The objective of this study was to examine the B lymphocyte subsets in primary immunodeficiency that progress with antibody deficiency.
MethodsThe patients’ naive, memory, class-switched memory and non-switched memory B cells were compared with those of healthy individuals of matching ages using flow cytometry.
ResultsA total of 67 patients with antibody deficiency and 28 healthy children of matching ages were included in the study. The median age of the patients was six years (min–max: 1–24) and 40 (59.7%) were male. The median age of the healthy controls was again six years (min–max: 1–17) and 12 (42.8%) were male. Patients with common variable immunodeficiency had higher relative counts of naive cells when compared with the control group; however, they were found to have lower relative counts of memory, relative and absolute counts of non-switched and relative counts of switched B lymphocytes (p=0.001, 0.023, 0.003–0.003, 0.001, respectively). In patients with selective IgA deficiency, similar to patients with common variable immunodeficiency, the relative counts of naive cells were found to be higher, while the relative counts of memory and relative and absolute counts of non-switched B lymphocytes were found to be lower when compared with the control group (p=0.011, 0.032, 0.006–0.009, respectively). Although patients with selective IgM deficiency had higher relative counts of naive B cells when compared with the control group, they had lower relative and absolute counts of non-switched B lymphocytes (p=0.008–0.016).
ConclusionsThe B lymphocyte subsets of patients with selective IgA deficiency are largely similar to those of patients with common variable immunodeficiency. Both illness groups exhibit low levels of memory B cells.
The early stages of B cell development occur in the bone marrow. B cells then continue to mature in the peripheral lymphoid organs, where they encounter foreign antigens.1 Antigenic stimulation triggers the proliferation and differentiation of antigen-specific cells. Successive steps in B cell differentiation result in the generation of two types of affinity-matured B cells: memory B cells and antibody-secreting plasma cells.2,3 Memory B cells continuously circulate between the blood and lymphoid organs and can rapidly differentiate into effector cells following cognate antigen recognition. By contrast, long-living plasma cells can reside in the bone marrow and produce high-affinity antibodies without antigenic stimulation.4–6
Antibody deficiency is defined as a decrease of 2SD (two standard deviations) in the levels of at least one of the immunoglobulin (Ig) isotypes compared to the mean values of age.7. The objective of this study was to analyse the memory B cell subsets of patients with antibody deficiencies, such as partial IgA deficiency (pIgAD), selective IgA deficiency (sIgAD), selective IgM deficiency (sIgMD), common variable immunodeficiency (CVID), unclassified hypogammaglobinaemia (UCH), and transient hypogammaglobinaemia in infancy (THI).
Materials and methodsStudy populationThe study had a prospective design. Patients who were followed by our paediatric immunology and allergy department between March 2012 and March 2014 were included in the study. Out of the 67 patients with antibody deficiencies, 20 patients with THI, 18 patients with UCH, 13 patients with CVID, 7 patients with sIgAD, 5 patients with sIgMD, and 4 patients with pIgAD participated in the study. Twenty-eight healthy, age-matched children were included in the study as the control group. Sixty-seven patients with antibody deficiencies were grouped according to their diagnoses. Each patient group was compared with the children in the control group. On the condition that their ages matched, some children from the control group were used to compare data with more than one of the patient groups.
Definition of antibody deficiencyThe patients’ serum Ig levels were measured using nephelometry. Normal values were interpreted according to healthy, age-matched Turkish children, as reported by Tezcan et al.8 Patients who used antiepileptics and corticosteroids, or who had antibody deficiencies that were caused by other chronic diseases, immunodeficiencies, and congenital anomalies, were excluded from the study.
Definition of primary immunodeficienciesTransient hypogammaglobinaemia in infancy was diagnosed according to the following criteria:
- •
Low serum IgG levels that were accompanied by low IgA and/or IgM levels upon admission.
- •
Normalisation of low Ig levels during follow-up.
