β-glucans are a group of biologically active polysaccharides of natural origin with a proven pleiotropic immunomodulation effect. Their efficacy has been confirmed in the therapeutic treatment and prevention of various infectious diseases, secondary immune defects and also of oncologic disorders. Allergic diseases are one of the most frequent diseases and their prevalence continues to increase. They develop as a consequence of dysregulation of the immune system, especially when there is failure in the equilibrium of the response of TH1/TH2 lymphocytes towards TH2. New therapeutic approaches in the treatment of immunopathological conditions (e.g. allergic or oncologic) are directed to restoring the equilibrium among different T lymphocyte subpopulations. Based on in vitro experiments, and also on animal and human clinical studies, there is much evidence for the importance of β-glucans in the treatment and also prevention of allergic diseases; this opens new perspectives on the use of this widespread and popular group of natural substances.
In recent years, the prevalence of various forms of allergic diseases has continued to increase, and this fact underlines the need for more intensive medical and also social attention to be given to them. Allergic diseases present a pressure for both the individual and society and are associated with ongoing efforts to find the most effective treatment options. From an epidemiological point of view, the prevalence of allergic diseases is still increasing globally. More than 40% of all people suffer from various forms of allergic rhinitis. It is possible that very soon 50% will be suffering from some form of clinical allergy, and this fact places allergic diseases, together with cardiovascular and oncologic disorders, into the group of the most important disease conditions, termed collectively lifestyle diseases (or diseases of civilisation). The frequency of allergic diseases varies according with age. In small children, the most prevalent are allergic symptoms of the digestive tract (e.g. food allergies), followed by skin symptoms (especially atopic eczema). The older the child, the more prevalent are symptoms of the respiratory tract, especially bronchial asthma and allergic rhinitis (rhinoconjunctivitis). The gradual onset of allergic symptoms in various organ systems is known by the name ‘atopic march’. The substrate for the development and persistence of allergic diseases is the emerging disbalance of the immune system, which can be effectively modulated and corrected through therapy and prevention.
Immunomodulation with natural substancesThe use of natural substances and products in the treatment and prevention of many diseases has been well known for many years. Specific products differ not only in efficacy but also in the level of evidence for their effects, and especially in knowledge of their precise composition and mechanism of action. Many are a mixture of several substances which contribute to different extents to the final biological activity of the products. One of the most studied groups of natural substances with proven biological activity (especially in the immune system) is the group of biologically active polysaccharides (BAPs), e.g. β-glucans, β-fructans or chitin. BAPs are a mixture of non-cellulose polymers of glucose units connected by the linear beta(1→3) and lateral beta(1→6) glycosidic linkages. There are several known natural sources of BAPs, such as fungi (e.g. pleuran, scleroglucan, lentinan, schizophylan, grifolan, glomerellan), bacteria (e.g. curdlan), moulds and yeasts (e.g. betafectin, yeast glucan, zymosan, zymocel), seaweed (e.g. laminaran, chrysolaminaran) and certain cereals (molecules of cereal-derived glucans contains different beta(1→4) glycosidic linear linkages between the glucose units). BAPs are typical modifiers of biological reactions, with evident immunomodulation activity in both directions: positive (immunostimulation) and also negative (immunosuppression).1 Their potent immunomodulation effect has been confirmed in many animal experiments, in vitro studies and also in several human clinical studies, and has predominantly been studied in the treatment and prevention of infectious and oncologic diseases.2 There are several trials which have been performed recently and which study the possible effect of BAPs in the treatment and prevention of allergic diseases. They have opened new perspectives on the clinical use of BAPs.
The mechanism of BAP action in the organism is mediated through several receptors, especially the Dectin-1 receptor, Toll-like receptors (TLR-2, 4, 6), complement receptor 3 (CR3), scavenger receptor and lactosylceramid.3 The most important is the Dectin-1 receptor, which is highly expressed in many immunocompetent cells such as dendritic cells (DC), neutrophils, eosinophils, macrophages, monocytes, several T lymphocytes and, in humans, also in C lymphocytes. After the binding of β-glucan to Dectin-1 receptor, it stimulates production of many cytokines or other mechanisms of immune and non-immune reactions.
The effect of BAPs depends on the form of application (per oral, intravenous, intramuscular, subcutaneous) and also on many other characteristics and circumstances e.g. the source, solubility, size of molecules and conformational space or purity of product.4 There are several described and confirmed effects of β -glucans in the literature: immunomodulation, anti-infectious, anti-tumorous, anti-mutagenic, anti-allergic, regenerative, anti-thrombogenic, anti-coagulative, antioxidant, hypolipidemic and radioprotective.
