Immunotherapy selectively modulates the allergen-specific immune response. It involves the gradual administration of increasing amounts of allergen for the purpose of inducing protective immunological changes and it is the only curative approach for specific type I allergy.
Aim- -
Description of the allergic inflammation.
- -
Comprehension of the early cellular changes after specific immunotherapy has been initiated.
- -
Exposure of the mechanisms involved in tolerance induction by regulatory T cells (Treg) with the inhibition of the Th2 responses.
- -
Comprehension of IL-10 and transforming growth factor-β (TGF-β) roles.
- -
Explanation of specific IgE, IgG and IgA changes.
- -
Description of the suppression of inflammatory responses during immunotherapy.
Treatment of allergic diseases consists in allergen avoidance and the use of pharmacotherapy. This includes antihistamines, corticosteroids, antileukotrienes and beta-2 agonists1. Although effective at controlling symptoms and inflammation, these treatments can have side effects if used for a long time. In patients, where it has been demonstrated and documented that symptoms appear on exposure to specific allergens, allergen-specific immunotherapy (SIT) should be indicated. This treatment selectively modulates the allergen-specific immune response.
Allergen-specific immunotherapy involves the gradual administration of increasing amounts of allergen for the purpose of inducing protective immunological changes. SIT is currently the only treatment that alters the abnormal immune response underlying allergic disease. It is the only curative approach for specific type I allergy.2 Unlike pharmacotherapy, allergen immunotherapy provides long-term clinical benefits. These include long-term disease remission, prevention of new atopic sensitisations, and a reduction in disease progression from rhinitis to asthma.3
