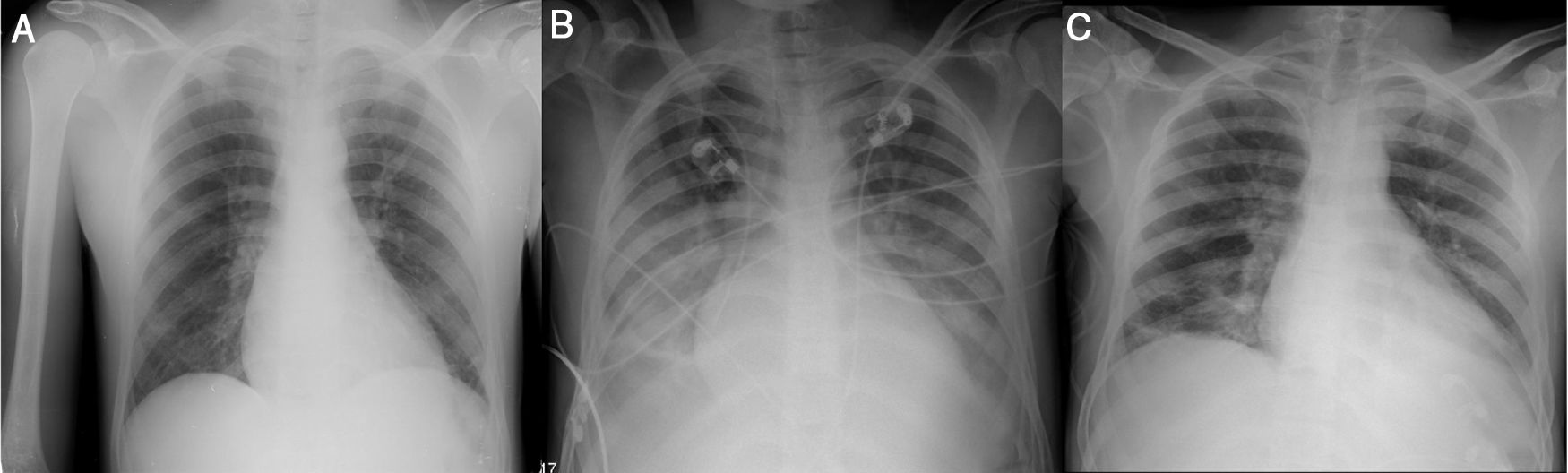
The authors describe a case of an acute chest syndrome (ACS) in whom fast recognition of the diagnosis and fast referral to the Intensive Care Unit (ICU) with early instituition of exchange transfusion (ExT) were fundamental to avoid the need for invasive mechanical ventilation (IMV) and clinical worsening.
A 26-year-old black man with sickle cell disease (SCD, homozygous) on regular treatment with hydroxyurea presented to the emergency department with a worsening 5-day pleuritic chest pain of sudden onset and nonproductive cough. The physical examination was normal. Laboratory tests revealed hemoglobin (Hb) 10.4g/dL (his basal), normal platelet count (307×109/L), leukocytosis (16.26×109/L) and C-reactive protein (CRP) 1.8mg/dL. Chest radiography (CxR) showed no consolidation (Fig. 1A). He was treated with intravenous (IV) hydration, paracetamol, diclofenac, tramadol and morphine 20mg with no control of pain. With the diagnosis of vaso-occlusive crisis, he was admitted in Internal Medicine ward. He mantained IV hydration, IV opioids and antibiotic therapy (AB) was started with amoxicilin/clavulanic acid 1.2g tid. Within 72h the patient showed a progressive clinical worsening with fever (38.5°C), uncontrolled chest pain with perfusion of morphin, purulent sputum, dyspnea, type I respiratory failure (FiO2 40% by venturi mask, PaO2/FiO2=217mmHg), CRP 29.1mg/dL, decrease in Hb (7.5g/dL, HbS 74%) with hemolysis (LDH 2299U/L, total bilirrubin 5.51mg/dL, direct bilirrubin 2.04mg/dL), thrombocytopenia (118×109/L) and new bilateral pulmonary infiltration on CxR (Fig. 1B). With the diagnosis of ACS he was transferred to the ICU where he underwent two ExT with removal of 450 cc of blood from internal jugular vein catheter and blood replacement after with good clinical tolerance. Within 24h HbS reduced to 58.9% (Hb 8.8g/dL) with improvement of chest pain and respiratory failure (FiO2 40% by venturi mask, PaO2/FiO2=300mmHg). The FiO2 was progressively reduced, IV hydration and pain control were mantained and AB was switched to piperacilin/tazobactam 4.5g q6h and azythromycin 500mg once daily. Blood cultures and urine antigen tests were negative. Haemophilus influenzae was isolated from sputum culture.