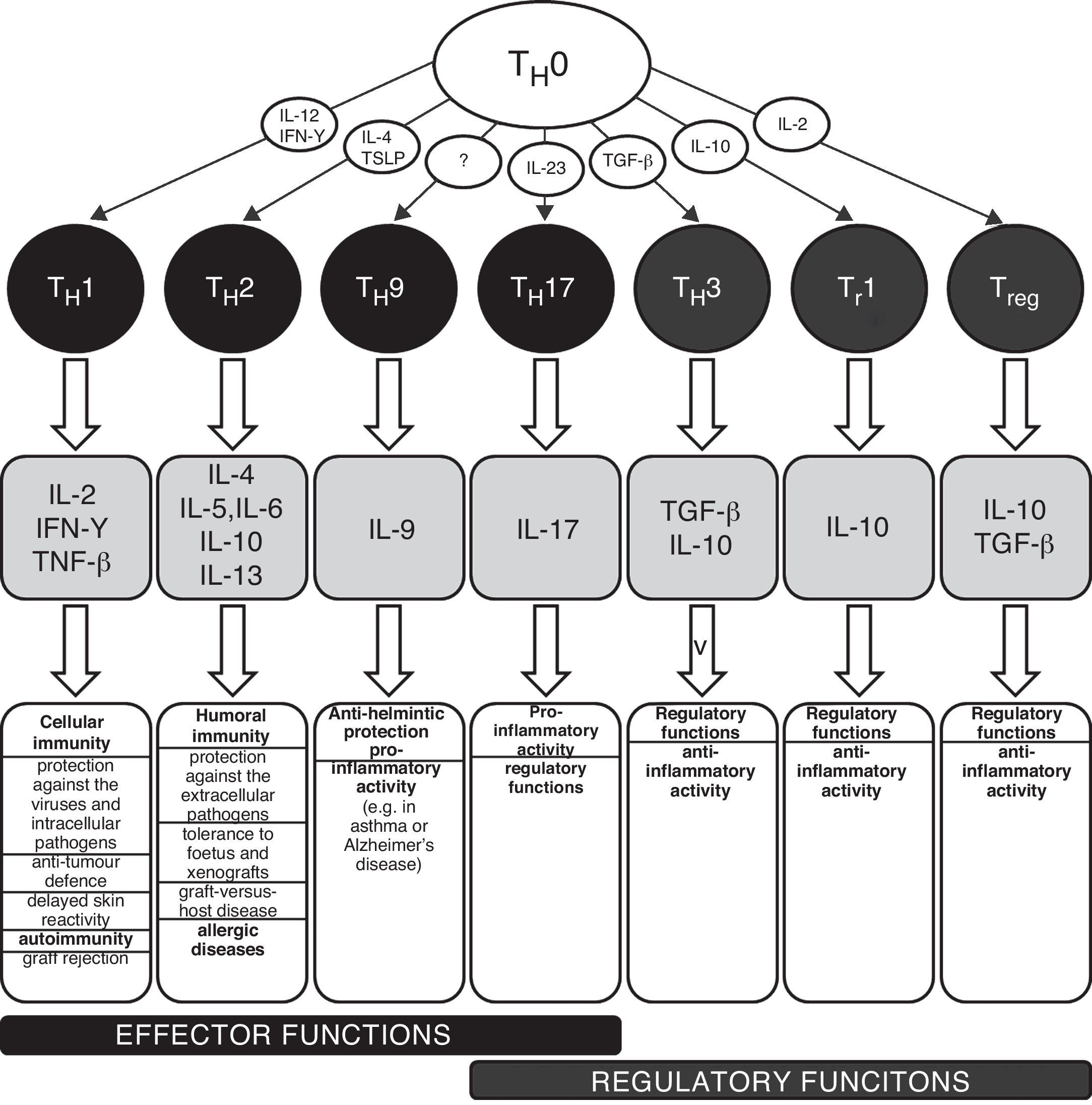
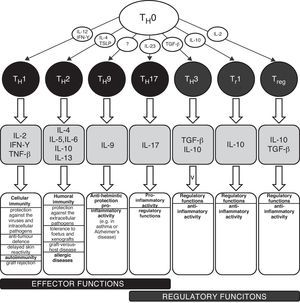
TH1/TH2 equilibrium and immunopathological conditionsThe carriers of specific cellular immunity are T lymphocytes. We can discriminate T helpers (TH), T cytotoxic and T regulatory lymphocytes. To date, several subpopulations of TH lymphocytes have been detected, based on the characteristic spectrum of produced cytokines (Fig. 1). The two best-known and important subpopulations are TH1 and TH2 lymphocytes. TH1 characteristically produce and secrete interleukin 2 (IL-2), interferon gamma (IFN-γ) and tumour necrosis factor beta (TNF-β), which induce immune tolerance and the reactions of cellular immunity. The other important subpopulation is TH2 lymphocytes, which produce interleukins 4, 5, 6, 10 and 13 and activate humoral immunity and typically contribute to the development and persistence of allergic inflammation. TH0 lymphocytes simultaneously produce IL-4 and also IFN-γ. There are several newly discovered lymphocyte subpopulations with an important role in the development of immunopathological conditions and in the regulation of immune response: TH3 with regulatory functions (producing transforming growth factor beta, TGF-β); TH17 participating in the development of allergic diseases (producing IL-17, 21 and 22); and TH9 (secreting IL-9). The theory used to explain the development of various diseases associated with disturbances in the immune system is based on a disbalance between various T lymphocyte populations, especially between TH1 and TH2, which arises as a consequence of the failure of regulatory mechanisms (e.g. production of regulatory IL-1β and IL-18 from antigen-presenting cells, APC). The changes in the cytokine microenvironment lead to the dominance of the TH1 or TH2 response.5 The TH1/TH2 paradigm was established in 1986 and until the present day has undergone many changes and modifications, including the implementation