- •
Normal production of an antibody specific to isohaemagglutinins.
- •
Intact cellular immunity.
UCH was diagnosed according to the following criteria:
- •
Low serum IgG levels that were accompanied by low IgA and/or IgM levels upon admission.
- •
Low Ig levels by the end of follow-up.
- •
Normal production of an antibody specific to isohaemagglutinins.
- •
Intact cellular immunity.
Selective IgA deficiency is defined as IgA levels <7mg/dL in children older than four years of age.
Partial IgA deficiency is defined in children who are older than four years of age as IgA levels <2SD of the age-matched normal values.
Selective IgM deficiency is defined as IgM levels <2SD of the age-matched normal values.
The criteria for the diagnoses of common variable immunodeficiencies included the presence of a low value of at least one of the IgM or IgA levels and all of what follows in male or female patients with IgG levels that were clearly low (average levels were lower than at least 2SD of age):
- •
Onset of immune deficiencies after the age of two.
- •
Absence of isohaemagglutinin and/or a weak immune response to vaccines.
- •
Exclusion of other factors that cause antibody deficiency.7,9,10
Total serum Ig levels were measured using commercially available nephelometry kits (Dade Behring Marburg GmbH, Marburg, Germany). For the antibody response, the patients’ poliovirus responses and isohaemagglutinin levels were studied. Three cc of blood was taken from each patient and stored in tubes containing ethylenediamine tetraacetic acid (EDTA). Immunophenotyping was performed using the following monoclonal antibodies: IgD PE, CD19 APC, and CD27 FITC (BD Biosciences, Pharmingen, Germany). The percentages of the lymphocyte subsets in the CD19 complex were analysed using flow cytometry (BD FACS Calibur; BD Biosciences, San Jose, USA). The peripheral CD19+ B cell subsets were defined as follows: memory B cells as CD19+CD27+, naive mature B cells as CD19+CD27−IgD+, non-switched B cells as CD19+CD27+IgD+, and class-switched memory B cells as CD19+CD27+IgD−.11
Statistical analysesAll statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS, Version 15, Windows). All data was expressed as the median or percentages that were caused by distributions that were not considered normal. A Mann–Whitney U test was used, and values of p<0.05 were accepted to be statistically significant.
Ethical disclosureEthical approval was granted in decision no. OMU KAEK 2012/544 (dated 23.02.2012) by Ondokuz Mayis University's Ethics Committee of Medical Research.
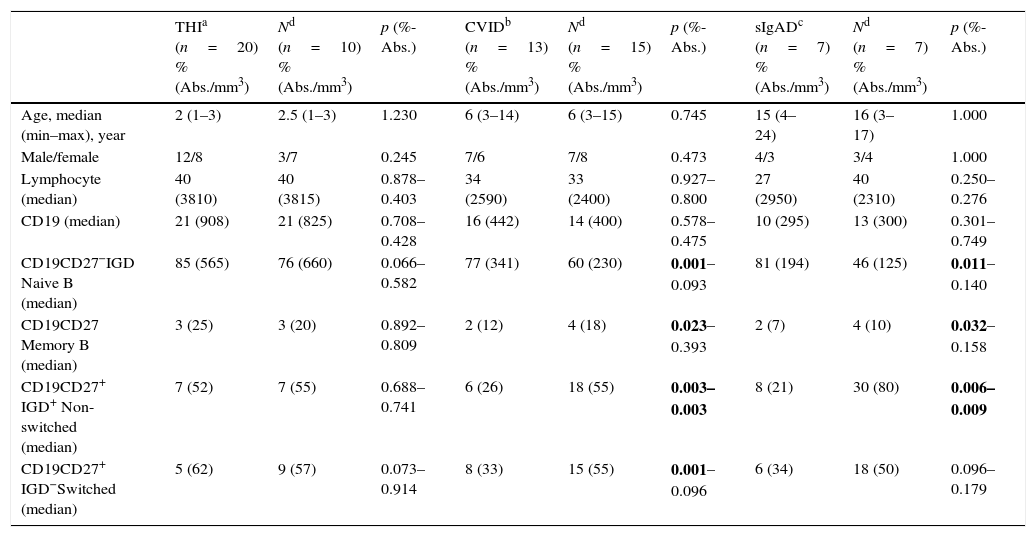