Since the first use of SIT in the early 20th century (a hundred years ago), a large amount of clinical trials have been published. These studies have led SIT to emerge as an evidence-based treatment with the knowledge of some of its mechanisms of action.4
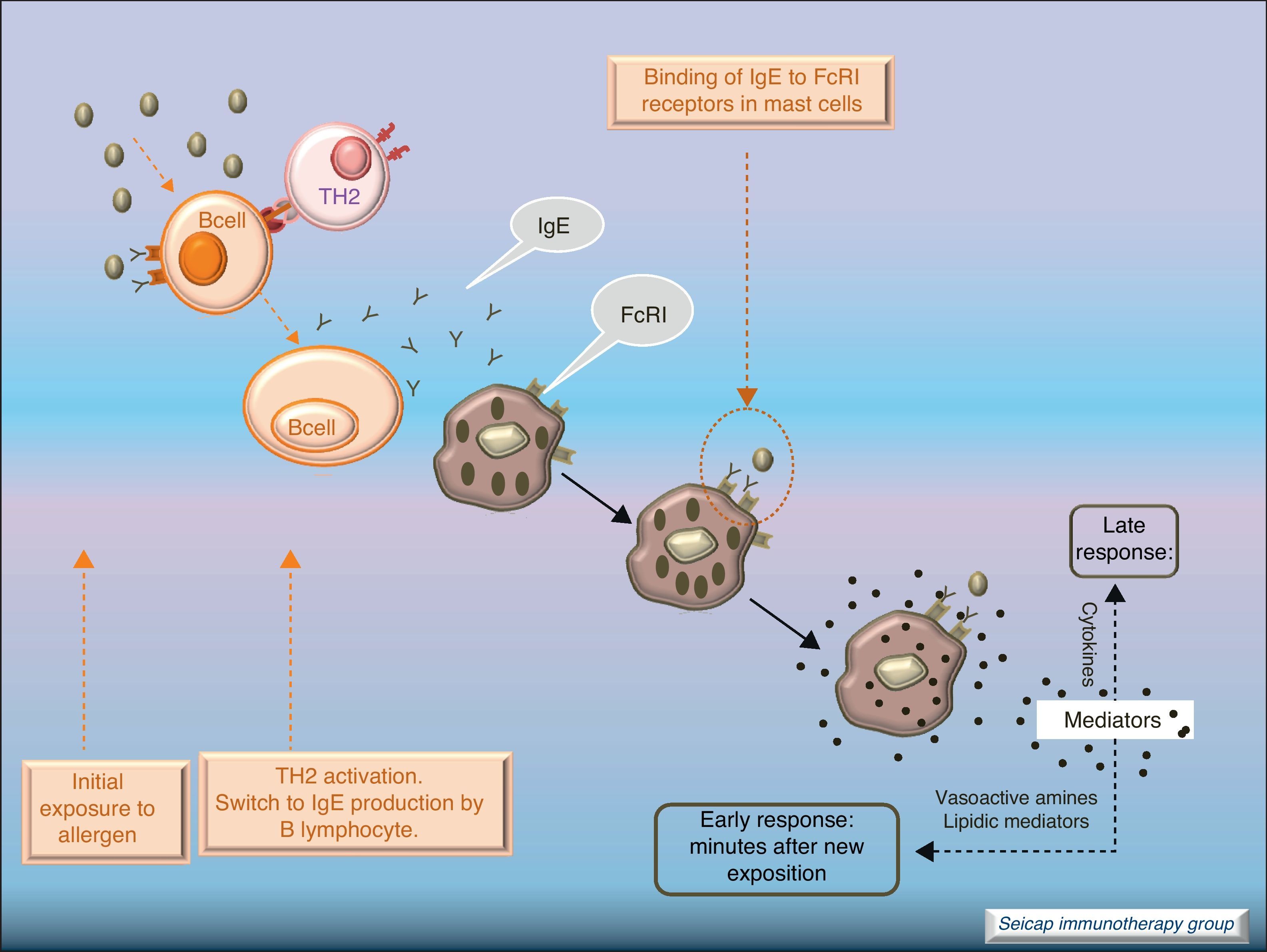
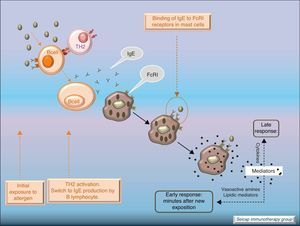
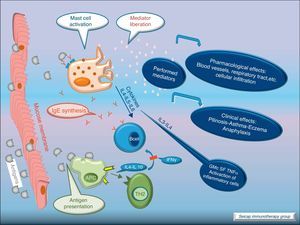
Allergic inflammationAfter a previous exposure to an allergen in susceptible individuals, IgE antibodies are generated. These antibodies bind to high affinity IgE Fc receptors on blood basophils and mucosal tissue mast cells. On reexposure to the specific allergen, a biphasic response is elicited.1 Allergic patients, who are challenged with the antigen implicated in their allergic inflammation, often respond with this biphasic reaction. There is an early phase (peak at 15–30min after allergen exposure) with the release of mediators from local tissue mast cells and circulating basophils. These mediators include histamine, kinins, prostaglandin D2, cytokines, chemokines and leukotrienes. Their function is the recruitment of cells to the zone leading to the arrival of inflammatory cells that include eosinophils, T lymphocytes and additional basophils which release specific inflammatory mediators (late phase, 6–12h after allergen exposure). Thus, the mechanisms that contribute to the allergic inflammation persist. Fig. 1.
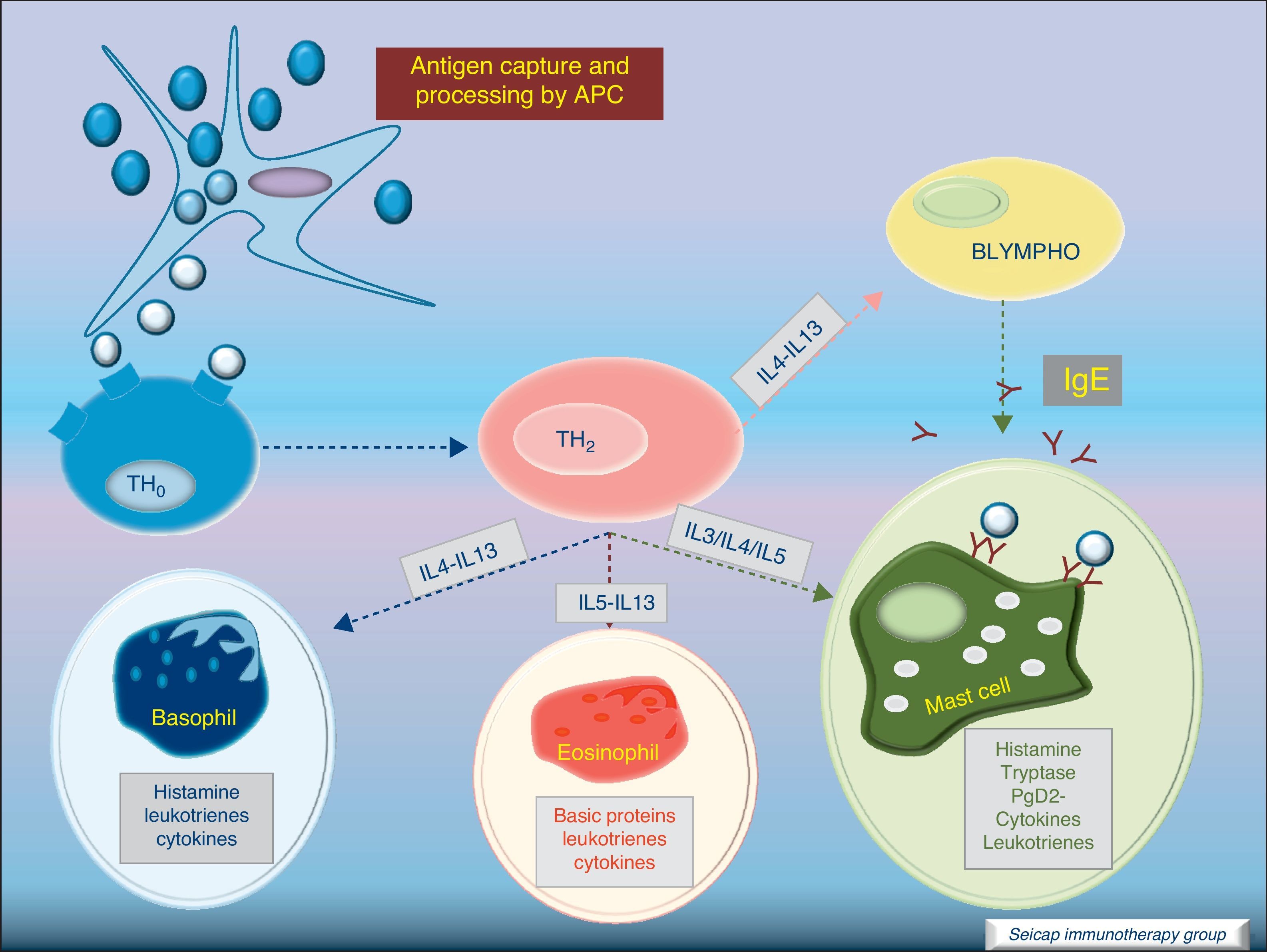
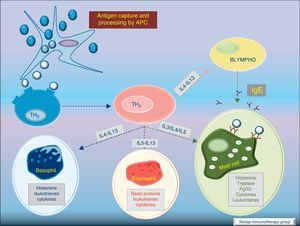
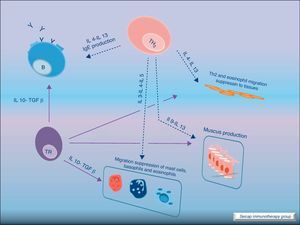