of newly discovered cellular populations and cytokines (e.g. TH17 lymphocytes, regulatory T lymphocytes). The implementation of new cellular populations into the TH1/TH2 paradigm allows better understanding of particular immunopathological conditions which could not be explained only by simple disturbance in the TH1/TH2 equilibrium.6–8 Neither is it possible to explain the origin of allergic diseases only through simple dominance of the TH2 response. The pathogenesis consists of complex dysregulation of the immune system with contribution from many cellular populations (the role of T regulatory cells and TH17 lymphocytes) and cytokines (e.g. IL-25 and IL-33).9,10 However, dysregulation of the TH2 response remains one of the most important aspects of the development and persistence of allergic inflammation.
Schematic classification of the main T lymphocyte subpopulations with characteristic production of cytokines and their effects in the human body.
IL – interleukin, IFN – interferon, TGF – transforming growth factor, TH – T helper lymphocyte, TNF – tumour necrosis factor, Tr – induced T regulatory lymphocyte, Treg – natural T regulatory lymphocyte, TSLP – thymic stromal lymphopoetin.
New therapeutic approaches in the treatment of immunopathological conditions (e.g. allergic and oncologic diseases) are focused on the restoration of the disbalance among different T lymphocyte subpopulations.3
Anti-allergic effect of biologically active polysaccharides – in vitro studiesVarious BAPs could have different effects on the TH1/TH2 equilibrium, and also on the production of TH1- or TH2-dependent antibody subclasses. This depends strongly on the specific type of the studied polysaccharide and the experimental conditions. It should also be noticed that some effects observed in vitro have not been confirmed in animal and human in vivo studies. Therefore the reported experimental observations should be interpreted with caution.