ResultsA total of 67 patients with antibody deficiencies and 28 healthy, age-matched children were included in the study. Forty (59.7%) of the patients were male and had a median age of six years (min–max: 1–24), and twelve (42.8%) of the healthy controls were male and had a median age of six years (min–max: 1–17). The clinical data and patients’ serum Ig levels are summarised in Tables 1 and 2, respectively. Based on patient's diagnoses, 20 patients with THI (12 males and 8 females; aged 1–3; median 2 years), 18 patients with UCH (10 males and 8 females; aged 3–8; median 5 years), 13 patients with CVID (7 males and 6 females; aged 3–14; median 6 years), 7 patients with sIgAD (4 males and 3 females; aged 4–24; median 15 years), 4 patients with pIgAD (2 males and 2 females; aged 4–9; median 7 years), and 5 patients with sIgMD (5 males; aged 3–15; median 13 years) were included in the study. There was no significant difference between the control groups and the THI and UHC patients’ B lymphocyte subsets (p>0.05). While patients with CVID had a higher relative count of naive cells than the control group, their relative counts of memory B lymphocytes, relative and absolute counts of non-switched B lymphocytes, and relative counts of switched B cells were found to be lower than the control group's (p=0.001, 0.023, 0.003–0.003, and 0.001, respectively). Similar to patients with CVID, sIgAD patients were found to have higher relative counts of naive cells, lower relative counts of memory B cells, and lower relative and absolute counts of non-switched B cells (p=0.011, 0.032, 0.006–0.009, respectively). Unlike patients with CVID, patients with sIgAD were found to have a normal rate of switched memory B cells (p>0.05). Patients with pIgAD had a higher relative count of naive B cells than the control group (p=0.02). Although patients with sIgMD also had higher relative counts of naive B cells than the control group, their relative and absolute counts of non-switched B cells were lower (p=0.008 – 0.016) (Tables 3 and 4; Figs. 1 and 2).
Clinical data of patients with antibody deficiencies.
| n | Age, year median (min–max) | Male (%) | URTI (%) | Pneumonia (%) | Diarrhoea (%) | Otitis (%) | Parotitis (%) | Atopy (%) | Abscess (%) | HSM (%) | LAP (%) | Anaemia (%) | Thrombocytopenia (%) | Neutropenia (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Transient hypogammaglobulinaemia | 20 | 2 (1–3) | 60 | 50 | 5 | 5 | 20 | – | 30 | – | – | 5 | – | – | – |
| Unclassified hypogammaglobulinaemia | 18 | 5 (3–8) | 55 | 94 | 11 | 11 | 16 | – | 22 | – | – | – | – | – | – |
| Common variable immunodeficiency | 13 | 6 (3–14) | 54 | 92 | 46 | 38 | 46 | 7 | 23 | – | 30 | 38 | – | 14 | 7 |
| Selective IgA deficiency | 7 | 15 (4–24) | 57 | 71 | 30 | 14 | 28 | – | 28 | – | 14 | 14 | – | – | – |
| Selective IgM deficiency | 5 | 13 (3–15) | 100 | 40 | – | – | 20 | – | 20 | – | – | 20 | – | – | – |
| Partial IgA deficiency | 4 | 7 (4–9) | 50 | 100 | – | – | – | – | 25 | – | – | – | – | – | – |
URTI: upper respiratory tract infection; HSM: hepatosplenomegaly; LAP: lymphadenopathy.
