We report the case of a 17-year-old woman from the Dominican Republic who was diagnosed with sickle cell disease at the age of 9 months due to a vaso-occlusive crisis (VOC).
She attended the emergency department with severe abdominal and rib pain 72h after arriving in Spain. She reported no fever or other associated symptoms. Hemodynamics and peripheral oxygen saturation were normal. On physical examination, conjunctival jaundice and epigastric and right hypochondrial pain at palpation were noted.
Hemolytic markers (decreased haptoglobin, indirect hyperbilirubinemia, elevated LDH, and reticulocytosis), normocytic anemia (hemoglobin [Hb] 6.9g/dL) and mild leukocytosis with neutrophilia were detected. The peripheral blood smear showed anisopoikilocytosis, sickle cells, and polychromatophilia. Capillary electrophoresis revealed that sickle Hb (HbS) was 93.5%.
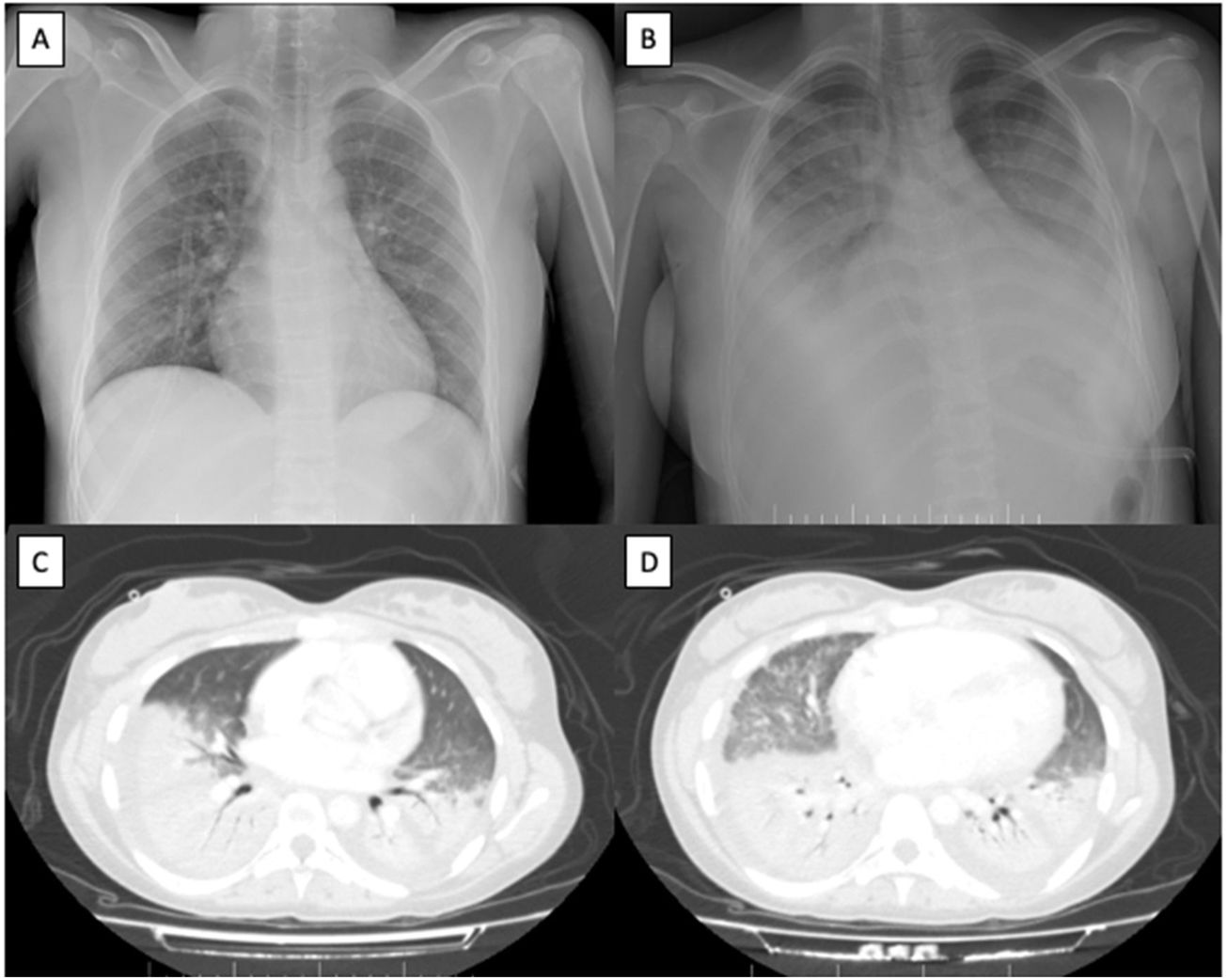
No pleuroparenchymal alterations were observed on the chest radiograph (Fig. 1A). Electrocardiogram and abdominal ultrasound were normal. The SARS-CoV-2 polymerase chain reaction test was positive. Given the suspicion of VOC and severe anemia, two red cell concentrates were transfused, and fluid replacement therapy, intravenous morphine and oxygen therapy with nasal cannulae were initiated.
