Lithiasis in renal graft recipients might be a dangerous condition with a potential risk of organ function impairment.
Evidence acquisitionA systematic literature search was conducted through February 2023. The primary objective was to assess the incidence of lithiasis in kidney transplant (KT) recipients. The secondary objective was to assess the timing of stone formation, localization and composition of stones, possible treatment options, and the incidence of graft loss.
Evidence synthesisA total of 41 non-randomized studies comprising 699 patients met our inclusion criteria. The age at lithiasis diagnosis ranged between 29–53 years. Incidence of urolithiasis ranged from 0.1–6.3%, usually diagnosed after 12 months from KT. Most of the stones were diagnosed in the calyces or in the pelvis. Calcium oxalate composition was the most frequent. Different treatment strategies were considered, namely active surveillance, ureteroscopy, percutaneous/combined approach, or open surgery. 15.73% of patients were submitted to extracorporeal shock wave lithotripsy (ESWL), while 26.75% underwent endoscopic lithotripsy or stone extraction. 18.03% of patients underwent percutaneous nephrolithotomy whilst 3.14% to a combined approach. Surgical lithotomy was performed in 5.01% of the cases. Global stone-free rate was around 80%.
ConclusionsLithiasis in kidney transplant is a rare condition usually diagnosed after one year after surgery and mostly located in the calyces and renal pelvis, more frequently of calcium oxalate composition. Each of the active treatments is associated with good results in terms of stone-free rate, thus the surgical technique should be chosen according to the patient’s characteristics and surgeon preferences.
La litiasis en el receptor del injerto renal puede ser una enfermedad peligrosa cuyo riesgo potencial es el deterioro de la función renal.
Adquisición de la evidenciaSe realizó una búsqueda sistemática de la literatura hasta febrero de 2023. El objetivo primario era evaluar la incidencia de litiasis en receptores de trasplante renal (TR). El objetivo secundario era evaluar el momento de formación, la localización y la composición de la litiasis, las opciones de tratamiento disponibles y la incidencia de la pérdida del injerto.
Síntesis de la evidenciaUn total de 41 estudios no aleatorizados compuestos por 699 pacientes cumplieron los criterios de inclusión. La edad en el momento del diagnóstico de litiasis oscilaba entre 29 y 53 años. La incidencia de urolitiasis se encontraba entre el 0,1 y 6,3%, siendo diagnosticada generalmente a los 12 meses del TR. La mayoría de litiasis diagnosticadas se localizaron en los cálices o en la pelvis. La composición más frecuente fue la de oxalato cálcico. Se consideraron diferentes estrategias de tratamiento como vigilancia activa, ureteroscopia, abordaje percutáneo/combinado o cirugía abierta. Del total de pacientes, 15,73% fueron sometidos a litotricia extracorpórea por ondas de choque (LEOCh) y 26,75% se sometieron a litotricia endoscópica o extracción quirúrgica. De estos sujetos, 18,03% se abordaron mediante nefrolitotomía percutánea, mientras que el 3,14% se sometieron a un abordaje combinado. Se realizó litotomía quirúrgica en 5,01% de los casos. La tasa libre de litiasis (TLL) global se situó en torno al 80%.
ConclusionesLa litiasis en el trasplante renal es una patología poco frecuente que suele diagnosticarse al año de la cirugía. Su localización más frecuente son los cálices y la pelvis renal, y en la mayoría de los casos está compuesta de oxalato cálcico. Todos los tratamientos activos han demostrado resultados satisfactorios en términos de TLL, por lo que la elección de la técnica quirúrgica se debe basar en las características del paciente y las preferencias del cirujano.
Renal transplant graft lithiasis might be a dangerous scenario with a potential risk of organ failure and graft loss.1,2 In fact, due to the partial denervation of the graft, the typical cholic flank pain associated with lithiasis formation may be missed, leading to a late diagnosis of graft hydronephrosis and functional impairment. Furthermore, it is a challenging condition because it involves a single-functioning kidney in patients who often have high comorbidity status that can lead to severe complication as sepsis. Treatment of de novo lithiasis in kidney transplant (KT) recipients also presents many technical challenges owing to the heterotopic position of the renal graft on the iliac vessels and the diverse urinary anastomosis techniques. Only small and retrospective series are available in the Literature reporting the incidence and characteristics of urolithiasis in transplanted kidneys.3 Consequently, there is a lack of high-level evidence regarding the correct management of these patients. Therefore, we performed a systematic review to assess the incidence of lithiasis in KT recipients, the type of stone composition, and the more frequent used treatment.
