted for 67% of variation in language, associated with descriptive speech, word and sentence repetition, visual confrontation naming, and semantic category fluency (animals) tasks. Uptake was highly lateralised to the left, with foci in the left superior and middle temporal gyri, left precuneus, and bilateral supramarginal gyri. Uptake in the right lingual gyrus predicted 85% of variance in visuospatial performance in object perception and spatial perception tasks and the ROCF copy task. The authors also report extensive uptake with peaks throughout the medial parietal and posterior occipitotemporal areas bilaterally. The AD-typ group displayed a similar pattern of uptake, which was most marked in the left precuneus and posterior cingulate, right posterior superior temporal and inferior parietal regions, and bilateral middle temporal areas. The associated eigenvector included segments of the right inferior temporal gyrus, cuneus, hippocampus, and amygdala. |
| 35 | Phonological verbal fluency was preserved in patients with AD-typ and PCA but not in the lvPPA group. The marked phonological deficit observed in patients with lvPPA also had an impact on word retrieval. | The pattern of atrophy in AD-typ involved the medial and lateral temporal cortex and the precuneus and posterior cingulate cortex. The PCA group presented greater atrophy in the occipital lobe, inferior and posterolateral temporal regions, lateral parietal areas, and precuneus/posterior cingulate cortex, with slight predominance in the right hemisphere. In contrast, the lvPPA group presented more pronounced atrophy of lateral temporal and lateral parietal regions and the precuneus and posterior cingulate cortex, with predominance in the left hemisphere. |
| 36 | Patients with PCA presented acalculia, impaired laterality (left-right indistinction), simultanagnosia, and limb apraxia. Similarly, these patients performed poorly in working memory tasks, particularly those testing visual working memory. To a lesser extent, the authors also observed complete Bálint syndrome, complete Gerstmann syndrome, visual agnosia, and aphasia predominantly affecting reading and writing. However, they performed significantly worse than patients with AD-typ in verbal episodic memory tasks, in both free and cued recall. | Comparison between patients with AD-typ and PCA revealed that PCA is associated with severe occipitoparietal hypoperfusion and greater perfusion of frontal and mesiotemporal regions and the anterior cingulate. In AD-typ, the pattern of atrophy observed in initial and progressive stages of the disease remained constant, mainly affecting posterior regions. In turn, PCA was associated with marked left inferior parietal and bilateral dorsal occipitoparietal atrophy. |
| 37 | Comparisons between AD-typ and PCA revealed that the latter group presented greater deficits in shape detection, object decision, fragmented letters, dot-counting, and letter cancellation. Patients with PCA performed worse in a matrix reasoning task. Patients with AD-typ presented more severe deficits in memory tasks involving verbal recognition. | AD-typ was associated with marked hippocampal atrophy, sparing the parasubiculum. In PCA, atrophy was pronounced in both the left (prebiculum) and right hemispheres (subiculum, dentate gyrus, hippocampus, and amygdala). |
| 38 | Patients with PCA presented considerable impairment of visuospatial skills. They also scored lower overall in the MoCA test than patients with AD-typ. | Patients with PCA presented severe posterior atrophy; left medial temporal lobe atrophy was less severe than in the AD-typ group. The PCA group also presented considerable hypometabolism in the temporal, parietal, and occipital lobes, and severe right occipital lobe involvement. |
| 39 | The 7 patients with PCA presented deficits in object and face recognition, as well as difficulty reading. Visual agnosia and alexia were the earliest symptoms in all but one of the patients. Three patients presented prosopagnosia and 3 displayed language deficits and ideomotor apraxia over the course of the disease. | The authors recorded selective damage to the ventral visual network (occipitotemporal regions, inferior longitudinal fasciculus, and fronto-occipital fasciculus), particularly in the left hemisphere. Patients with PCA displayed bilateral grey matter atrophy in the posterior temporal, parietal, and occipital regions. They also presented marked neuronal loss in the middle and inferior occipital lobe, ventral occipitotemporal lobe, inferior parietal lobe, posterior and middle temporal lobes, and hippocampus. In the right hemisphere, atrophy was observed in the superior parietal gyrus and thalamus. The left calcarine cortex, cuneus, precuneus, superior temporal gyrus, and posterior cingulate showed grey matter loss. The ventral occipitotemporal region presented white matter involvement bilaterally. |