Most of the allergens which contact the respiratory mucosa are cleared by the physical barrier. Some will penetrate the epithelium and will be captured by antigen presenting cells, especially immature dendritic cells (DC). In healthy individuals this results in the induction of tolerance.5 In atopic individuals, this initial encounter results in the switch of T-cells to Th2 cells. Specific IgE bound to low affinity IgE Fc receptors on DC play a role in facilitating allergen uptake.1 This allergen-loaded DC arrives to lymph nodes where they present processed allergen as peptides to T cells with allergen-specific receptors. It is then when activated T cells release cytokines which facilitate allergen-specific B cell activation and differentiation to antibody producing plasma cells. The persistence of allergic disease depends on CD4+ Th2 lymphocytes and their production of IL-4, IL-5, IL-9 and IL-13 (and probably other recently identified interleukines such as IL-25, IL-31, and IL-336) which drive B cell immunoglobulin class switching to IgE, recruit and activate allergic inflammatory cells. Fig. 2.
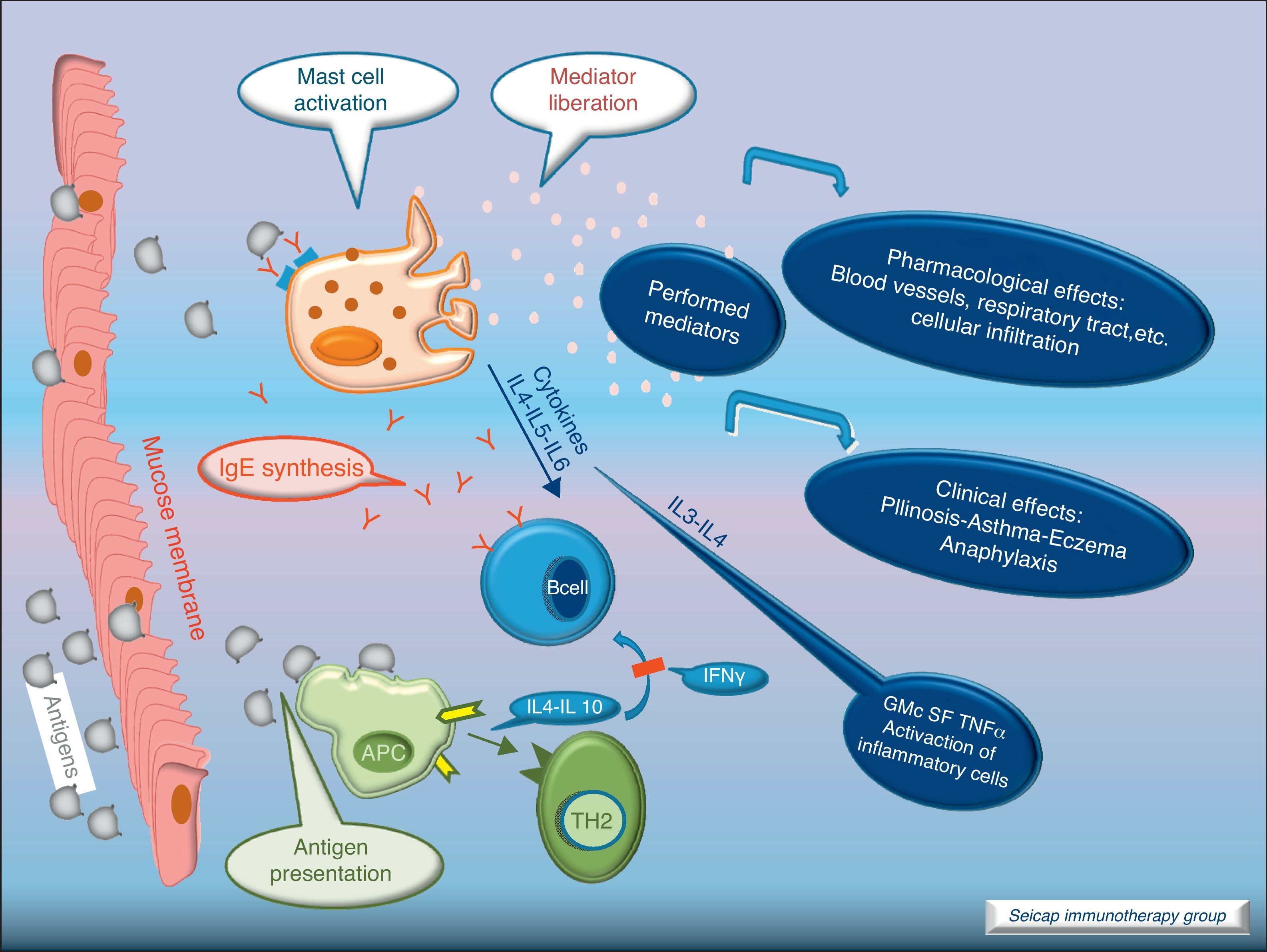
With the release of cytokines and mediators, the pharmacological effects on blood vessels, respiratory tract, etc., with cellular infiltration, and the clinical effects (pollinosis, asthma, eczema, anaphylaxis) become evident. Fig. 3.
Cytokine functionIL-4 – Induces the production of IgE and IgG4, and the expression of adhesion molecules by endothelial cells. Inhibits Th1 cell development and promotes Th2 cell development. Induces mucus production. Up-regulates B cell co-stimulatory molecules and the expression of low affinity IgE receptor.
IL-5 – Induces eosinophil differentiation, survival and growth.