Sonifilan (β-glucan isolated from Sclerotinia sclerotiorumi) induces differentiation of TH1 cells, followed by synthesis of IFN-γ by the stimulation of IL-12 production.9 Forland et al.11 also observe that the response of TH1 cytokines is characterised by the production of IL-2, IFN-γ and TNF-α. Zymosan (yeast β-glucan) stimulates the dendritic cells (the most important antigen-presenting cells) through its interaction with the Dectin-1 receptor and TLR-2 to produce important cytokines (especially IL-10) with a subsequent tollerogenic T-cell response.12
Curdlan possesses the ability to induce differentiation of TH17 lymphocytes through the dendritic cells.13Zymosan (complex of protein-polysaccharides from yeast), after interaction with the Dectin-1 receptor, induces the activation of antigen-presenting cells (followed by production of IL-23) and T lymphocytes (followed by secretion of IL-17).14β-glucan from Agaricus blazei stimulates the secretion of pleiotropic and regulatory cytokine IL-17 from dendritic cells.15 The β-glucan fraction from the fungus Ganoderma lucidum (reishi mushroom) activates and stimulates the differentiation of dendritic cells whereby, at the level of genes, it is possible to detect inhibition of the genes connected with phagocytosis with simultaneous activation of the genes for cytokines and co-stimulatory molecules. The result of the increased IL-12 production is stimulation of the proliferation and differentiation of T lymphocytes, predominantly towards TH1. IL-23 and IL-27 have a similar effect.16
Nathan et al.17 studied in vitro the secretion of the chemokine CCL20 from human airway epithelial cells after exposure to house dust mite allergens. Exposure of the cells to the allergens caused specific and rapid secretion of CCL20, a potent chemotactic factor for immature dendritic cells, which play an important role in the development of airway allergic inflammation. The use of various β -glucans (laminarin, zymosan, curdlan) inhibited chemokine release, and none of the β-glucans by itself demonstrated the capacity to induce CCL20 secretion. This observation also contributes to clarification of the complex anti-allergic activity of BAPs.17
Another possible mechanism of the additive effect of BAPs in the treatment of allergic diseases is their antioxidant activity through the neutralisation of hydroxyl and superoxide radicals. The anti-inflammatory effect is mediated by the inhibition of nitric oxide synthase and cyclooxygenase.18
Sakurai et al.19 have analysed the effect of β-glucan isolated from Sclerotinia sclerotiorum on alveolar macrophages. Intravenous application of β-glucan modified the biological activity of alveolar macrophages without provoking chronic inflammation in the alveoli.19
Holck et al.20 investigated the capacity of different β-glucans (curdlan, laminarin, scleroglucan) on the histamine release from human leucocytes. The results obtained by their experiments did not indicate direct interaction of β-glucans with human basophiles leading to histamine release. However, they revealed a potentiating effect of β-glucans (regardless of the branching of the molecules) on histamine release induced by anti-IgE antibody as well as by specific antigen in patients allergic to house dust mites. They suggest that in certain conditions β-glucans may lead to the development or aggravation of allergic inflammation. However, the clinical meaning of this observation is unclear.20
Anti-allergic effect of biologically active polysaccharides – animal experimentsThere are several experimental animal models of allergic and oncologic diseases which, besides the series of in vitro studies, showed that β-glucans possess an anti-allergic and immunomodulation effect.
Baran et al.21 observed an increased proportion of T lymphocytes producing IL-4 in the group of mammary cancer-bearing animals in the absence of the application of β-glucan (from Saccharomyces cerevisiae). The addition of β-glucan led to a significant increase of T lymphocytes producing IFN-γ. The switch from TH2 to TH1 was probably mediated by macrophages in the intestinal wall which, after activation by β-glucan, started to produce important regulatory and differentiation cytokine interleukin 12.21 The effect of lentinan on the TH1/TH2 equilibrium was also studied in human clinical research concerning patients with gastrointestinal cancer. β-glucan reduced the TH2 response with concomitant stimulation of an anti-tumour TH1 reaction.22 In animal experiments, intraperitoneal application of lentinan improved the capacity of peritoneal macrophages to produce IL-12, which directed the immune response towards TH1 and stimulated the T lymphocytes to produce IFN-γ.23 Earlier animal studies also confirmed the capacity of BAPs to modify the TH1/TH2 equilibrium towards TH1.24
Inoue et al.25 investigated the effect of the β -glucan fraction from Grifola fondosa (maitake mushroom) on the cytokine balance from TH1 and TH2 lymphocytes. Application of grifolan led to a decreased activation of B lymphocytes with subsequent T helper activation. The result of administering grifolan was TH1 dominance with parallel suppression of the TH2 response, probably due both to the stimulation of the differentiation of naive T cells towards TH1 and to inhibition of the conversion of TH1 to TH2 cells.
Vetvicka et al.26 analysed the effect of β-glucans isolated from fungi and yeast on the immune system and cytokine production. In an animal model, they demonstrated that application of β-glucan caused a significant increase of various cytokines, especially of IL-8. The inhibitory effect of β-glucans on TH2 with parallel stimulation of the TH1 response was also proven in another animal study performed by Saito et al.27. Administration of β-glucan (from Acetobacter species) inhibited the production of ovalbumin-specific IgE in vitro with simultaneous increase of IL-12 concentration in vivo.