| 40 | PCA was associated with poor performance in verbal executive function tasks, language (lexical/semantic), and verbal episodic memory (coding/retrieval), with the latter domain being identified as a potential biomarker of the disease. | Patients presented atrophy of the bilateral posterior parietal lobe, medial and inferior parietal regions, precuneus, retrosplenial cortex, intraparietal sulcus, lateral temporal lobe, and occipital cortex, with more severe involvement in the left hemisphere. |
| 41 | The semantic PPA and PCA groups presented broad lexical/semantic deficits in word category processing tasks (colour, shape, numbers, spatial prepositions, function words, and pseudowords). The PCA group performed worse for spatial prepositions and better for colours in the lexical decision task. | The poor linguistic performance in the lexical decision task in the PCA group was correlated with the semantic circuits in the posterior parietal cortex. Interestingly, the pattern of atrophy observed in this region tends to be bilateral, with a slight right predominance. |
| 42 | Cognitive performance was compared between patients categorised by quartile according to CSF total tau (t-tau) value, with the high t-tau group performing worse in verbal abstraction and non-verbal delayed recall. In the MoCA test, the high t-tau group performed significantly worse than other groups in the clock-drawing, vigilance, serial 7s, verbal fluency, and sentence repetition subtests. When the highest and lowest t-tau quartiles were compared, global MoCA test scores were 2 points higher in the latter group. | Among patients with clinical and biochemical markers suggesting underlying AD, patients with highly elevated CSF t-tau levels presented a higher likelihood of non-amnestic forms (lvPPA, bvAD, and posterior-biparietal variant AD). The most common non-amnestic presentation in the high t-tau group was lvPPA. Mean CSF t-tau and phosphorylated tau levels were higher in patients with bvAD than in those with PCA, with lvPPA and AD-typ being associated with intermediate levels compared to PCA. CSF tau levels decreased in advanced stages of AD-typ. |
| 43 | Patients with bvAD presented anosognosia, and scored 2 standard deviations lower than patients with AD-typ in measures of executive function. They also presented marked decreases in information processing speed and planning, as well as cognitive rigidity and visuoconstructive deficits. | Patients with bvAD presented frontal hypoperfusion and white matter hyperintensities in the fronto-subcortical circuits compared to patients with AD-typ. |
| 44 | Patients with CBS presented mild cognitive problems with executive function and working memory, and Gerstmann syndrome with predominant agraphia. In terms of language, they presented agrammatism, paraphasia-type phonological errors, dysarthria, and oral apraxia. | CBS was associated with bilateral generalised hypoperfusion of the associative temporal-parietal cortex, precuneus, and posterior cingulate, predominantly on the left side. The authors also observed hypoperfusion in perirolandic, premotor, and dorsolateral prefrontal areas of the right hemisphere, with no involvement of the hippocampus or mesiotemporal areas. |
| 45 | The group of patients with likely AD-related CBS performed worse in attention, episodic memory, and visuospatial tasks due to preferential involvement of the parietal-dorsal stream, in addition to myoclonus and hallucinations, which were less frequent. | Patients with likely AD-related CBS presented temporoparietal hypometabolism, including the temporal-parietal association cortex, posterior cingulate, and cuneus, frequently presenting an asymmetric profile. |
AD: Alzheimer disease; AD-typ: typical variant of Alzheimer disease; BNT: Boston Naming Test; bvAD: behavioural/dysexecutive or frontal variant Alzheimer disease; CBS: corticobasal syndrome; CSF: cerebrospinal fluid; FTD: frontotemporal dementia; HATA: hippocampus-amygdala transition area; lvPPA: logopenic variant primary progressive aphasia; MoCA: Montreal Cognitive Assessment; PCA: posterior cortical atrophy; PET: positron emission tomography; PiB: Pittsburgh compound-B; PPA: primary progressive aphasia; Ref.: reference; ROCF: Rey-Osterrieth Complex Figure Test; WAB: Western Aphasia Battery.