IL-9 – Promotes mast cell and basophil growth and eosinophil development. Up-regulates expression of high affinity IgE receptor on mast cells.
IL-13 – Induces the production of IgE and IgG4, and the expression of adhesion molecules by endothelial cells. Induces mucus production.6
SIT inhibits the allergic early phase as well as the late response with a significant reduction in the number of infiltrating cells, eosinophils and basophils. It promotes the reduction of inflammatory mediators at sites of allergen exposure.7,8 To understand the underlying mechanisms for these tissue changes, many studies have examined changes in immunological parameters accompanying clinically effective SIT.2,9
Early phase cellular changesFrom the beginning of SIT (sometimes after the first injection), there is a decrease in the activity of basophils and mast cells with a fall in their degranulation and capacity of inducing anaphylaxis.10 The suppression of the activity of these cells, which implies early desensitisation, can also be enhanced by changes in other immunological parameters such as the appearance of allergen specific regulatory T-cells (Treg) and the fall in specific IgE.11
Changes in humoral immunity- 1.
Changes in IgE: when SIT is initiated, allergen-specific IgE levels usually show an initial increase and then a gradual decrease during the years of treatment. This parameter correlates poorly with clinical efficacy.
- 2.
Changes in IgG: SIT generally results in an increase in allergen-specific IgG antibodies which can persist for many years after SIT is discontinued.12 This rise does not necessarily correlate with improvement in symptoms but if the levels do not increase, there is a lack of clinical response.12
- 3.