Serum immunoglobulin levels of patients with antibody deficiencies.
| Diagnosis | IgG | IgA | IgM | |
|---|---|---|---|---|
| Case 1 | THI | L | N | L |
| Case 2 | THI | L | L | L |
| Case 3 | THI | L | L | N |
| Case 4 | THI | L | L | L |
| Case 5 | THI | L | L | L |
| Case 6 | THI | L | N | L |
| Case 7 | THI | L | L | L |
| Case 8 | THI | L | L | L |
| Case 9 | THI | L | N | L |
| Case 10 | THI | L | N | N |
| Case 11 | THI | L | N | L |
| Case 12 | THI | L | L | N |
| Case 13 | THI | L | L | L |
| Case 14 | THI | L | L | L |
| Case 15 | THI | L | L | N |
| Case 16 | THI | L | L | N |
| Case 17 | THI | L | N | N |
| Case 18 | THI | L | N | N |
| Case 19 | THI | L | N | L |
| Case 20 | THI | L | L | N |
| Case 21 | UCH | L | L | N |
| Case 22 | UCH | L | N | N |
| Case 23 | UCH | L | L | N |
| Case 24 | UCH | L | N | N |
| Case 25 | UCH | L | N | L |
| Case 26 | UCH | L | N | L |
| Case 27 | UCH | L | L | N |
| Case 28 | UCH | L | N | N |
| Case 29 | UCH | L | L | L |
| Case 30 | UCH | L | N | N |
| Case 31 | UCH | L | L | L |
| Case 32 | UCH | L | L | L |
| Case 33 | UCH | L | N | N |
| Case 34 | UCH | L | L | L |
| Case 35 | UCH | L | N | L |
| Case 36 | UCH | L | N | N |
| Case 37 | UCH | L | L | L |
| Case 38 | UCH | L | N | N |
| Case 39 | CVID | L | L | N |
| Case 40 | CVID | N | L | N |
| Case 41 | CVID | L | N | L |
| Case 42 | CVID | L | L | N |
| Case 43 | CVID | L | N | N |
| Case 44 | CVID | L | N | L |
| Case 45 | CVID | L | L | N |
| Case 46 | CVID | L | N | L |
| Case 47 | CVID | L | N | L |
| Case 48 | CVID | L | N | L |
| Case 49 | CVID | L | L | N |
| Case 50 | CVID | L | L | L |
| Case 51 | CVID | L | N | L |
| Case 52 | SIgAD | N | La | N |
| Case 53 | SIgAD | N | La | N |
| Case 54 | SIgAD | N | La | N |
| Case 55 | SIgAD | N | La | N |
| Case 56 | SIgAD | N | La | N |
| Case 57 | SIgAD | N | La | N |
| Case 58 | SIgAD | N | La | N |
| Case 59 | SIgMD | N | N | L |
| Case 60 | SIgMD | N | N | L |
| Case 61 | SIgMD | N | N | L |
| Case 62 | SIgMD | N | N | L |
| Case 63 | SIgMD | N | N | L |
| Case 64 | PIgAD | N | Lb | N |
| Case 65 | PIgAD | N | Lb | N |
| Case 66 | PIgAD | N | Lb | N |
| Case 67 | PIgAD | N | Lb | N |
L: low; N: normal.
Comparison of B cell subsets in healthy children and patients with THI, CVID, and sIgAD.