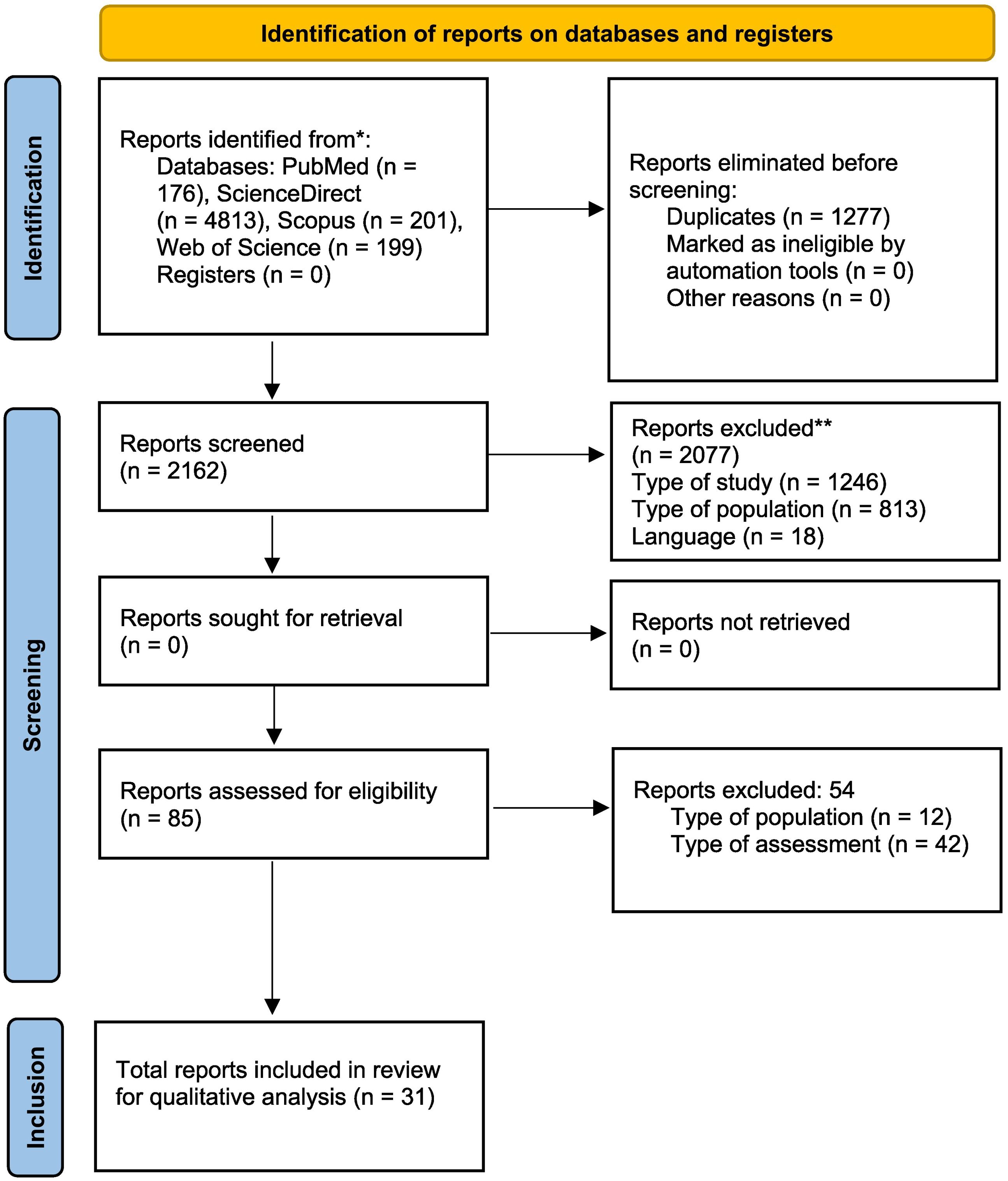
The article selection process is illustrated in the PRISMA flow chart (Fig. 1).13 We identified the titles and abstracts of 5389 articles, 1227 of which were duplicates. After applying the inclusion/exclusion criteria to the 2162 articles screened, 2077 articles were eliminated: 1246 due to the type of study, 813 due to the type of population, and 18 due to the language of publication. Thus, the full texts of 85 articles were analysed to assess their eligibility. Of these, 54 articles were eliminated: 12 due to the type of population and 42 due to the type of evaluation. Finally, a total of 31 articles were included for review.