Changes in IgG1 and IgG4 antibodies: allergen-specific IgG1 and IgG4 antibody levels may increase. IgG4 are thought to act as “blocking” antibodies. They inhibit IgE-facilitated allergen uptake by DC and prevent IgE-mediated allergen activation of basophils and mast cells with the subsequent inhibition of the release of inflammatory mediators.13 Thus, the blocking effects of IgG4 antibodies may have an important role in suppressing IgE-mediated T cell activation. However, the SIT-induced IgG4 levels fail to correlate with the clinical response to treatment.14
Chronic stimulation with allergen (SIT) induces a predominantly IgG4 response, while limited allergen exposure results in an IgG1 response.13 It is thought that these allergen-specific IgG4 antibodies compete for allergen with IgE bound to mast cells. It is also believed that allergen-specific IgG4 antibodies may reduce the sensitivity of antigen-presenting B cells and therefore T cells to allergen by competing with IgE.12
Most recently, a parallelism between the inhibition of early skin response, histamine release, IgG binding to B cells and IgG4 increase was demonstrated after SIT.15 Nevertheless, as stated before, the measurement of the levels of allergen-specific IgG4 does not seem to correlate exactly with clinical success and some studies14,15 failed to find an association between increased IgG4 and clinical benefit.15
- 4.
Changes in IgA: specific IgA2 levels are also increased (although in a more modest way) after SIT,16 and secreted specific IgA seems to play a protective role at mucosal surfaces. The isotype IgA2 may also act as “blocking antibody” at the mucosal surface. More studies are needed to confirm the protective action of high levels of this antibody after SIT.
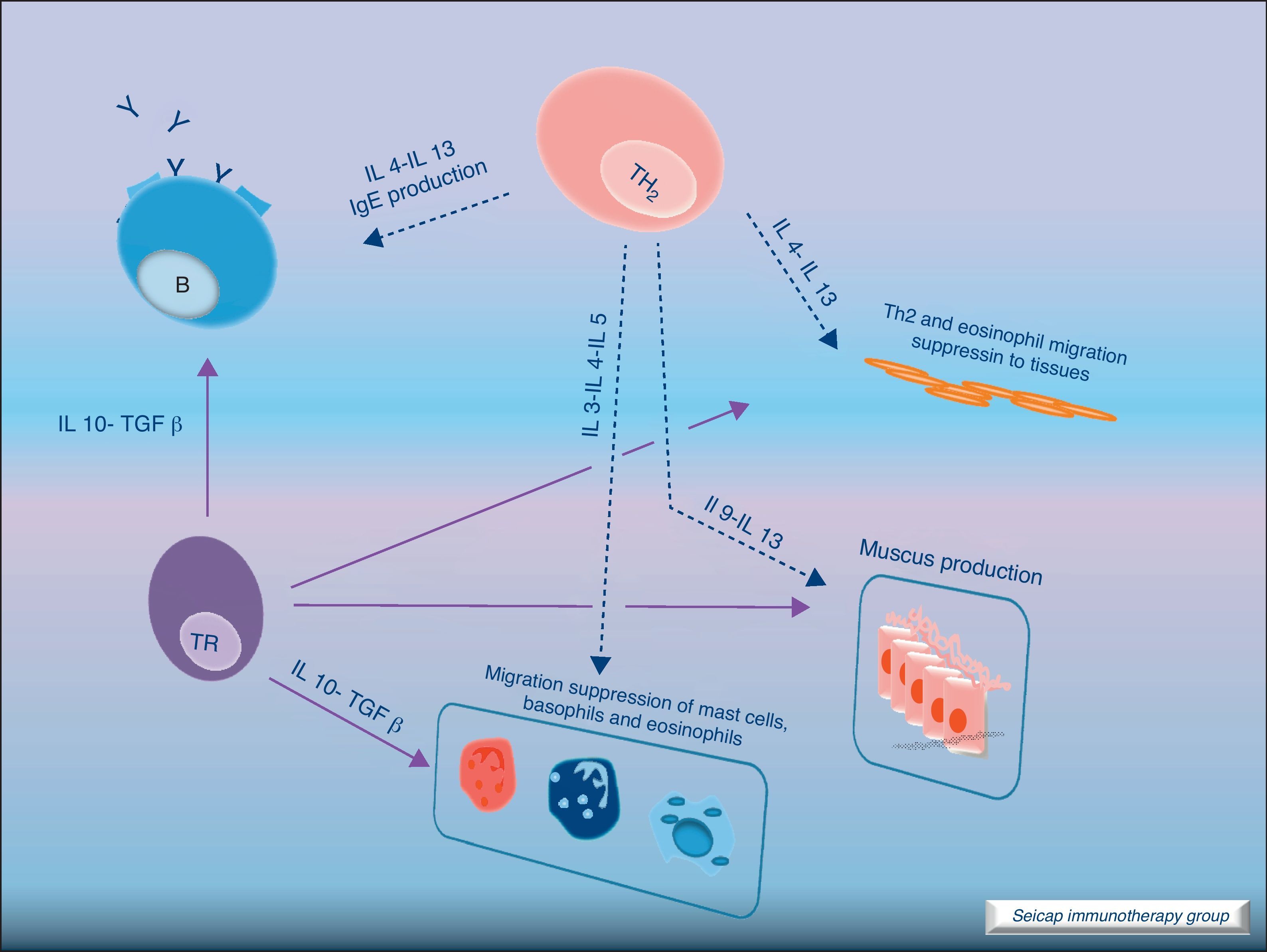
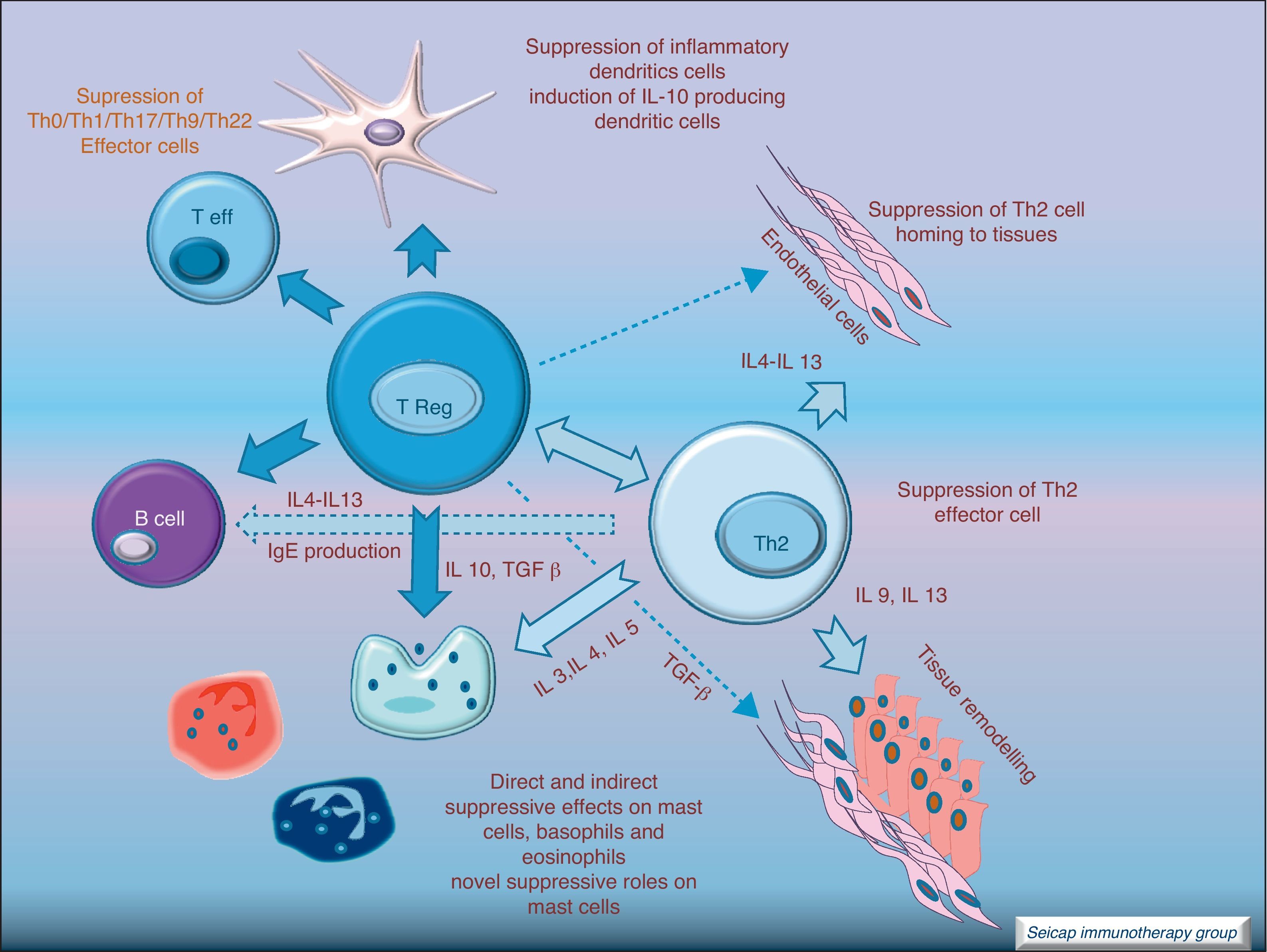
The induction of tolerance in peripheric T-cells is an essential step in SIT. This induced tolerance depends mainly on the generation of allergen-specific Treg cells17 and their production of IL-10 and TGF-β.17 Atopic patients have a decrease in their Treg function. In recent years there have been many studies in this field. Some of these studies have been performed in patients with hymenoptera venom allergy. These patients are a homogeneous group in which the effects of SIT on T cells are easier to study. There are also studies of SIT with grass pollen extract18 and house dust mites17 in which it can be demonstrated that there is a shift of CD4+ helper T cells from producing Th2 cytokines (IL-4, IL-5) to Th1 cytokines (interferon gamma, IL-10) following stimulation with allergen. Early production (soon after the beginning of SIT) of IL-10 and maintained levels of TGF-β are related to an efficacious SIT. Fig. 4.