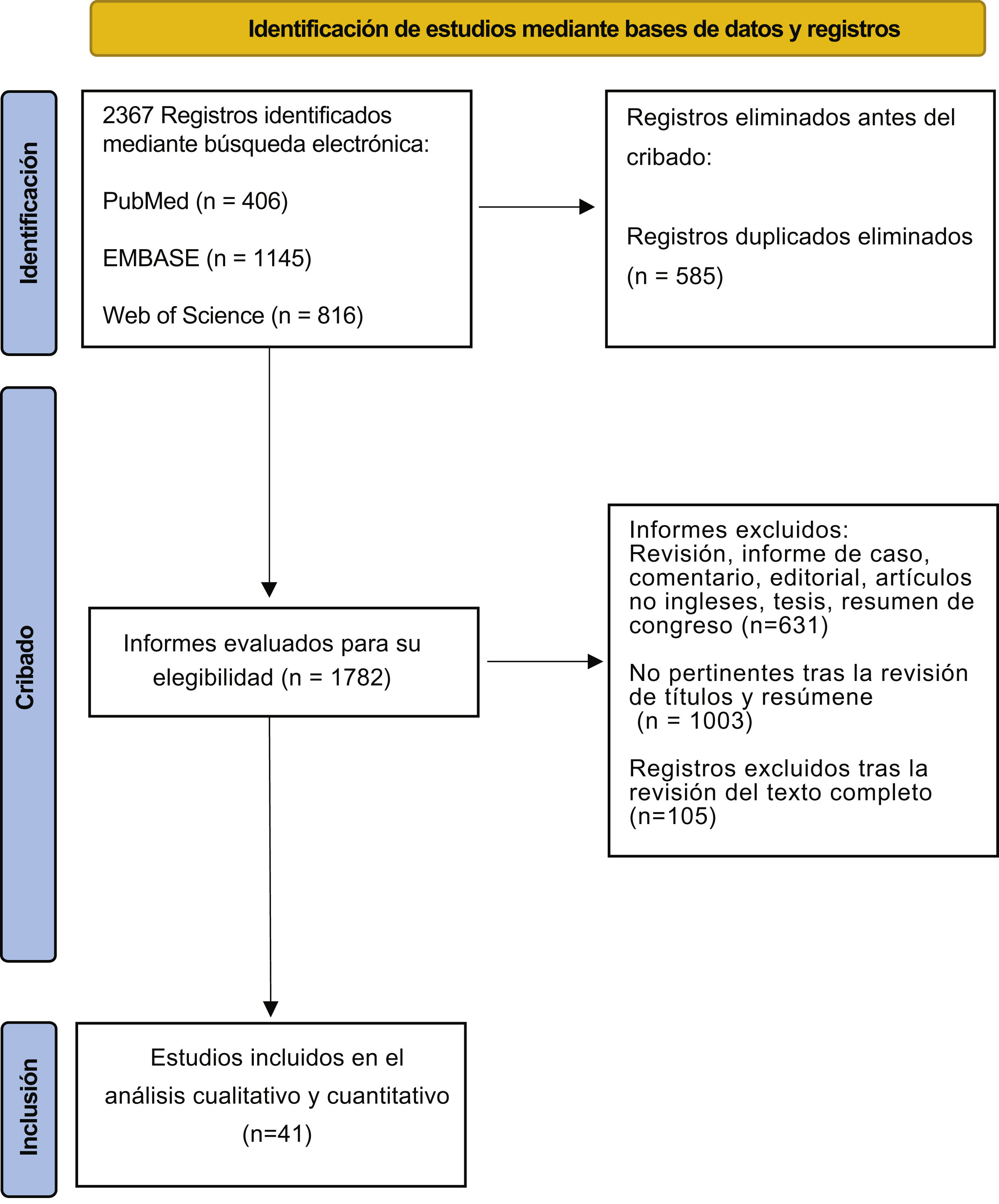
Materials and methodsSearch strategyWe conducted a systematic review in line with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines4 (Fig. 1). This protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (Registration Number: CRD42023409259). The literature search was performed in PubMed/Medline, Embase, and Web of Science databases, to identify reports published through February 2023 reporting on lithiasis of kidney transplant recipients. The search strategy used the combination of the following terms grouped according to the Boolean operators (AND, OR, NOT): kidney transplant, renal transplant, stone, lithiasis. The primary objective of this systematic review is to assess the incidence of lithiasis in kidney transplant (KT) recipients. The secondary objective is to assess the timing of stone formation, localization and composition of stones, possible treatment options, and the incidence of graft loss.
Study selectionStudies were deemed eligible if they included patients who underwent renal transplantation developing urolithiasis in the graft (P), submitted to active treatment (I) or conservative management (C) in order to assess the incidence of lithiasis, the time of stone formation, the composition of the stone, peri-operative outcomes, and graft loss (O). All articles with data of interest were selected: only those articles pertinent with PICO strategy, full text in English were included. Abstracts, editorials, commentaries, reviews, book chapters, non-English language, single case reports and articles reporting experimental studies on animals or cadavers were excluded.
Data extractionThe articles were independently reviewed by two of the authors (A.P. and G.B.) on the basis of inclusion and exclusion criteria. Titles and abstracts were analyzed. After this initial screening, a full-text review was conducted to confirm the selected articles’ eligibility for inclusion. Finally, references from the selected articles were reviewed in order to identify other possible sources of data. Disagreements regarding study selection were resolved by a third reviewer (A.T.).
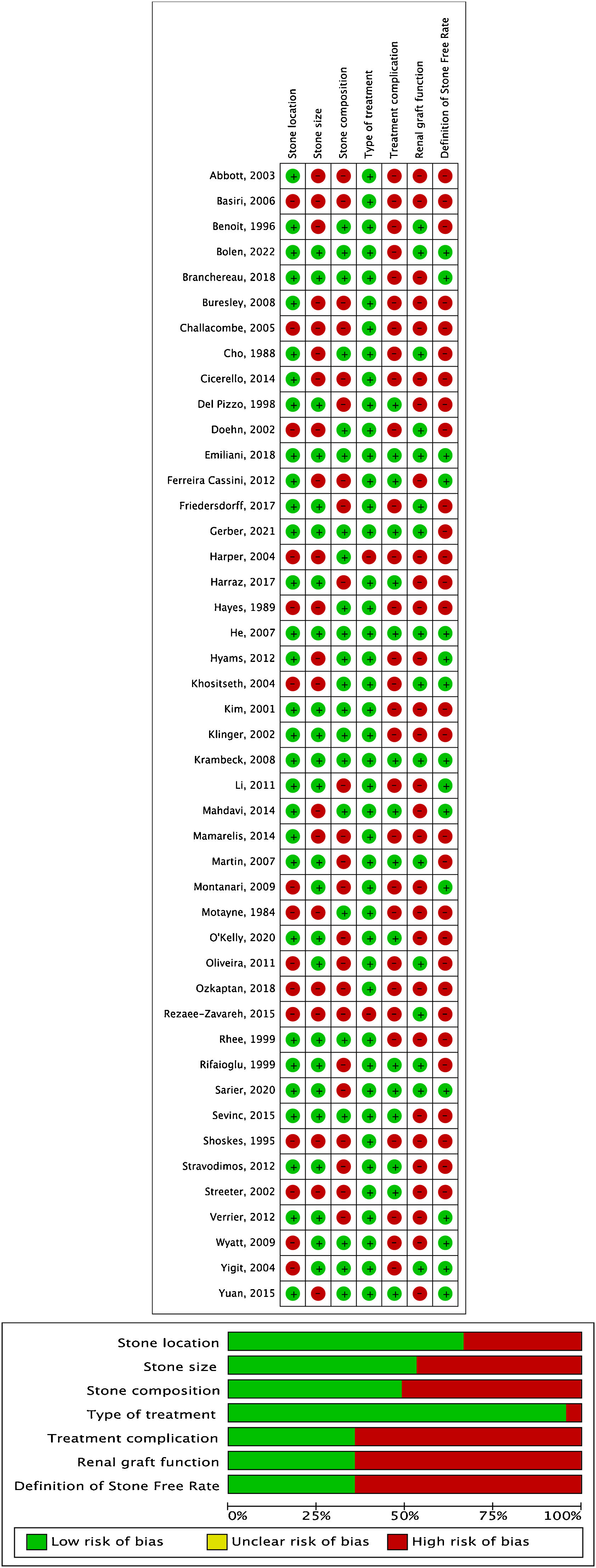
Risk-of-Bias assessmentThe risk of bias assessment was performed independently by two authors (T. P. and A.P.) using the Prediction Model Study Risk of Bias Assessment Tool (PROBAST).5 The eventual disagreement was solved by a third author (A. T.). The risk of bias was measured over four domains of interest (participants, predictors, outcome, and analysis) (Fig. 2).