PRISMA flow diagram.
*Consider, if feasible to do so, reporting the number of records identified from each database or register searched (rather than the total number across all databases/registers).
**If automation tools were used, indicate how many records were excluded by a human and how many were excluded by automation tools.
Table 1 shows the general characteristics of the 31 articles included for review.15–45 The years in which the highest number of articles were published were 2019 (5 articles), 2020 (5 articles), and 2021 (4 articles); in contrast, only 2 articles per year were published between 2011 and 2018, with the exception of 2014, in which 1 article was published. The total sample included 2133 elderly patients, with a mean age of 66.2 years. The most frequently used neuropsychological assessment tools and measures for the different profiles of AD included the Addenbrooke’s Cognitive Examination (ACE), Boston Naming Test (BNT), Clinical Dementia Rating Scale (CDR), F-A-S verbal fluency test (FAS), Mini–Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA), Rey-Osterrieth Complex Figure (ROCF), Trail-Making Test (TMT), Visual Object and Space Perception (VOSP) battery, and the Wechsler Adult Intelligence Scale (WAIS). The most commonly used imaging techniques were MRI, PET, and SPECT.
Neuropsychological profiles and neural correlatesTable 2 shows how the neuropsychological profiles of the different subtypes of AD share deficits in episodic memory, working memory, executive function, language (verbal fluency), and visuospatial/visuoconstructive skills. In turn, the neural correlates identified share patterns of atrophy in the frontal, temporoparietal, and occipital areas, as well as the cuneus and precuneus.
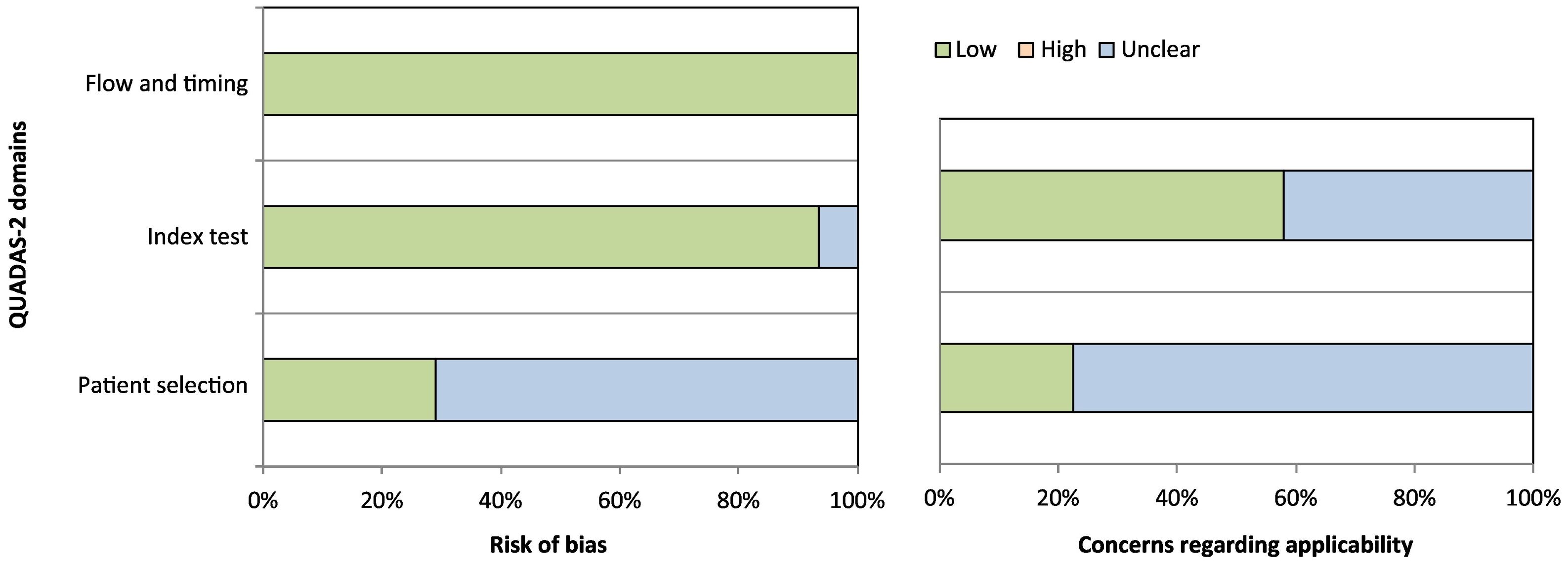
Evaluation of methodological quality and risk of biasThe 31 articles selected were analysed according to the QUADAS-2 guidelines (Fig. 2).14 In the bias analysis, all articles were categorised as low risk in the flow and timing domain; in the index test domain, 94% were classed as low risk and 6% as unclear risk; and in the patient selection domain, 71% were categorised as unclear risk and 20% as low risk. In turn, in the analysis of methodological quality, 58% presented high quality and 42% unclear quality for the applicability of results in the index test domain, while 77% of studies presented unclear quality and 23% high quality for applicability of results in the patient selection domain.