The clinical, laboratory (Hb 9.4g/dL, HbS 54.9%, LDH 866U/L, total bilirrubin 0.98mg/dL, direct bilirrubin 0.58mg/dL, platelets 303×109/L, CRP 4.57) and radiological courses were favorable (Fig. 1C). He was discharged after completing the AB.
SCD is a genetic disorder affecting Hb caused by a single mutation in the gene encoding the β-globin chain which formes HbS. The homozygous form is the most common form.1 HbS is less soluble than normal Hb. On desoxygenation it polymerizes within the cell inducing a sickle shape of red blood cells (RBC). Their shorter lifespan promotes hemolysis within the vessels and they occlude small and sometimes larger vessels causing vaso-oclusive events responsible for acute episodes of severe pain, stroke, avascular necrosis, kidney, hepato-billiary and pulmonary complications.1
ACS is a distinctive complication and the leading cause of death of SCD.2 ACS is characterized by new pulmonary infiltrate in CxR consistent with alveolar consolidation plus either cough, fever, hypoxia, chest pain or dyspnea.
Our patient met the criteria for the diagnosis but with late radiologic findings. In fact, most patients are admitted with vaso-occlusive crises with no radiographic and clinical symptoms and develop ACS after a few days.2,3 A single physical examination or radiograph may not be adequate for early diagnosis. Therefore it should be strongly suspected when pulmonary symptoms and signs occur even with normal CxR findings specially in an isolated episode of vaso-occlusive crisis.
The different causes of ACS may coexist progressing to a common final pathway of reduced ventilation with hypoxia and increased sickling. One of them is pulmonary infection caused most frequently by Chlamydia, Mycoplasma and viral infections.2 On the other hand, severe vaso-occlusive crisis of multiple bones can cause fat embolism with pulmonary vascular obstruction and pulmonary infarction. When it affects the thorax may cause regional alveolar hypoventilation.4,5
ACS can rapidly progress to severe respiratory failure within 24h and severe hypoxia is a predictor of poor outcome.6 Radiographic evidence of extensive lobar involvement and platelet count inferior to 200000/mm3 at diagnosis were found to be as well independent predictors of respiratory failure.3 Although no hemodynamic impairment was reported in the patient, the occurrence of shock is not uncommon. Thus vigilance should be maintained closely for early treatment.
The aim of treatment is to prevent or reverse acute respiratory failure.The mainstays of management are pain control, rehydration, oxygenation with close monitoring for worsening respiratory failure with possibility of invasive mechanical ventilation (IMV) and treatment of any identifiable precipitating cause. The clinical presentation is similar whether due to infectious or non-infections causes whereby empirical AB should be initiated with coverage for atypical bacteria. Adequate pain control usually requires initially high doses of opiates. IV hydration is preferred when patient is not able to drink adequate amounts aiming for 3–4l a day. However, it should be taken carefully to not cause fluid overload which will exacerbate lung injury.
A simple transfusion or an ExT with removal of the patient's own blood and transfusing blood cause improvements in clinical and radiologic outcomes in the hypoxic patient.7 Yet ExT seems to be more beneficial in severe clinical features, namely worsening hypoxia and dyspnea, decreasing Hb, multilobal disease and neurological complications.6 ExT is a potentially life-saving procedure that allows improvement of anemia by replacing sickle cells without increasing blood viscosity and tissue oxygenation whilst reducing microvascular sickling. The target Hb should be 10–11g/dL7 but there is no evidence for an optimal volume of RBC exchange and/or HbS% target. ExT can be done manually with minimal equipment in ICU. In our patient, the amount of RBC to be exchanged was decided based on the clinical response to the treatment.
The fast clinical's deterioration and the delayed admission to the ICU are responsible to ACS as the leading mortality cause of SCD.4 Therefore we want to alert the clinicians to maintain tight clinical vigilance in these patients for early recognition of ACS as potential complex and near-fatal condition. The fast ICU referral, even if no organ failure is present, with appropriate therapeutic modalities prevents as well the needing of IMV and its associated complications.
Conflicts of interestThe authors have no conflicts of interest to declare.