Data extraction and analysisBaseline demographics (age, body mass index, prostate specific antigen (PSA), cause of end stage renal disease (ESRD)), peri-operative variables (operating time, estimated blood losses [EBL], complications, length of stay), postoperative complications (>30 post-operative days) were recorded whenever available.
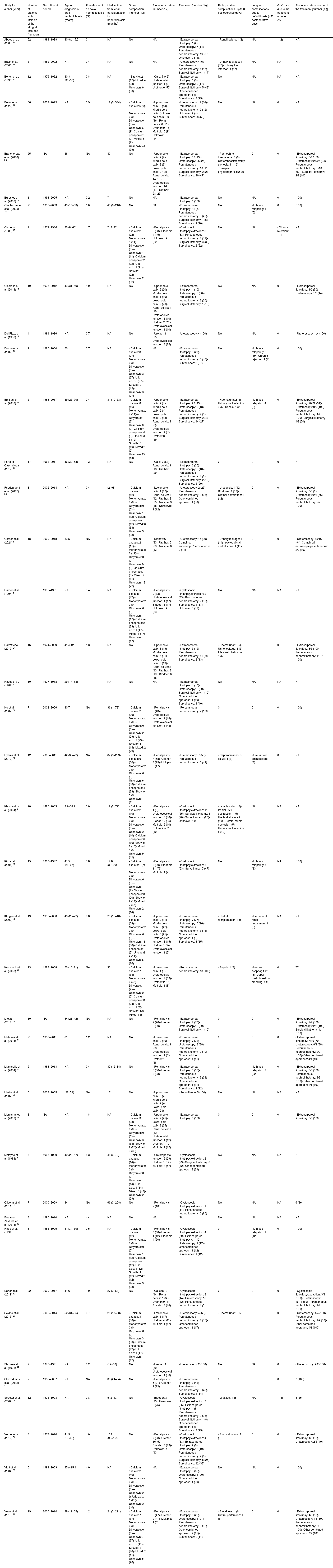
Evidence synthesisStudy characteristicsThe Literature search included 2367 records. After screening and eligibility assessment, 41 studies met the inclusion criteria. In total, 699 KT recipients with urolithiasis were included with an age at the diagnosis ranging between 29 and 53 years-old.6,7Table 1 summarizes data extracted from each study. One of the studies included in this review was conducted on a pediatric population with a median age of 9.2 (standard deviation 4.7) years.8 The study publication year varied considerably, ranging from 19849 to 2021.6 In addition, all the studies included single-center retrospective cohorts.
Characteristics of studies included in the systematic review.
| Study first author (year) | Number of patients with lithiasis of the allograft included (number) | Recruitment period | Age on diagnosis of graft nephrolithiasis (years) | Prevalence of de novo nephrolithiasis (%) | Median time from renal transplantation to nephrolithiasis (months) | Stone composition [number (%)] | Stone localization [number (%)] | Treatment [number (%)] | Peri-operative complications (up to 30 postoperative days) | Long term complications due to nefrolithiasis (>30 postoperative days) | Graft loss due to the treatment number (%) | Stone free rate according to the treatment [number (%)] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbott et al. (2003) 14 | 52 | 1994−1998 | 40.8+/-15.6 | 0.1 | NA | NA | NA | - Extracorporeal lithotripsy: 1 (2)- Ureteroscopy: 7 (14)- Percutaneous nephrolithotomy: 19 (37)- Unknown: 25 (48) | - Renali failure: 1 (2) | NA | 1 (2) | NA |
| Basiri et al. (2006) 15 | 6 | 1989−2002 | NA | 0.4 | NA | NA | NA | - Ureteroscopy: 4 (67)- Percutaneous nephrolithotomy: 1 (17)- Surgical litothomy: 1 (17) | - Urinary leakage: 1 (17)- Urinary tract infection: 1 (17) | NA | NA | NA |
| Benoit et al. (1996) 13 | 12 | 1976−1992 | 40.3 (30−50) | 0.8 | NA | - Struvite: 2 (17)- Mixed: 4 (33)- Unknown: 6 (50) | - Calix: 5 (42)- Ureteropelvic junction: 1 (8)- Urether: 6 (50) | - Extracorporeal lithotripsy: 1 (8)- Ureteroscopy: 2 (17)- Surgical litothomy: 5 (42)- Other combined approach: 1 (8)- Surveillance: 3 (25) | NA | NA | NA | NA |
| Bolen et al. (2022) 12 | 56 | 2009−2019 | NA | 0.9 | 12 (0−384) | - Calcium oxalate: 6 (9)--- Monohydrate: 0 (0)--- Dihydrate: 0 (0)--- Unknown: 6 (9)- Calcium phosphate: 1 (2)- Mixed: 5 (9)- Unknown: 44 (79) | - Upper pole calix: 8 (14)- Middle pole calix: ()- Lower pole calix: 20 (36)- Renal pelvis: 6 (11)- Urether: 9 (16)- Multiple: 5 (9)- Unknown: 8 (14) | - Ureteroscopy: 19 (34)- Percutaneous nephrolithotomy: 7 (12)- Unknown: 2 (4)- Surveillance: 28 (50) | NA | NA | 0 | NA |
| Branchereau et al. (2018) 38 | 95 | NA | 48 | NA | 40 | NA | - Upper pole calix: 7 (7)- Middle pole calix: 3 (3)- Lower pole calix: 27 (28)- Renal pelvis: 14 (15)- Ureteropelvic junction: 16 (17)- Urether: 28 (29) | - Extracorporeal lithotripsy: 12 (13)- Ureteroscopy: 25 (26)- Percutaneous nephrolithotomy: 10 (11)- Surgical litothomy: 2 (2)- Surveillance: 46 (47) | - Perinephric haematoma: 8 (8)-Ureteroneocistostomy stenosis: 11 (12)- Transplant phyelonephritis: 2 (2) | 0 | 0 | - Extracorporeal lithotripsy: 6/12 (50)- Ureteroscopy: 21/25 (84)- Percutaneous nephrolithotomy: 9/10 (90)- Surgical litothomy: 2/2 (100) |
| Buresley et al. (2008) 11 | 1 | 1993−2005 | NA | 0.2 | 7 | NA | NA | - Extracorporeal lithotripsy: 1 (100) | NA | NA | 0 | (100) |
| Challacombe et al. (2005) 16 | 21 | 1997−2003 | 43 (15−63) | 1.0 | 43 (6−216) | NA | NA | - Extracorporeal lithotripsy: 12 (57)- Percutaneous nephrolithotomy: 6 (29)- Surgical litothomy: 1 (5)- Surveillance: 2 (10) | NA | - Lithiasis relapsing: 1 (5) | 0 | (100) |