| THIa (n=20) %(Abs./mm3) | Nd (n=10) %(Abs./mm3) | p (%-Abs.) | CVIDb (n=13) %(Abs./mm3) | Nd (n=15) %(Abs./mm3) | p (%-Abs.) | sIgADc (n=7) %(Abs./mm3) | Nd (n=7) %(Abs./mm3) | p (%-Abs.) | |
|---|---|---|---|---|---|---|---|---|---|
| Age, median (min–max), year | 2 (1–3) | 2.5 (1–3) | 1.230 | 6 (3–14) | 6 (3–15) | 0.745 | 15 (4–24) | 16 (3–17) | 1.000 |
| Male/female | 12/8 | 3/7 | 0.245 | 7/6 | 7/8 | 0.473 | 4/3 | 3/4 | 1.000 |
| Lymphocyte (median) | 40 (3810) | 40 (3815) | 0.878–0.403 | 34 (2590) | 33 (2400) | 0.927–0.800 | 27 (2950) | 40 (2310) | 0.250–0.276 |
| CD19 (median) | 21 (908) | 21 (825) | 0.708–0.428 | 16 (442) | 14 (400) | 0.578–0.475 | 10 (295) | 13 (300) | 0.301–0.749 |
| CD19CD27−IGD Naive B (median) | 85 (565) | 76 (660) | 0.066–0.582 | 77 (341) | 60 (230) | 0.001–0.093 | 81 (194) | 46 (125) | 0.011–0.140 |
| CD19CD27 Memory B (median) | 3 (25) | 3 (20) | 0.892–0.809 | 2 (12) | 4 (18) | 0.023–0.393 | 2 (7) | 4 (10) | 0.032–0.158 |
| CD19CD27+ IGD+ Non-switched (median) | 7 (52) | 7 (55) | 0.688–0.741 | 6 (26) | 18 (55) | 0.003–0.003 | 8 (21) | 30 (80) | 0.006–0.009 |
| CD19CD27+ IGD−Switched (median) | 5 (62) | 9 (57) | 0.073–0.914 | 8 (33) | 15 (55) | 0.001–0.096 | 6 (34) | 18 (50) | 0.096–0.179 |
Bolded p-values indicate statistical significance.
Comparison of B cell subsets in healthy children and patients with UCH, pIgAD, and sIgGM.
| Unclassified (n=18) %(Abs./mm3) | Nc (n=16) %(Abs./mm3) | p (%-Abs.) | pIgADa (n=4) %(Abs./mm3) | Nc (n=4) %(Abs./mm3) | p (%-Abs.) | sIgMDb (n=5) %(Abs./mm3) | Nc (n=5) %(Abs./mm3) | p (%-Abs.) | |
|---|---|---|---|---|---|---|---|---|---|
| Age, median (min–max), year | 5 (3–8) | 3 (3–8) | 0.177 | 7 (4–9) | 7.5 (5–8) | 0.766 | 13 (3–15) | 13 (3–15) | 0.831 |
| Male/female | 10/8 | 6/11 | 0.479 | 2/2 | 2/2 | 1.000 | 5/0 | 2/3 | 0.167 |
| Lymphocyte (median) | 33 (3200) | 36 (3560) | 0.908–0.692 | 28 (2685) | 27 (3025) | 0.773–0.564 | 30 (2300) | 33 (1850) | 0.834–0.175 |
| CD19 (median) | 15 (517) | 17 (556) | 0.528–0.817 | 15 (383) | 15 (385) | 1.000–1.000 | 12 (252) | 14 (250) | 0.172–0.917 |
| CD19CD27−IGD Naive B (median) | 74 (372) | 69 (380) | 0.457–0.974 | 66 (254) | 46 (210) | 0.020–0.248 | 74 (186) | 56 (150) | 0.009–0.165 |
| CD19CD27 Memory B (median) | 3 (15) | 4 (20) | 0.229–0.234 | 3 (13) | 6 (20) | 0.078–0.076 | 2 (6) | 3 (10) | 0.107–0.461 |
| CD19CD27+ IGD+ Non-switched (median) | 7 (45) | 11 (60) | 0.144–0.053 | 9 (34) | 29 (105) | 0.078–0.083 | 6 (18) | 20 (55) | 0.008–0.016 |
| CD19CD27+ IGD−Switched (median) | 10 (56) | 10 (66) | 0.753–0.668 | 14 (61) | 15 (75) | 0.885–0.248 | 11 (21) | 19 (20) | 0.293–0.251 |
Bolded p-values indicate statistical significance.