The high-single dose application of β-glucan in animal experiments improves asthmatic symptoms and lung abnormalities.28 Xie et al.29 confirm that polysaccharide isolated from the fungus Cryptoporus volvatus has a preventive effect on the development of ovalbumin-induced allergic rhinitis, on the basis of the inhibition of eotaxin mRNA in nasal mucosa and lung tissue. This effect was mediated through decline of IgE elevation and stimulation of IFN-γ. A similar preventive effect on β-glucans in the development of allergic disease was also noticed in β-fructans, e.g. levan (β-2,6-fructan), arising by the fermentation of soy by the micro-organism Bacilus subtilis. Peroral application of levan caused a decline of ovalbumin-specific IgE in serum and inhibition of the TH2 response.30 On the other hand, high concentration of β-glucans in environment on murine model can promote airway eosinophil infiltration.31
The anti-allergic effect of β-glucans has also been examined in the animal model of food allergy. Addition of 0.5 – 1 .0% β-glucan (isolated from the fungus Aureobasidium pullulans) into the diet of ovalbumin-allergic mice resulted in a decline of specific IgE, with coincidental increase of production of IFN-γ and IL-12 from splenocytes in vitro after stimulation with concanavalin A.32 The effect depends on the application method and on the form of applied β-glucan, because a stronger effect was noticed with increasing degree of dispersion.32,33 Correspondingly, peroral application of herbal extract with β-glucan content (from Ganoderma lucidum) resulted in a reduction of clinical symptoms in mice allergic to peanuts34 and house dust mite allergens.35
In the animal model of atopic eczema, application of paramylon (β-glucan from the unicellular flagellated protist Euglena gracilis) caused responses of both TH1 and TH2 to be blocked. Whereas in the serum a significant decline of IL-4 and IFN-γ was observed, a concentration of IL-18 and IL-12 was detected in skin lesions. The effect of paramylon was also documented by a significant decline in symptom score.36 The anti-allergic and anti-inflammatory effect of BAPs was proven also in an animal model of contact hypersensitive dermatitis. The effect was mediated by inhibition of the function of L- and P-selectins which are involved in leukocyte extravasation and development of local inflammatory infiltrate.36
Anti-allergic effect of biologically active polysaccharides – clinical studiesThe anti-allergic mechanisms of BAPs indicated in vitro have also been investigated by several clinical studies, which gave some evidence for anti-allergic activity of β-glucans. Some of these studies, especially the epidemiological ones, gave also contradictory results. Application of BAPs or natural mixtures with β-glucan content could lead to improvement and control of various allergic symptoms.37,38 Peroral administration of the polysaccharide fraction from Agaricus blazei attenuated the clinical symptoms of bronchitis, probably though increase of IFN-γ.39
In the study of Yamada et al.,33 peroral administration of lentinan over two months resulted in a reduction of spontaneous production of specific and total IgE in serum. Clinical response to the therapy was positively correlated with the degree of IgE decline and also with the capacity of monocytes to bind the β-1,3-glucan. The use of lentinan also improved the clinical symptoms of allergic rhinoconjunctivitis. This improvement also continued for six months after the end of the application, suggesting that positive changes in the immune system persist for a long time after the end of therapy. The authors concluded their results by stating the hypothesis that the peroral application of lentinan could resolve the epidemic increase of allergic rhinoconjunctivitis.33