| Cho et al. (1988) 17 | 9 | 1972−1986 | 30 (8−65) | 1.7 | 7 (3−42) | - Calcium oxalate: 2 (22)--- Monohydrate: 1 (11)--- Dihydrate: 0 (0)--- Unknown: 1 (11)- Calcium phosphate: 2 (22)- Uric acid: 1 (11)- Struvite: 2 (22)- Unknown: 2 (22) | - Renal pelvis: 3 (33)- Bladder: 4 (45)- Unknown: 2 (22) | - Cystoscopic lithotripsy/extraction: 3 (33)- Percutaneous nephrolithotomy: 1 (11)- Surgical litothomy: 3 (33)- Surveillance: 2 (22) | NA | NA | - Chronic rejection: 1 (11) | NA |
| Cicerello et al. (2014) 18 | 10 | 1995−2012 | 43 (31−59) | 1.0 | NA | NA | - Upper pole calix: 2 (20)- Middle pole calix: 1 (10)- Lower pole calix: 2 (20)- Renal pelvis: 1 (10)- Ureteropelvic junction: 1 (10)- Urether: 2 (20)- Ureterovescical junction: 1 (10) | - Extracorporeal lithotripsy: 1 (10)- Ureteroscopy: 6 (60)- Percutaneous nephrolithotomy: 2 (20)- Surgical litothomy: 1 (10) | NA | NA | 0 | - Extracorporeal lithotripsy: 1/2 (50)- Ureteroscopy: 1/7 (14) |
| Del Pizzo et al. (1998) 19 | 4 | 1991−1996 | NA | 0.7 | NA | NA | - Urether: 1 (25)- Ureterovescical junction: 3 (75) | - Ureteroscopy: 4 (100) | NA | NA | 0 | - Ureteroscopy: 4/4 (100) |
| Doehn et al. (2002) 20 | 11 | 1985−2000 | 50 | 0.7 | NA | - Calcium oxalate: 3 (27)--- Monohydrate: 0 (0)--- Dihydrate: 0 (0)--- Unknown: 3 (27)- Uric acid: 3 (27)- Struvite: 2 (19)- Unknown: 3 (27) | NA | - Extracorporeal lithotripsy: 3 (27)- Percutaneous nephrolithotomy: 5 (46)- Surveillance: 3 (27) | NA | - Lithiasis relapsing: 2 (19)- Chronic rejection: 1 (9) | 0 | (100) |
| Emiliani et al. (2018) 21 | 51 | 1983−2017 | 49 (26−70) | 2.4 | 31 (10−63) | - Calcium oxalate: 8 (16)--- Monohydrate: 7 (14)--- Dihydrate: 1 (2)--- Unknown: 0 (0)- Calcium phosphate: 4 (8)- Uric acid: 6 (12)- Struvite: 5 (10)- Mixed: 1 (2)- Unknown: 27 (53) | - Upper pole calix: 2 (4)- Middle pole calix: 2 (4)- Lower pole calix: 9 (18)- Renal pelvis: 4 (8)- Ureteropelvic junction: 2 (4)- Urether: 30 (59) | - Extracorporeal lithotripsy: 22 (43)- Ureteroscopy: 9 (18)- Percutaneous nephrolithotomy: 4 (8)- Surgical litothomy: 2 (4)- Surveillance: 14 (27) | - Haematuria: 2 (4)- Urinary tract infection: 3 (6)- Sepsis: 1 (2) | - Lithiasis relapsing: 4 (8) | 0 | - Extracorporeal lithotripsy: 20/22 (91)- Ureteroscopy: 9/9 (100)- Percutaneous nephrolithotomy: 4/4 (100)- Surgical litothomy: 1/2 (50) |
| Ferreira Cassini et al. (2012) 22 | 17 | 1968−2011 | 46 (32−63) | 1.3 | NA | NA | - Calix: 9 (53)- Renal pelvis: 3 (18)- Urether: 5 (29) | - Extracorporeal lithotripsy: 6 (35)- Ureteroscopy: 3 (18)- Percutaneous nephrolithotomy: 1 (6)- Surgical litothomy: 2 (12)- Surveillance: 5 (29) | 0 | 0 | 0 | NA |
| Friedersdorff et al. (2017) 23 | 8 | 2002−2014 | NA | 0.4 | (2−98) | - Calcium oxalate: 1 (12)--- Monohydrate: 0 (0)--- Dihydrate: 0 (0)--- Unknown: 1 (12)- Calcium phosphate: 1 (12)- Mixed: 3 (38)- Unknown: 3 (38) | - Lower pole calix: 1 (12)- Renal pelvis: 1 (12)- Urether: 2 (25)- Multiple: 3 (38)- Unknown: 1 (12) | - Ureteroscopy: 2 (25)- Percutaneous nephrolithotomy: 2 (25)- Other combined approach: 4 (50) | - Urosepsis: 1 (12)- Blod loss: 1 (12)- Urether perforation: 1 (12) | 0 | 0 | - Extracorporeal lithotripsy: 0/3 (0)- Ureteroscopy: 2/3 (66)- Percutaneous nephrolithotomy: 2/2 (100) |
| Gerber et al. (2021) 6 | 18 | 2009−2018 | 53.5 | NA | NA | - Calcium oxalate: 2 (11)--- Monohydrate: 2 (11)--- Dihydrate: 0 (0)--- Unknown: 0 (0)- Calcium phosphate: 1 (5)- Mixed: 2 (11)- Unknown: 13 (72) | - Kidney: 6 (33)- Urether: 6 (33)- Multiple: 6 (33) | - Ureteroscopy: 16 (89)- Combined endoscopic/percutaneous: 2 (11) | - Urinary leakage: 1 (11)- Ipacted distal uretral stone: 1 (11) | 0 | 0 | - Ureteroscopy: 15/16 (94)- Combined endoscopic/percutaneous: 2/2 (100) |
| Harper et al. (1994) 1 | 6 | 1990−1991 | NA | 3.4 | NA | - Calcium oxalate: 1 (17)--- Monohydrate: 0 (0)--- Dihydrate: 0 (0)--- Unknown: 1 (17)- Calcium phosphate: 2 (33)- Uric acid: 1 (17)- Mixed: 1 (17)- Unknown: 1 (17) | - Renal pelvis: 2 (33)- Ureterovescical junction: 1 (17)- Bladder: 1 (17)- Unknown: 2 (33) | - Cystoscopic lithotripsy/extraction: 2 (33)- Percutaneous nephrolithotomy: 2 (33)- Surveillance: 1 (17)- Unknown: 1 (17) | NA | NA | NA | NA |