DiscussionOur review suggests the existence of neuropsychological symptoms common to AD-typ and the various atypical profiles, particularly in the areas of memory, executive function, and language. Global cognitive performance is also influenced by deficits in attention and visuospatial/visuoconstructive skills. The pattern of cortical atrophy in these clinical conditions shows a trend towards damage in frontal, temporal, parietal, and occipital areas, as well as marked atrophy of the cuneus and precuneus.
Neuropsychological profile of typical Alzheimer diseaseSeveral articles46–49 report similar findings regarding memory deficits in AD-typ, highlighting early difficulties with the registration and consolidation of verbal and visual information. Meng et al.48 propose using the Common Objects Memory Test (COMT)50 for the assessment of episodic memory, due to its sensitivity in early stages. The semantic and episodic memory deficits reported in AD-typ are consistent with those described in previous studies.51,52
Executive function deficits are common in the early stages of AD-typ, and have been proposed as a predictor of development and progression.53 Kashiwa et al.54 describe how executive dysfunction promotes significant deficits in the inhibitory response. One study emphasised that this dysfunction can also increase symptoms of apathy and even the appearance of affective disorders, complicating the initial diagnosis of AD-typ and bvAD.55
Semantic fluency is severely affected in AD-typ, unlike phonological fluency.56,57 Henry et al.51 note that phonological fluency does not constitute an additional characteristic in this disorder; however, other authors58–60 suggest that it should be considered a predictor in differential diagnosis. Recent studies61–63 suggest evaluating spontaneous language in early AD-typ, with specific emphasis on prolonged pauses during speech.
Neuropsychological profile of logopenic variant primary progressive aphasiaSimilarly to our own findings, Eikelboom et al.64 report that lvPPA presents with deficits in episodic and working memory. Win et al.65 suggest that the significant decline in verbal and autobiographical episodic memory is similar to that reported in AD-typ. Other studies66,67 suggest that the decline in short-term and working memory is minimal in early stages.
Studies suggest that executive function is frequently affected not only in lvPPA, but also in the semantic and non-fluent/agrammatic variants of PPA.68,69 Executive dysfunction in lvPPA affects information processing speed, sustained attention, and visuospatial/visuoconstructive skills.25,70–72 Similarly, Kamath et al.73 report that the magnitude of the deficit may be equally significant as that of linguistic alterations in lvPPA.