The effect of β-glucans on the cytokine microenvironment was investigated in a group of 24 patients with allergic rhinitis in a double-blind, placebo-controlled study. Individuals were treated, in addition to classical anti-allergic treatment, with β-glucan (from Saccharomyces cerevisiae) for 12 weeks, and showed a significant decline of IL-4 and IL-5, with parallel increase of IL-12, in the nasal lavage fluid. They did not observe any change in cytokine level in the placebo-treated group. Authors also detected a significant decline of eosinophil quantities in nasal lavage but with an unchanged concentration in the peripheral blood of the patients treated additionally with β-glucan.40
Subcutaneous application of β-glucan (imunoglucan, a biologically active polysaccharide complex with majority of pleuran isolated from Pleurotus ostreatus) in 20 children with allergic, partially controlled asthma, caused a significant increase of serum levels of anti-inflammatory cytokine IL-10, with a simultaneous decline of daily and nocturnal symptom scores. The observed increase of serum IL-10 after subcutaneous administration of BAPs could modulate allergic sensitisation with the restoration of the TH1/TH2 equilibrium, given the fact that low serum levels of IL-10 are associated with the dysregulation of T lymphocytes.4
In a recent study, the effect of baker's yeast β-glucan on various cytokines in blood during and after exercise was examined. The 10-day supplementation of yeast β-glucan increased the potential of blood leucocytes for the production of IL-2, IL-4, IL-5 and IFN-γ. Authors concluded that β-glucan may have potential to alter immunity following a strenuous exercise session.41
Other than the studies performed on selected groups of patients, there are several published epidemiological studies which confirm that early exposure to high doses of β-glucans in the domestic environment is associated with lower risk of wheezing in children of atopic parents, especially in sensitised children.42 A similar effect was also evidenced in an animal study.43 The preventive effect of early exposure to β-glucans on the development of allergic diseases in childhood was also observed in another study,44 but there are also some negative observations published.45–47 It was shown that daily vacuum cleaning of mattresses over time significantly reduces the concentration of β-glucans and decreases the exposure to them.48
The association between environmental exposure to β-glucans and the development of respiratory allergy was examined in detail in two recently-published papers in which the authors analyse the influence of environmental impacts on the development of respiratory allergic diseases. The presence of visible moulds in homes was associated with the development of allergic diseases of respiratory tracts. In contrast, the presence of β-glucans and extracellular polysaccharides had a preventive effect on the development of respiratory allergies. In a cohort of 260 children followed up for 10 years from birth, the environmental exposure to β-glucans did not increase the risk for bronchial asthma, bronchial hyperreactivity or allergic sensitisation.49 One epidemiological study showed that long-term and massive exposure to organic dust (containing high concentration of β-glucans) may cause disruption of normal immune response and allow the development of various immunopathological conditions.47 High level of β-glucans in the environment is