| Harraz et al. (2017) 24 | 16 | 1974−2009 | 41+/-12 | 1.3 | NA | NA | - Upper pole calix: 3 (19)- Middle pole calix: 5 (31)- Lower pole calix: 3 (19)- Renal pelvis: 2 (13)- Urether: 3 (19)- Bladder: 6 (38) | - Extracorporeal lithotripsy: 3 (19)- Percutaneous nephrolithotomy: 11 (68)- Surveillance: 2 (13) | - Haematuria: 1 (6)- Urine leakage: 1 (6)- Intestinal obstruction: 1 (6) | 0 | 0 | - Extracorporeal lithotripsy: 3/3 (100)- Percutaneous nephrolithotomy: 11/11 (100) |
| Hayes et al. (1989) 7 | 10 | 1977−1988 | 29 (17−53) | 1.1 | NA | NA | NA | - Extracorporeal lithotripsy: 1 (10)- Ureteroscopy: 3 (30)- Surgical litothomy: 1 (10)- Other combined approach: 1 (10)- Surveillance: 4 (40) | NA | NA | NA | NA |
| He et al. (2007) 39 | 7 | 2002−2006 | 40.7 | NA | 36 (1−72) | - Calcium oxalate: 2 (29)--- Monohydrate: 0 (0)--- Dihydrate: 0 (0)--- Unknown: 2 (29)- Uric acid: 2 (29)- Struvite: 1 (14)- Mixed: 2 (29) | - Renal pelvis: 3 (43)- Ureteropelvic junction: 1 (14)- Ureterovescical junction: 3 (43) | - Percutaneous nephrolithotomy: 7 (100) | 0 | 0 | 0 | (100) |
| Hyams et al. (2012) 40 | 12 | 2006−2011 | 42 (36−72) | NA | 87 (8−209) | - Calcium oxalate: 6 (50)--- Monohydrate: 0 (0)--- Dihydrate: 0 (0)--- Unknown: 6 (50)- Calcium phosphate: 4 (33)- Struvite: 1 (8)- Unknown: 1 (8) | - Renal pelvis: 7 (58)- Urether: 3 (25)- Multiple: 2 (17) | - Ureteroscopy: 7 (58)- Percutaneous nephrolithotomy: 5 (42) | - Nephrocutaneous fistula: 1 (8) | - Uretral stent encrustation: 1 (8) | 0 | NA |
| Khositseth et al. (2004) 8 | 20 | 1896−2003 | 9.2+/-4.7 | 5.0 | 19 (2−72) | - Calcium oxalate: 2 (10)--- Monohydrate: 0 (0)--- Dihydrate: 0 (0)--- Unknown: 2 (10)- Calcium phosphate: 6 (30)- Struvite: 2 (10)- Mixed: 1 (5)- Unknown: 9 (45) | - Renal pelvis: 1 (5)- Ureterovescical junction: 8 (40)- Bladder: 7 (35)- Multiple: 2 (10)- Suture line: 2 (10) | - Cystoscopic lithotripsy/extraction: 11 (55)- Surgical litothomy: 4 (20)- Surveillance: 4 (20)- Unknown: 1 (5) | - Lymphocele 1 (5)- Partial UVJ obstruction 1 (5)- Urethral stricture 2 (10)- Ureteral stump necrosis 1 (5)- Urinary tract infection 8 (40) | NA | NA | NA |
| Kim et al. (2001) 25 | 15 | 1980−1997 | 41.5 (28−67) | 1.8 | 17.8 (3−109) | - Calcium oxalate: 1 (7)--- Monohydrate: 0 (0)--- Dihydrate: 0 (0)--- Unknown: 1 (7)- Calcium phosphate: 3 (20)- Struvite: 2 (14)- Mixed: 7 (46)- Unknown: 2 (14) | - Renal pelvis: 3 (20)- Bladder: 11 (73)- Multiple: 1 (7) | - Cystoscopic lithotripsy/extraction: 8 (53)- Surveillance: 7 (47) | NA | - Lithiasis relapsing: 5 (33) | NA | (100) |
| Klingler et al. (2002) 26 | 19 | 1993−2000 | 48 (26−72) | 0.8 | 28 (13−48) | - Calcium oxalate: 11 (58)--- Monohydrate: 0 (0)--- Dihydrate: 0 (0)--- Unknown: 11 (58)- Calcium phosphate: 1 (5)- Uric acid: 2 (11)- Unknown: 5 (26) | - Upper pole calix: 2 (11)- Middle pole calix: 8 (42)- Lower pole calix: 4 (21)- Ureteropelvic junction: 3 (15)- Urether: 1 (5)- Ureterovescical junction: 1 (5) | - Extracorporeal lithotripsy: 7 (37)- Ureteroscopy: 5 (26)- Percutaneous nephrolithotomy: 3 (16)- Other combined approach: 1 (5)- Surveillance: 3 (15) | - Uretral reimplantation: 1 (5) | - Permanent renal impairment: 1 (5) | NA | NA |
| Krambeck et al. (2008) 41 | 13 | 1988−2008 | 50 (16−71) | NA | 33 | - Calcium oxalate: 7 (54)--- Monohydrate: 6 (46)--- Dihydrate: 1 (7)--- Unknown: 0 (0)- Calcium phosphate: 3 (23)- Uric acid: 1 (8)- Struvite: 1(8)- Mixed: 1 (8) | - Lower pole calix: 1 (8)- Ureteropelvic junction: 9 (69)- Urether: 2 (15)- Multiple: 1 (8) | - Percutaneous nephrolithotomy: 13 (100) | - Sepsis: 1 (8) | - Herpes esophagitis: 1 (8)- Upper gastrointestinal bleeding: 1 (8) | 0 | 77 |
| Li et al. (2011) 45 | 10 | NA | 34 (21−42) | NA | NA | NA | - Renal pelvis: 2 (20)- Urether: 8 (80) | - Extracorporeal lithotripsy: 7 (70)- Ureteroscopy: 2 (20)- Surgical litothomy: 1 (10) | 0 | 0 | 0 | - Extracorporeal lithotripsy: 7/7 (100)- Ureteroscopy: 2/2 (100)- Surgical litothomy: 1/1 (100) |