The most relevant deficits in lvPPA are problems with phonological fluency, phonological paraphasia, difficulties with sentence repetition and comprehension, and word-finding difficulties.74–76 Although our results are consistent with these difficulties, the literature also suggests an alteration in semantic fluency, similar to that reported in AD-typ.77,78
Neuropsychological profile of posterior cortical atrophyDeficits are reported in the coding and retrieval of verbal information in early stages of PCA,79–81 similar to those reported in AD-typ.82 Memory deficits are not limited to working memory and conceptual reasoning,83–85 but rather also affect episodic and autobiographical memory, which constitutes a challenge for differential diagnosis with AD-typ.86,87 Kas et al.36 showed that episodic memory performance in PCA is better in the first 3 years after diagnosis.
Putcha et al.40 report that the executive deficit in PCA has an impact on verbal episodic memory, and Li et al.88 note that it is more severe in PCA than in AD-typ, also reporting significant involvement of language and visuospatial, visuoconstructive, and visuoperceptual skills. Several other studies83,89–91 describe deficits in visuospatial skills in PCA, which in turn lead to such progressive disorders as visual agnosia,92 oculomotor alexia,93 acalculia, and simultanagnosia.94
Anomia is frequent and marked in PCA, manifesting early in the course of the disease.94,95 Migliaccio et al.96 found no significant differences in naming and phonological fluency performance between PCA and lvPPA. Similarly, Crutch et al.97 report alterations in both groups in auditory processing, repetition, and phonological fluency, with less severe involvement of spontaneous language and comprehension in PCA.
Neuropsychological profile of frontal or behavioural/dysexecutive variant Alzheimer diseaseLehingue et al.98 studied patients with bvAD and AD-typ, reporting poorer episodic memory performance in the latter group. Li et al.99 report a progressive deterioration of memory in bvAD, mainly affecting delayed recall; this is a key aspect of the differential diagnosis of bvAD and FTD, as its onset is early and severe in bvAD.100–103 In contrast, Ossenkoppele et al.104 suggest that bvAD and FTD do not present significant differences in memory performance.
Several studies7,43,103,105 suggest that the pronounced executive dysfunction in bvAD tends to be generalised and therefore has an impact on global cognitive performance. According to Woodward et al.,106 such instruments as the Frontal Assessment Battery may detect executive function alterations; however, this instrument is more effective for differentiating between bvAD and AD-typ than between bvAD and FTD.
Toloza-Ramírez et al.12 note that phonological fluency is severely affected in bvAD, whereas semantic fluency is preserved. These symptoms, in addition to the syntactic/grammatical alterations, are essential in differential diagnosis with AD-typ.
Neuropsychological profile of corticobasal syndromeShelley et al.107 suggest that episodic memory is impaired early in CBS. They also suggest that this form of memory impairment is essential to identifying this entity; in fact, Sakae et al.108 report that nearly 26% of patients with CBS present memory problems in early stages.
Several studies44,108–110 report significant impairment of visuospatial skills and the development of ideomotor apraxia. Boyd et al.111 indicate that VOSP subtests are sensitive and specific for detecting CBS, helping in differential diagnosis with AD-typ. In contrast, Constantinides et al.112 suggest that differential diagnosis of CBS should not only be based on neuropsychological testing but rather should also include cerebrospinal fluid analysis.