the risk factor for allergic sensitisation50 and airway inflammation.51 Organic dust also contains the polysaccharide chitin, which is usually associated with the presence of β-glucans. The fungal chitin derived from home environments also showed capacity to induce eosinophilic allergic inflammation in the lungs and therefore they can modify the effect of β-glucans.52 It was shown that pollen from birch and other plant species contains β-glucans that might contribute to pollen sensitivity.53 However, short term exposure to β-glucans does not increase the allergic inflammation in nasal mucosa.54
According to the published studies, it can be hypothesized that the presence of these natural immunomodulators in the home (e.g. β-glucans) is an important factor affecting the development and achievement of immune system homeostasis and contributes to understanding the hygienic hypothesis. The final influence on the immune system probably strongly depends on several factors and aspects, such as the concentration of β-glucans (low vs. high), the duration of exposure (short-term vs. long-term), way of exposure (respiratory tract vs. per oral supplementation), or the presence of other organic compounds in the environment with the capacity to modify the effect of β-glucans (e.g. chitin, endotoxins).44,55 The results of the available published studies should be confirmed through other well-designed trials with higher number of subjects. The conflicting results of the epidemiological studies could be due to the relative small sample population sizes and other factors which can bias the results.
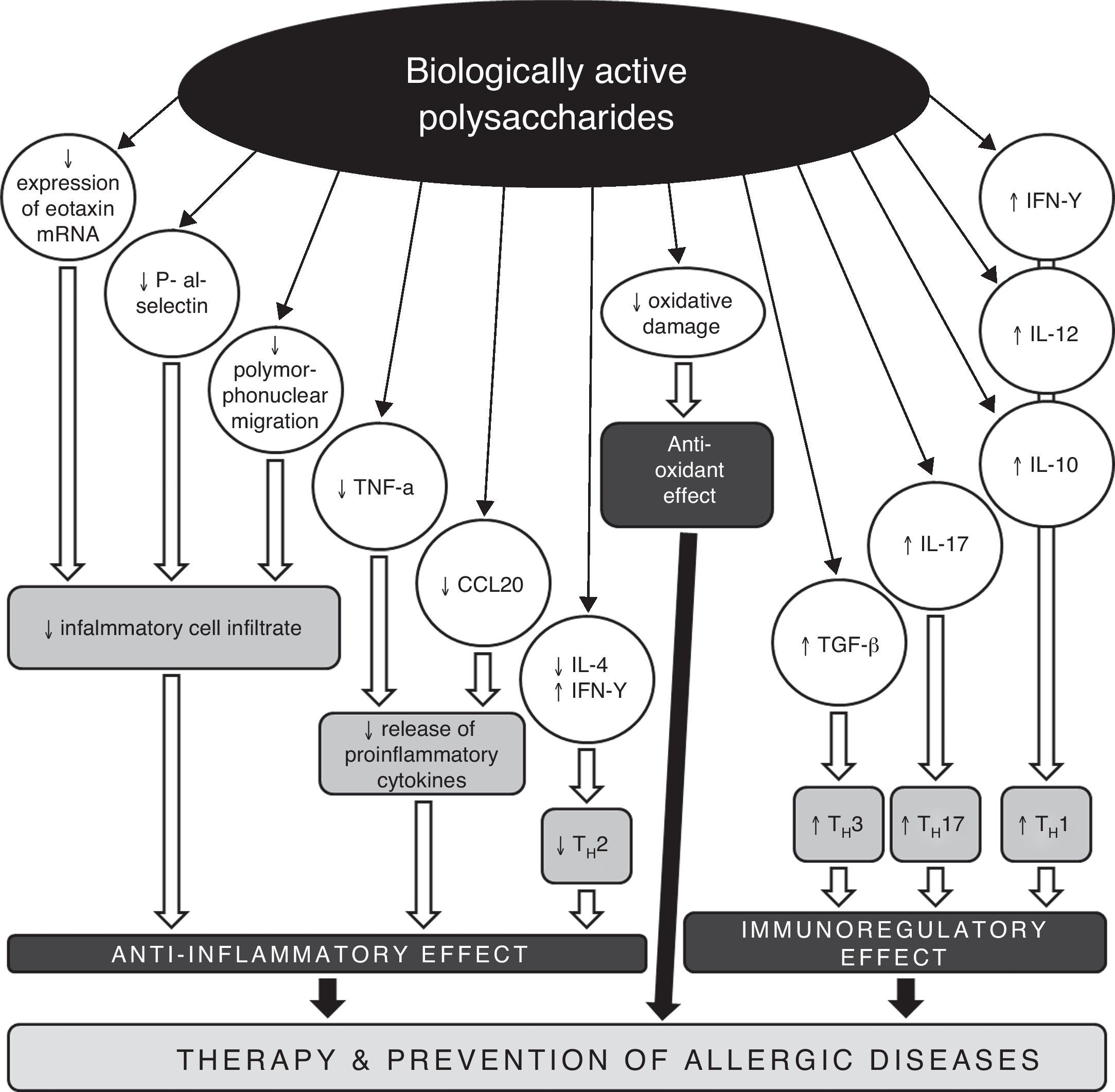
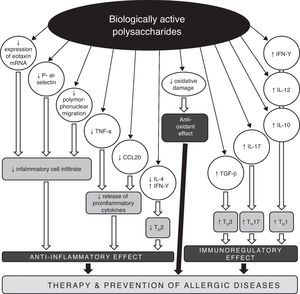
Conclusions and perspectivesRegarding the increasing prevalence of allergic diseases, it is necessary to search for new therapeutic strategies and approaches, with the aim of impacting on the development and persistence of asthma and other allergic diseases. One possible target could be a disturbed TH1/TH2/TH17 equilibrium. Biologically active polysaccharides appear to be suitable immunomodulators with the capacity to restore the disbalance in the cytokine TH1/TH2 response (Fig. 2). Their impact on the treatment and prevention of allergic diseases is twofold. On the one hand, they restore the disturbed TH1/TH2 equilibrium, and on the other hand, they decrease morbidity through the pleiotropic immunomodulation effect, with improvement of disease control and a decrease in the risk of acute exacerbations typically associated with respiratory infections. The use of biologically active polysaccharides and other immunomodulation agents of natural origin opens new perspectives on the treatment and prevention of allergic diseases, on the basis of activation of the TH1 response with parallel suppression of allergic immune reactions, and with restoration of the TH1/TH2/TH17 equilibrium. Immunomodulation presents an effective future approach in the prevention and treatment of bronchial asthma and other allergic diseases.
Possible mechanisms of anti-allergic and anti-inflammatory actions of biologically active polysaccharides.
CCL20 – chemokine (C-C motif) ligand 20, IL – interleukin, IFN – interferon, mRNA – messenger ribonucleic acid, TGF – transforming growth factor, TH – T helper lymphocyte, TNF – tumour necrosis factor.
The authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of DataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Conflicts of interestThe authors have no conflicts of interest to declare.
This work was supported by the project Centre of Experimental and Clinical Respirology II, which is co-funded by EU sources.