Imaging tests. (A) Chest radiograph with no pleuroparenchymal alterations at admission. (B) Extensive bilateral pulmonary condensations on chest radiograph 48h after admission. (C and D) Extensive consolidative foci affecting the lower, middle and lingula lobes with air bronchogram and associated pleural effusion on computed tomography scan 48h after admission.
The patient suffered acute respiratory failure 48h after admission, requiring a Venturi mask at 8L of oxygen. She had fever spikes and elevated inflammatory parameters were detected; accordingly, empirical antibiotic therapy and intravenous remdesivir were initiated. Chest radiograph showed extensive bilateral pulmonary condensations (Fig. 1B) and computed tomography demonstrated extensive consolidative foci affecting the lower, middle and lingula lobes with associated pleural effusion (Fig. 1C and D).
Given her adverse progress, the patient was transferred to the intermediate respiratory care unit for monitoring and respiratory support with high-flow oxygen therapy. In view of her history and clinical and radiological progress, she was diagnosed with acute chest syndrome (ACS) triggered by SARS-CoV-2.
Since the patient was worsening and HbS was above 90%, a red blood cell exchange was performed. After the procedure, consisting of a replacement volume of 1292–1488mL and 30–32% of desired final HbS, the patient improved significantly and oxygen therapy was de-escalated. She was discharged after few days with outpatient follow-up.
We used remdesivir because it acts as an analog of adenosine triphosphate (ATP) and competes with natural ATP substrate for incorporation into nascent RNA chains by RNA-polymerase, which contributes in the production of RNA SARS-CoV-2, resulting in delayed chain termination during viral RNA replication. Remdesivir is indicated in children older than 12 years and adults with COVID-19, regardless of disease severity.1 Since the patient continued to worsen at an alarming rate with increasing oxygen requirements and because HbS has a lower affinity for oxygen, we decided to perform a red blood cell exchange with the aim of improving her severe hypoxia.
ACS is the most common cause of death in adults with sickle cell disease and the second leading cause of hospitalization. It is defined as a pulmonary infiltrate accompanied by fever, chest pain, tachypnea, cough, hypoxemia and wheezing. Patients diagnosed with ACS are at increased risk of recurrence. The most common causes are VOC, usually triggered by infections (bacterial, viral and/or fungal, including SARS-CoV-2).2–4
The pathophysiology of ACS is based on occlusion within the pulmonary microvasculature. Triggering events precipitate deoxygenation of HbS, its polymerization and the formation of sickle cells, which cause vascular occlusion, ischemia and resulting endothelial lesion. The clinical presentation is non-specific. As observed in our case, radiological involvement usually does not appear until some days after admission. Cardiorespiratory monitoring is therefore essential. It is common to find basal predominant pulmonary consolidations and ground-glass opacities. Lung ischemia may cause fibrosis and pulmonary hypertension in 4% of patients with ACS.5,6
In summary, ACS is a rare disease in which a high rate of suspicion in the appropriate clinical context is key. We would like to emphasize the importance of early diagnosis, including the determination of the percentage of HbS, and early treatment, which probably saved our patient's life. We recommend close follow-up of these patients and monitoring of symptoms and HbS levels, in order to prevent VOC and ACS episodes. Transfusion therapy should be done every 4–6 weeks to maintain an HbS percentage below 50%, but ideally below 30%.
For further consideration, it would probably be interesting to investigate future alternative therapies. It is of utmost importance to provide the best possible respiratory support and hemodynamic treatment until red blood cell exchange can be performed.
Informed consentInformed consent has been obtained from the patient.
FundingNo funding has been required.
Authors’ contributionsAll four authors were similarly involved in the development of the project. The order of appearance has been determined by their active participation in the management of the patient.
Conflicts of interestAuthors report no conflict of interest.