| Mahdavi et al. (2014) 27 | 21 | 1989−2011 | 31 | 1.2 | NA | NA | - Lower pole calix: 2 (10)- Renal pelvis: 8 (38)- Ureteropelvic junction: 1 (5)- Urether: 10 (48) | - Extracorporeal lithotripsy: 7 (33)- Ureteroscopy: 8 (38)- Percutaneous nephrolithotomy: 2 (10)- Other combined approach: 4 (11) | 0 | 0 | 0 | - Extracorporeal lithotripsy: 7/10 (70)- Ureteroscopy: 8/9 (88)- Percutaneous nephrolithotomy: 2/2 (100)- Other combined approach: 4/4 (100) |
| Mamarelis et al. (2014) 28 | 9 | 1983−2013 | NA | 0.4 | 37 (12−84) | NA | - Renal pelvis: 6 (66)- Urether: 3 (33) | - Extracorporeal lithotripsy: 3 (33)- Percutaneous nephrolithotomy: 3 (33)- Other combined approach: 1 (11)- Surveillance: 2 (22) | 0 | - Lithiasis relapsing: 2 (22) | 0 | - Extracorporeal lithotripsy: 3/3 (100)- Percutaneous nephrolithotomy: 3/3 (100)- Other combined approach: 1/1 (100) |
| Martin et al. (2007) 42 | 5 | 2003−2005 | (28−51) | NA | 17 | NA | - Upper pole calix: 3 ()- Middle pole calix: 2 ()- Lower pole calix: 2 () | - Surveillance: 5 (100) | NA | NA | NA | NA |
| Montanari et al. (2009) 29 | 8 | NA | NA | 1.8 | NA | - Calcium oxalate: 3 (38)--- Monohydrate: 0 (0)--- Dihydrate: 0 (0)--- Unknown: 3 (38)- Struvite: 2 (25)- Mixed: 3 (38) | - Upper pole calix: 2 (25)- Lower pole calix: 2 (25)- Renal pelvis: 1 (12)- Ureteropelvic junction: 1 (12)- Urether: 1 (12)- Multiple: 1 (12) | - Extracorporeal lithotripsy: 8 (100) | 0 | 0 | 0 | - Extracorporeal lithotripsy: 8/8 (100) |
| Motayne et al. (1984) 9 | 7 | 1965−1980 | 42 (23−57) | 6.3 | 48 (6−72) | - Calcium oxalate: 1 (14)--- Monohydrate: 0 (0)--- Dihydrate: 0 (0)--- Unknown: 1 (14)- Uric acid: 1 (14)- Mixed: 3 (43)- Unknown: 2 (29) | - Ureteropelvic junction: 2 (29)- Urether: 1 (14)- Multiple: 4 (57) | - Cystoscopic lithotripsy/extraction: 2 (29)- Surgical litothomy: 3 (42)- Other combined approach: 2 (29) | NA | NA | NA | NA |
| Oliveira et al. (2011) 43 | 7 | 2000−2009 | 44 | NA | 66 (3−208) | NA | - Renal pelvis: 7 (100) | - Cystoscopic lithotripsy/extraction: 1 (14)- Percutaneous nephrolithotomy: 6 (86) | NA | NA | NA | 6 (86) |
| Rezaee-Zavareh et al. (2015) 30 | 31 | 1990−2010 | NA | 4.4 | NA | NA | NA | NA | NA | NA | NA | NA |
| Rhee et al. (1999) 31 | 8 | 1984−1995 | 51 (34−60) | 0.5 | NA | - Calcium oxalate: 1 (12)--- Monohydrate: 0 (0)--- Dihydrate: 0 (0)--- Unknown: 1 (12)- Calcium phosphate: 1 (12)- Uric acid: 1 (12)- Struvite: 1 (12)- Mixed: 1 (12)- Unknown: 3 (38) | - Renal pelvis: 3 (38)- Urether: 1 (12)- Bladder: 4 (50) | - Cystoscopic lithotripsy/extraction: 4 (50)- Extracorporeal lithotripsyy: 1 (12)- Ureteroscopy: 1 (12)- Other combined approach: 1 (12)- Surveillance: 1 (12) | 0 | - Lithiasis relapsing: 1 (12) | 0 | (100) |
| Sarier et al. (2019) 32 | 22 | 2009−2017 | 41.6 | 1.0 | 27 (3−67) | NA | - Caliceal: 3 (14)- Renal pelvis: 7 (32)- Urether: 9 (41)- Bladder: 3 (14) | - Cystoscopic lithotripsy/extraction: 3 (14)- Ureteroscopy: 18 (82)- Percutaneous nephrolithotomy: 1 (5) | 0 | 0 | 0 | - Cystoscopic lithotripsy/extraction: 3/3 (100)- Ureteroscopy: 16/18 (89)- Percutaneous nephrolithotomy: 1/1 (100) |
| Sevinc et al. (2015) 33 | 6 | 2008−2014 | 52 (31−65) | 0.7 | 28 (17−58) | - Calcium oxalate: 3 (50)--- Monohydrate: 0 (0)--- Dihydrate: 0 (0)--- Unknown: 3 (50)- Calcium phosphate: 1 (17)- Uric acid: 1 (17)- Unknown: 1 (17) | - Lower pole calix: 1 (17)- Urether: 4 (66)- Multiple: 1 (17) | - Ureteroscopy: 4 (66)- Percutaneous nephrolithotomy: 1 (17)- Other combined approach: 1 (17) | - Haematuria: 1 (17) | 0 | 0 | - Ureteroscopy: 4/4 (100)- Percutaneous nephrolithotomy: 1/2 (50)- Other combined approach: 1/1 (100) |
| Shoskes et al. (1995) 34 | 2 | 1975−1991 | NA | 0.2 | (12−60) | NA | - Urether: 1 (50)- Ureterovescical junction: 1 (50) | - Ureteroscopy: 2 (100) | NA | NA | 0 | - Ureteroscopy: 2/2 (100) |
| Stravodimos et al. (2012) 44 | 7 | 1983−2007 | NA | NA | 38 (24−84) | NA | - Renal pelvis: 5 (71)- Urether: 2 (29) | - Extracorporeal lithotripsy: 3 (43)- Percutaneous nephrolithotomy: 3 (43)- Surveillance: 1 (14) | 0 | 0 | 0 | 7 (100) |
| Streeter et al. (2002) 35 | 12 | 1975−1998 | NA | 0.8 | 5 (2−43) | NA | - Bladder: 3 (25)- Unknown: 9 (75) | - Cystoscopic lithotripsy/extraction: 3 (25)- Extracorporeal lithotripsy: 1 (8)- Percutaneous nephrolithotomy: 3 (25)- Surgical litothomy: 1 (8)- Other combined approach: 1 (8)- Surveillance: 3 (25) | - Graft lost: 1 (8) | NA | 1 (8) | 8 (66) |