IL-10 is a potent immunosuppressive cytokine involved in tolerance induction and maintenance. It reduces proinflammatory cytokine release from mast cells, eosinophils, and T cells; and elicits tolerance in T cells by means of selective inhibition of the CD28 costimulatory pathway.19 It is produced by T regulatory cells (Treg) which also have the ability of producing transforming growth factor-beta (TGF-β), another immunosuppressive cytokine which inhibits specific IgE and IgA production. It also suppresses Th1 and Th2 cells.20 SIT-induced IL-10 production is not limited to T cells. It is also produced by B cells, monocytes and macrophages.
There are different types of Treg cells with characteristic phenotypes and mechanisms of action. Among these Treg cells, the natural subset, expressing CD4 and CD5 and the transcription factor fox p 3 (FOXP3+CD4+CD5+ cells), is the most studied in recent years.21 The expression of the transcription factor Foxp3 is required for natural Treg function and Treg development and expansion is dependent on TGF-β.22 Other subtypes of Treg cells such as Tr1 and Tr3 can be induced by different stimulus.23
It is noteworthy that rather than having reduced total Treg numbers, allergic patients have intrinsic defects in the ability of these cells to suppress pathogenic allergen-specific effector cell responses. Tregs are an essential component of the normal immune system for maintaining immune homeostasis to exogenous antigens.
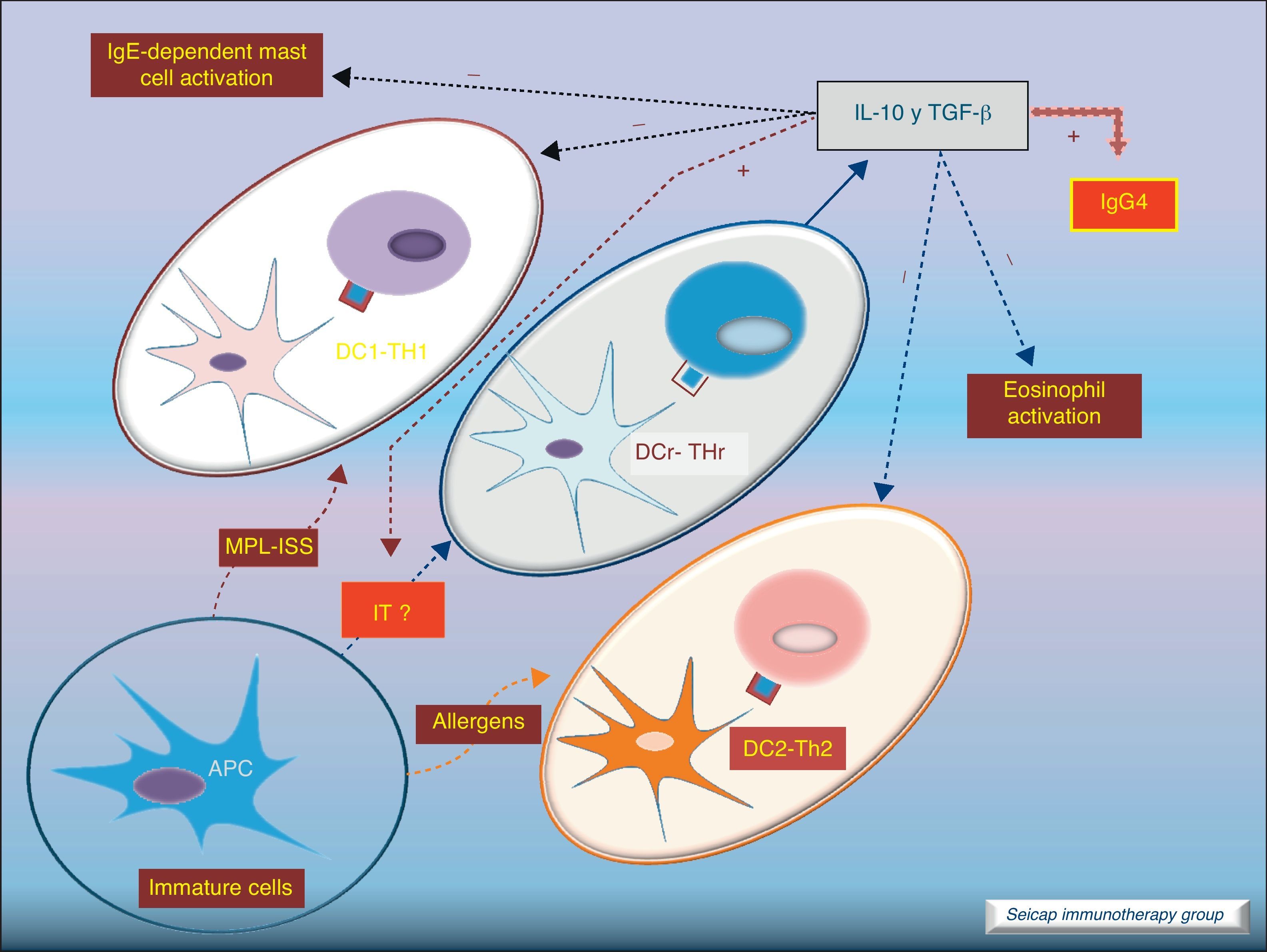
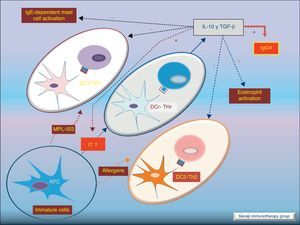
Dendritic cells also play an important role stimulating IL-10-producing Treg cells (these DC are named DCr). The other subtype DC1 directs Th1 responses and DC2 addresses the Th2 pathway. It is thought that the allergen extracts used for SIT could act directly on DC to induce a tolerogenic phenotype.8Fig. 5.
Role of dendritic cells: DC2 direct Th2 responses. Bacterial components (MPL, ISS; used as adjuvant for SIT) promote differentiation of DC to DC1 which direct Th1 responses. SIT directs the development of regulatory dendritic cells (DCr) that stimulate the development of Treg cells.
During SIT with grass pollen and venom an increase in Treg cell activation was also shown. These changes take place as soon as six hours after the first SIT dose which contrasts with the modification in Ig concentrations.11,16,18
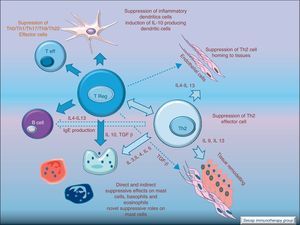