B lymphocyte subgroups of patients with transient hypogammaglobinaemia in infancy and unclassified hypogammaglobinaemia are similar to those found in the healthy control groups. While patients with CVID have higher relative counts of naive cells than the control group, they were found to have lower relative counts of memory cells, relative counts of switched cells, and relative and absolute counts of non-switched cells. C: Control; L: Lymphocyte; N: Naive; M: Memory; S: Switched; NS: Non-switched.
Relative counts of the naive cells in patients with partial IgA deficiencies, selective IgA deficiencies, and selective IgM deficiency was higher than in the healthy control group. Relative and absolute counts of non-switched cells and relative counts of memory cells were lower in patients with selective IgA deficiencies than in the healthy control group. Relative and absolute counts of non-switched cells in patients with selective IgM deficiencies were also lower than in the control group. C: Control; L: Lymphocyte; N: Naive; M: Memory; S: Switched; NS: Non-switched.
A CD27 with a surface expression of IgD can be used as a marker of human memory B cells. Memory B cells can be subdivided into two distinct subsets: non-switched cells, which predominantly synthesise IgM, and switched cells, which synthesise IgG, IgM, or IgA.7 In a previous study, it was shown that patients with CVID had five pathophysiologically different disorders. From most common to least common, these disorders included B cell activation and proliferation defect, germinal centre defects, B cell production defects, post germinal centre defects, and B cell maturation defects.12 Extremely low counts of switched memory B cells in patients with CVID were found to be associated with splenomegaly and granulomatous disease. In addition, extremely low counts of switched memory B cells were associated with germinal centre defects.11 The current study was informed by existing literature and found that patients with CVID had lower relative counts of switched memory B cells than the control group.
Recently, a group of patients who did not meet specific common variable immunodeficiency diagnosis criteria (reduction of two Ig isotypes and a reduced response to vaccination) were defined as idiopathic primary hypogammaglobinaemia. The most significant difference between these patients and patients with common variable immunodeficiencies was their slightly lower serum Ig levels. While these patients came to the hospital with serious infectious complications, their peripheral B cell subgroups were typically normal.13 For the current study, we used European Society of Immune Deficiencies (ESID) and Pan American Group for Immune Deficiency (PAGID) diagnosis criteria for CVID.9. Although 11 of our patients fully met the CVID diagnosis criteria, two were assessed with probable CVID. The two patients with probable CVID had lymphocyte subgroups that were similar to the lymphocyte subgroups in patients that fully met the CVID diagnosis criteria.
Most patients with selective IgA deficiencies are asymptomatic. Allergic and autoimmune diseases are especially common in these patients. Some patients may seek treatment for recurrent infections because they cannot generate antibodies against the antigens in carbohydrate structures.14. For patients with CVID, memory cell counts are used as prognostic markers of splenomegaly, autoimmunity, intestinal disease, respiratory disease, and granuloma formations. In CVID patients who have low counts of memory cells, these clinical entities are more commonly found. In some of the patients with selective IgA deficiencies, a transformation to CVID has been reported to occur in progressive stages.14,15. For our study, while the relative counts of naive cells were found to be higher in patients with CVID than in the control group, the relative counts of memory cells, relative counts of switched cells, and relative and absolute counts of non-switched cells, were found to be lower in the patients with CVID. The determination of CD27+IgD− cells might help predictions of the progression of sIgAD to CVID. Patients with low counts of CD27+IgD− cells may have a genetic predisposition to the development of CVID or at least be more prone to CVID than patients with normal counts of CD27+IgD− cells.16 In the current study, while relative counts of naive cells were found to be high in patients with sIgAD, switched cells were found to be normal, relative counts of memory cells were found to be low, and relative and absolute counts of non-switched cells were found to be low. According to these data, the B lymphocyte subsets of patients with sIgAD are largely similar to those of patients with CVID. Therefore, patients with selective IgA deficiencies, and who have low counts of memory cells, should be monitored closely for CVID.