| Verrier et al. (2012) 36 | 31 | 1978−2010 | 41.5 (19−68) | 1.0 | 102 (96−168) | NA | - Renal pelvis: 7 (23)- Urether: 16 (52)- Bladder: 4 (13)- Unknown: 4 (13) | - Cystoscopic lithotripsy/extraction: 4 (13)- Extracorporeal lithotripsy: 2 (6)- Ureteroscopy: 3 (10)- Percutaneous nephrolithotomy: 2 (6)- Surgical litothomy: 8 (26)- Surveillance: 12 (33) | - Surgical failure: 2 (6) | 0 | 0 | - Extracorporeal lithotripsy: 1/3 (33)- Ureteroscopy: 2/5 (40) |
| Yigit et al. (2004) 37 | 5 | 1999−2003 | 35+/-15.1 | 4.0 | NA | - Calcium oxalate: 2 (40)--- Monohydrate: 0 (0)--- Dihydrate: 0 (0)--- Unknown: 2 ()- Uric acid: 1 (20)- Unknown: 2 (40) | NA | - Extracorporeal lithotripsy: 3 (60)- Ureteroscopy: 1 (20)- Other combined approach: 1 (20) | NA | NA | 0 | (100) |
| Yuan et al. (2015) 10 | 19 | 2000−2014 | 39 (11−65) | 1.2 | 21 (3−211) | - Calcium oxalate: 7 (37)--- Monohydrate: 0 (0)--- Dihydrate: 0 (0)--- Unknown: 7 (37)- Uric acid: 2 (11)- Struvite: 3 (16)- Mixed: 2 (11)- Unknown: 5 (26) | - Renal pelvis: 9 (47)- Urether: 9 (47)- Multiple: 1 (6) | - Extracorporeal lithotripsy: 5 (26)- Ureteroscopy: 4 (21)- Percutaneous nephrolithotomy: 6 (32)- Other combined approach: 2 (11)- Surveillance: 2 (11) | - Blood loss: 1 (6)- Uretral perforation: 1 (6) | 0 | 0 | - Extracorporeal lithotripsy: 4/5 (80)- Ureteroscopy: 4/4 (100)- Percutaneous nephrolithotomy: 6/6 (100)- Other combined approach: 2/2 (100) |
The first objective of this review was the assessment of the incidence of urolithiasis in transplanted kidneys. 32/41 studies reported the incidence of graft lithiasis.1,7–37 Data from included studies were heterogeneous. Emiliani et al.21 reported an incidence of lithiasis of 2.4%, in contrast with Verrier et al. who showed a decreasing trend in the last 3 decades from 2.1% to 0.6%.36 A percentage of 1.29% on a Brazilian population of 1313 patients was reported by Cassini et al.22 Similarly, Kim et al. reported 1.8%.25 Rhee et al. described an incidence of 0.23%31 whilst Abbott et al.14 observed the lowest rate of urolithiasis with an incidence of 0.15% (84.2/100.000) in females and 0.11% (59.9/100.000) in males. On the other hand, Motayne et al. reported the highest incidence (6.3%).9 Similarly, Khositseth et al. observed a 5%8 whilst a 4.4% was observed by Rezaee-Zavareh et al.30 The other studies included in this review report incidence rates among the above-mentioned ranges.
Several studies do not report the time of the diagnosis of urolithiasis from renal transplantation or it was not clearly reported. In 24/41 studies,8–12,16,17,21–23,25,26,28,32–36,38–44 the time of diagnosis exceeded 12 months with a maximum of 66 months from kidney transplantation.43
Considering the localization of the stones,1,6,8–10,12,13,17–19,21–29,31–36,38–45 renal calyces are generally the most common localization, reaching more than 50% of the cases in several studies, such as those published by Montanari et al.,29 Bolen et al.12 and Harraz et al.24 Conversely, Sarier et al.32 observed 9/22 (41%) cases of ureteral lithiasis and Emiliani et al.21 reported 30/51 (59%) cases. The incidence of bladder lithiasis was reported in Table 1 but it was not considered among the objectives of this review.
The composition of the stones in the graft, reported in 20/41 studies,1,6,8–10,12,13,17,20,21,23,25,26,29,31,33,37,39–41 does not vary significantly among the included series, being calcium oxalate the most frequent finding in the studies included, whenever reported. Harper et al. observed 33% of calcium phosphate lithiasis (2/6 patients)1 whilst Kim et al.25 20% (3/15 patients). Also Khositeth et al.8 described a higher rate of calcium phosphate stones of 30% over the calcium oxalate. Uric acid lithiasis was preponderant over the other compositions, matching in incidence calcium oxalate in some of the studies considered.1,9,20,31,39 Finally, struvite stones were less frequently observed with an incidence between 8–25%.