Regulatory T cells (Tregs) are considered the “master regulators” of immune homeostasis.24 They play a major role in maintaining immune self tolerance in the periphery and protect against excessive activation and disease.25 They have an important role in inducing tolerance in allergen-specific T cells in healthy and in allergic subjects following SIT. Moreover, SIT alters allergen-specific T cell reactivity. These events include decreases in antigen-specific proliferation and IL-4 production and increased IL-10 and TGF-β production.26Fig. 6.
These changes make SIT act at each step of the allergic reaction: IgE and IgG production, mast cell and eosinophil homing, T cell activation, and antigen presentation. SIT drives the immune system to tolerate allergens without the ongoing allergic inflammation. This antigen-specific immunomodulation is a unique and inestimable tool to understand not only the treatment, but also the disease it cures.4
IL-10 and TGF-β function, summaryIL-10- -
Specific IgE suppression.
- -
Allergen-specific IgG4 induction.
- -
Inhibition of the CD28 costimulatory pathway.
- -
Allergen specific lymphocytes Th1 and Th2 suppression.
- -
Inhibition of dendritic cells maturation.
- -
Increased expression of the transcription factor fox p 3.
- -
Decrease in the liberation of proinflammatory mediators in mast cells.
- -
Specific IgE suppression.
- -
Allergen-specific IgA induction.
- -
Allergen specific lymphocytes Th1 and Th2 suppression.
- -
Decrease of IgE high affinity receptors in Langerhans cells.
- -
Increase of the expression of Fox p 3.
Another consequence of high-dose SIT on T cells is cell death by apoptosis. Blood lymphocytes, mainly Th2 cells, show an increased susceptibility to undergo apoptosis when stimulated with allergen in vitro. Achieving high dose allergen treatment during SIT is important for obtaining efficacy.
Sublingual immunotherapy (SLIT) has emerged as an effective and safe alternative to subcutaneous immunotherapy27 (SCIT). The dose should be high and the time long enough.28
During SLIT the antigen is captured by DC through the sublingual mucosa. These DC mature (Langerhans’ cells) and migrate to the local lymph nodes where they present the antigen to T cells. Production of IgG blocking antibodies and Treg cells drive to inhibition of the Th2 responses.29
Langerhans’ cells in oral mucosa express Fc¿R1 and high amounts of MHC class I and II, as well as co-stimulatory molecules which make them highly suitable for antigen presentation.27 Nowadays evidence suggests that SCIT and SLIT share similar mechanisms of action.
Conflict of interestThe authors have no conflict of interest to declare.