Memory B cells can be produced from either the classical germinal centre pathway or the less commonly understood germinal centre-independent route.17 In the current study, patients with CVID and selective IgA deficiencies were found to have lower relative counts of memory cells and relative and absolute counts of non-switched cells when compared with the healthy controls. In addition, patients with CVID had lower relative counts of switched cells than the healthy controls. Germinal centre defects have been previously demonstrated in patients with CVID.12 This finding confirms that patients with sIgAD may also have germinal centre defects.
Increases in naive cells that were caused by disorders in the early phases of B cell development were shown in such autoimmune diseases as Sjögren's syndrome, rheumatoid arthritis, systemic lupus erythematosus, and type 1 diabetes mellitus. An increase of naive cells is thought to have an effect on autoimmunity.18 In our study, relative counts of the naive cells in patients with CVID, SIgAD, SIgMD, and PIgAD were found to be higher than in the control groups. The incidence of autoimmune disease increases in patients with CVID, SIgAD, SIgMD, and PIgAD.19–22 Increases in naive B cells that are caused by these antibody disorders may be due to deficiencies during early phases of B cell development. Similarly, naive cells likelihood can increase the autoimmunity in patients with CVID, SIgAD, SIgMD, and PIgAD.
Bukowska-Strakova et al.23 reported that the development of B cell subsets was normal during cases of THI. In their study, Cipe et al.7 found their counts of naïve B lymphocytes in patients with THI to be higher than they were in the control group. The authors explained this finding by way of the delay in maturation. In the current study B cell sub-groups were found to be normal in patients with THI and UCH. Recently, Keleş et al.10 reported that patients with THI and UCH showed similar clinical and laboratory features. This confirms that THI and UCH have normal B cell sub-groups and that both immunodeficiencies may be caused by delays in maturation.
Cipe et al.7 found that the counts for the non-switched memory B cells were low in 16 patients with sIgMD. They stated that the low cell count resulted in the identification of low levels of IgM. Similarly, our study's relative and absolute counts of non-switched B cells in patients with sIgMD were found to be lower than in the control group. Unlike Cipe et al., we found that the relative counts of naive B memory cells were high in patients with sIgMD.
Recurrent sinopulmonary infections are the most common clinical findings in patients with sIgAD.20 Very little data in existing literature covers the clinical findings of patients with partial IgA deficiencies. In our study, all of the patients with partial IgA deficiencies were admitted to the hospital with recurrent upper respiratory tract infections. The importance of secretory IgA is a well-known element of mucosal defence.20 Serious infections were not seen in any of our patients. Selective IgA levels can correspond with clinical infections that are more serious than partial IgA levels. However, our number of cases was not large enough to confirm that patients with partial IgA levels could prevent the development of serious infections such as pneumonia. In Cipe et al.’s7 study, the B lymphocyte subsets of patients with pIgAD were found to be normal. Conversely, in our study, the relative counts of naive B lymphocytes were found to be higher in patients with pIgAD than in the control.
Recurrent infections are more common when IgG subgroup deficiencies accompany selective IgA deficiencies.14 One of the limitations of our study was that patients with selective IgA deficiencies were not checked for IgG subgroups deficiencies. This study was also limited by the small number of patients in each of the three disease groups (SIgAD, PIgAD and SIgMD). In other words, larger series will be needed to obtain healthier data.
To conclude, while the B lymphocyte subsets of patients with THI and UCH were found to be normal, the B lymphocyte subsets of patients with sIgAD were largely similar to those found in patients with CVID, and both groups contained low levels of memory B cells. This result demonstrates that patients with sIgAD should be monitored regularly for CVID.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
FundingNone declared.
ContributorsEach of the authors has contributed to, read, and approved this manuscript.
Conflict of interestThe authors have no conflict of interest to declare.