TreatmentOnly 1/41 study did not report any treatment data.30 Surveillance of intrarenal lithiasis was considered as an alternative to active treatment, except for Martin et al. where 100% (5/5 patients) of the patients were just observed.42 Generally, active treatments were preferred in most of the cases, reaching 47% (46/95 patients) in the study from Branchereau et al.38
Overall, 110/699 (15.73%) patients were submitted to extracorporeal shock wave lithotripsy (ESWL). 187/699 (26.75%) patients underwent endoscopic lithotripsy or stone extraction. The biggest cohort of patients treated with ureteroscopy was described by Branchereau et al.,38 who included 25/95 (26%) patients. 126/699 (18.03%) patients underwent percutaneous nephrolithotomy whilst in 22/699 (3.14%) a combined approach was preferred. Finally, surgical lithotomy was performed in 35/699 (5.01%) cases.
Stone free rateIn 14/41 studies,6,10,11,16,18–21,23–25,27–29,31–39,41,43–45 the stone-free rate (SFR) after treatment was not reported. 16 (64.0%) studies stratified the stone-free rate after each treatment. In most of the cases, a highly successful rate was observed after endoscopic surgery as well as after percutaneous approaches. The global SFR was around 80%.
Complications and graft loss due to lithiasisPerioperative complications (up to 30 post-operative days) were not reported in 17/41 studies. No complications were observed in 9 studies.22,27–29,31,32,39,44,45 In the remaining 15 studies,6,8,10,14,15,21,23,24,26,33,35,36,38,40,41 different complications were described, from hematuria to graft loss. In detail, hematuria was observed in 5 (19.2%) out of the 26 studies which reported perioperative complications. Urinary leakage was reported in 3 (11.5%) studies; infections in 15 (57.7%) with 3 (11.0%) episodes of sepsis; 2 (7.7%) cases of ureteral perforations; ureteral strictures were described in 15 (57.7%) cases, graft loss due to the treatment in 1 case (3.8%). Long-term complications (more than 30 postoperative days) were reported in 23/41 studies whilst no complications in 14/23 (60.9%). In the remaining 9 studies, lithiasis recurrence was described in 15 (65.2%) cases, chronic rejections and renal failure in 2 (8.7%).
DiscussionKidney transplant is considered the standard treatment for end-stage renal disease (ESRD) leading to improved overall survival and a reduction of patient morbidity in comparison with dialysis.46,47 The use of robotics48–51 and the introduction of new technologies have been proposed with the aim of improving surgical results of KT and reducing the invasiveness of the procedure.52–54
However, a transplanted kidney may develop several subsequent problems, regardless of the surgical approach, potentially leading to permanent organ failure and graft loss.54–56 Among these, urolithiasis represents a rare but potentially dangerous condition. Periodic clinical controls with ultrasound imaging in patients previously submitted to KT make this scenario uncommon but still experienceable.
The heterogeneous data from Literature make the assessment of the incidence of this condition hard to define, ranging from 0.1%14 to 6.3% of Motayne et al.9 This difference in incidence and time of de novo stones detection may be due to different follow-up protocols among Centers, Countries and Decades. In fact, the use of non-contrast CT scan changed the capability of early detection of intrarenal stones over the X-ray. In addition, changes in medical treatments for the modulation of the immune response against the graft was thought to be potentially responsible for the difference in lithiasis incidence in KT recipients.57
Regarding the stone composition, it should be underlined that patients submitted to KT are chronically exposed to immune-modulating medications which may cause metabolic alterations in human body. Ciclosporine A and glucocorticoids may induce hyperuricemia, making those patients at an increased risk of uric acid stones formation.21 In fact, several studies, including those published by Motayne et al.9 and Doehn et al.20 reported an incidence of uric acid stones almost matching Calcium Oxalate in up to about 30% of the cases,39 which is the most frequent composition observed in the non-transplanted population. In the other cohorts, Calcium Oxalate remains the main chemical composition of urinary stones, as reported by Klingler et al.26 with a prevalence of 58% in a cohort of 19 patients. Other composition of lithiasis, such as struvite or infected stones, were found to be less prevalent.
In the majority of the cases, intrarenal lithiasis was observed. In fact, thanks to standardized follow-up programs providing periodic US, a prompt diagnosis is usually done. Differently from another SR published in 2022,3 we decided to include patients with ureteral lithiasis since, surprisingly, ureteral stones are not a rare finding, as was reported by Emiliani et al. who observed 59% (30/51 patients) of ureteral lithiasis. Colic pain may miss in the allograft due to iatrogenic denervation, making this pathology devious and potentially much more dangerous than in a normal kidney because of the high risk of being unrecognized in time for prompt treatment.
Regarding treatment strategies, in most of the studies included in this revision, active treatment was chosen in consideration of the particular population investigated. Unfortunately, in several studies, we do not have data about the results of each treatment performed in terms of SFR. However, positive results are generally described for each treatment strategy, from endoscopic to open surgery, as it was reported by Emiliani et al. with a stone free rate of 100% (9/9 patients) for patients treated with ureteroscopy and 91% (20/22 patients) for those treated with ESWL21 or 84% (21/25 patients) after ureteroscopy in the manuscript published by Brancherau et al.38
At this point, attention should be paid to the invasiveness and possible complications of each procedure, considering a different endoscopic/surgical anatomy in a transplanted kidney with its consequent challenges, especially in case of a surgeon without a high experience in this field. Even if the open surgical approach provided optimal results in terms of SFR after treatment in sporadic cases, as described by Li et al.45 and Emiliani et al.,21 the percutaneous or combined endoscopic/percutaneous approach represent an optimum compromise for the treatment of large intrarenal stones, as it was reported in a study on 95 patients with 9/10 (90%) patients free from lithiasis after percutaneous treatment.38
Finally, complications are just round the corner in previously transplanted patients as in the standard population. Lithiasis recurrence should not be considered a surgical failure but a pitfall to be investigated during follow-up. Sepsis, strictures, fistulas and so on are fearsome but fortunately rare occurrences. Urinary tract infections (IVU) were showed often complicated postoperative course, as described by Khositseth in 2004 with 40% of IVU, especially in an immunocompromised patient due to the risk of life-threatening sepsis.
ConclusionsDespite lithiasis in KT recipients being a rare condition, it represents a challenging disease both for diagnosis and treatment reasons. Timely diagnosis is essential since lithiasis in KT recipients may potentially compromise organ function. Each treatment option seems to provide positive results in terms of stone-free rate, laying the surgeon the choice of a more or less invasive treatment considering the different anatomy of the graft and related complications.
Conflicts of interestThe authors declare that they have no conflicts of interest.