Language impairment is marked in CBS, and has been proposed as a biomarker for early diagnosis.113–115 Cotelli et al.116 report significant syntactic/grammatical impairment with preserved comprehension. Other studies95,117 emphasise that these deficits and verbal fluency contribute to the differential diagnosis of CBS and non-fluent/agrammatic variant PPA. Other reported symptoms are problems in phonological processing118 and action naming tasks,119 anomia,120 and semantic categorisation.121
Neural correlates of typical and atypical variants of Alzheimer diseasePatients with AD-typ present moderate alterations in limbic areas122 and marked atrophy of the precuneus and posterior cingulate gyrus.123–125 The heterogeneity of brain areas affected in AD-typ presents great challenges, as the classical neuropsychological symptoms do not constitute an “exclusivity” criterion for diagnosis.12,126 From a neuropathological perspective, a common criterion for identifying AD-typ is the early development of neurofibrillary tangles in the medial temporal lobe and hippocampal atrophy.127–129
In the context of lvPPA, Rohrer et al.130 report predominantly perisylvian and/or posterior parietal atrophy. Furthermore, the left inferior parietal lobe, left posterior temporal lobe, and temporoparietal junction are key areas that are vulnerable to neurodegeneration in lvPPA.131,132 Tau PET imaging suggests that the pattern of brain atrophy in lvPPA is not symmetrical.133 Another key aspect is atrophy of the hippocampus and anterior temporal lobe, which have been proposed as key findings in the differential diagnosis of lvPPA134,135 and frontotemporal lobar degeneration.133
PCA may hinder the diagnosis of AD, as neuropsychological symptoms and the pattern of brain atrophy overlap with such other profiles as AD-typ and lvPPA.81,94 In fact, our findings suggest that PCA presents significant atrophy of the occipital lobe, inferior and posterolateral temporal lobe, precuneus, and posterior cingulate cortex, areas involved in the diagnosis of AD-typ and lvPPA35; in contrast, Holden et al.90 argue that there are currently no specific biomarkers for the detection of PCA. Similarly, previous studies136,137 propose studying brain connections, as alterations in brain connectivity become more marked at more advanced stages of the disease, with greater white matter degeneration in the cingulate having a direct impact at the corticosubcortical level (frontostriatal connectivity). Indeed, Fredericks et al.138 note that changes to functional connectivity in PCA tend to be modulated by the participation of specific thalamic nuclei, such as the medial pulvinar nucleus.
The findings of our review suggest that the pattern of atrophy in CBS involves the superior temporal cortex, perirolandic region (motor and somatosensory cortex), temporoparietal region, hippocampus, subiculum, precuneus, and posterior cingulate. Previous studies44,108 have reported relative preservation of superior frontal regions, despite pronounced loss of volume in the occipital lobes and temporoparietal junction. Other studies112,139 report an asymmetrical pattern of hippocampal atrophy and significant hypoperfusion of the precuneus and posterior cingulate, as in AD-typ.
Finally, comparative studies of bvAD and AD-typ suggest that the former involves marked frontoparietal atrophy with relative preservation of medial temporal regions and subtle parietal atrophy.103,140 Stopford et al.141 suggest that the consideration of frontal hypometabolism as a biomarker in bvAD can lead to misdiagnosis as frontotemporal degeneration. Several other articles100,103,142,143 suggest that atrophy of the posterior cingulate, precuneus, hippocampus, medial temporal lobe, and anterior and posterior frontal regions should be analysed with caution due to the similarity with the patterns of atrophy reported in behavioural variant FTD.
Neuroimaging techniques: Diagnostic implicationsCurrent diagnostic criteria for typical and atypical AD focus on both molecular and neuroimaging evidence,144,145 based on such techniques as MRI, functional MRI (fMRI), and PET.
Graff-Radford et al.146 note that MRI achieves early identification of atrophy in AD-typ in medial and lateral temporal regions and in the parietal lobe. Furthermore, they report left temporoparietal atrophy in lvPPA, parieto-occipital atrophy in PCA, frontoparietal atrophy in bvAD, and pronounced atrophy of the left motor cortex in CBS. However, Toloza-Ramírez et al.12 note that while MRI identifies this atrophy in advanced stages, early identification of these profiles requires advanced neuroimaging techniques.
Functional MRI contributes to improving the precision of anatomical localisation of brain areas involved in cognitive/linguistic processing in typical and atypical AD by analysing changes in brain metabolism.147,148 Other lines of research149–152 focus on the role of fMRI in understanding AD-typ; however, the authors stress that their findings should be interpreted with caution due to the atypical profiles of AD.
PET imaging has also been used for the early identification of typical and atypical AD, detecting important biomarkers such as the tau and amyloid proteins.153 Tau-PET imaging is able to detect tau deposition in anatomical areas associated with atrophy in typical and atypical AD, and is sensitive in distinguishing different profiles. Amyloid PET imaging identifies amyloid deposition in AD-typ, especially in the neocortex and posteromedial cortex, as well as in the medial temporal cortex, sensorimotor cortex, and visual cortex. However, despite its increasingly widespread use, results should be analysed with caution as typical and atypical variants of AD tend to display similar patterns of amyloid deposition.154–156
It should be stressed that neuroscience research has increasingly focused on observing how the brain functions, using metabolic and haemodynamic tools (e.g., fMRI). In a study using dynamic causal modelling, Friston et al.157 stress the central role of functional integration in the study of disease, which should seek to understand not only isolated brain regions, but also the connections between them. Therefore, future lines of research should focus on studying patterns of effective connectivity in language and cognition, in both typical and atypical forms of AD.
LimitationsThis study does not present a meta-analysis of the articles reviewed; rather, we sought to provide a qualitative analysis. The literature search was limited to 4 databases. Future studies should expand the search to avoid bias in the selection and inclusion of articles. Finally, it should be noted that the age range of the study population was 60 years and older; however, current research suggests that early-onset AD should also be taken into account.158,159
Contributions and future perspectivesThe information presented in this review is relevant to both the clinical and research settings, summarising the neuropsychological symptoms and neural correlates of AD-typ, lvPPA, PCA, bvAD, and CBS. Our descriptions encourage caution in the diagnostic workup of these atypical profiles due to the considerable neuropsychological similarities between them. Visuospatial skills are proposed as a key domain for distinguishing between PCA and CBS. Language deficits are helpful in distinguishing lvPPA from CBS, whereas difficulties with working memory are vital in discriminating between bvAD and lvPPA. Neuropsychological assessment complemented with advanced neuroimaging studies is an essential approach in reaching an accurate diagnosis, thus improving treatment programmes for these entities.
Worldwide demographic changes and the increased prevalence of AD dementia constitute a challenge for proper diagnostic workup, given the heterogeneity of atypical variants. This review is intended to serve as a guideline for healthcare professionals involved in the management of patients with neurodegenerative diseases, as it provides a detailed neuropsychological and neuroanatomical profile. It should be noted that, to our knowledge, this review is the first to jointly address lvPPA, PCA, bvAD, and CBS, and to compare them to AD-typ. Another strength is that we offer important guidelines for improving the differential diagnosis of typical and atypical AD in clinical practice, as well as some suggestions on differentiation from other recurrent neurodegenerative disorders, such as FTD and non-fluent/agrammatic variant PPA.
ConclusionsThis study enabled the characterisation of AD-typ and 4 atypical presentations of AD (lvPPA, PCA, bvAD, and CBS) based on the construction of neuropsychological and neuroanatomical profiles, with a contribution on the clinical and differential diagnosis of this heterogeneous clinical picture. Regarding the neural correlates of the disease, our findings confirm the presence of marked atrophy of the hippocampus, cuneus, and precuneus, as well as involvement of frontal, temporal, parietal, and occipital areas, specific to each variant.
From a neuropsychological perspective, each variant showed similar patterns of pronounced impairment to episodic and working memory, executive function, attention, visuospatial/visuoconstructive skills, and language; this constitutes a hindrance to accurate diagnosis. The discrepancies in the current literature on typical and atypical profiles of AD are explained by the great heterogeneity of the instruments available for the assessment of cognitive and linguistic domains. Our findings may assist in the construction of new sensitive, specific batteries for the broad spectrum of AD, paying special attention to language from the perspective of verbal fluency and spontaneous language, which are considered important biomarkers.
Accurate differential diagnosis of typical and atypical variants of AD depends on the complementary use of advanced neuroimaging techniques, aiming not only to identify structural damage but also to assess the function of the affected areas, which may present atrophy early in the progression of the disease, even before a formal clinical diagnosis is reached.
FundingThis study has received no external funding of any kind.
Conflicts of interestThe authors have no conflicts of interest to declare.
This qualitative systematic review is part of the final project of Claudia Fredes-Roa, Felipe Gutiérrez-Barría, and Cristina Ramírez-Bruna, as part of their master’s degree in neuropsychology at Universidad Mayor, Chile. David Toloza-Ramírez thanks ANID-Subdirección de Capital Humano/Doctorado Nacional/2021-21212181. Dr Carolina Méndez-Orellana thanks the health sciences department of Pontificia Universidad Católica de Chile (project ID 190510002).