The impact of COVID-19 is changing with country wise and depend on universal immunization policies. COVID-19 badly affects countries that did not have universal immunization policies or having them only for the selective population of countries (highly prominent population) like Italy, USA, UK, Netherland, etc. Universal immunization of BCG can provide great protection against the COVID-19 infection because the BCG vaccine gives broad protection against respiratory infections. BCG vaccine induces expressions of the gene that are involved in the antiviral innate immune response against viral infections with long-term maintenance of BCG vaccine-induced cellular immunity. COVID-19 cases are reported very much less in the countries with universal BCG vaccination policies such as India, Afghanistan, Nepal, Bhutan, Bangladesh, Israel, Japan, etc. as compared to without BCG implemented countries such as the USA, Italy, Spain, Canada, UK, etc. BCG vaccine provides protection for 50–60 years of immunization, so the elderly population needs to be revaccinated with BCG. Several countries started clinical trials of the BCG vaccine for health care workers and elderly people. BCG can be uses as a prophylactic treatment until the availability of the COVID-19 vaccine.
The recent COVID-19 outbreak from Wuhan city in China and spread globally with 4,648,479 confirmed cases and 309,008 deaths (as of May 16, 2020).1 SARS-CoV2 is pathogenically stronger than the previous outbreaks of coronavirus (Middle East Respiratory Syndrome (MERS) and Severe Acute Respiratory Syndrome (SARS)).2 SARS-CoV2 is transmitted from one person to another during sneezing or coughing droplets, reported in family settings as well as hospitals3 and is also transmitted from contaminated surfaces or contaminated consumables by self-inoculation through the eyes, mouth and nose.4,5 SARS-Cov-2 is closely related to the previous SARS coronavirus and the origin of SARS-Cov-2 is from the same reservoir bat host.6 Zoonotic transmission of the SARS coronavirus between bat and human by intermediate hosts palm civets and raccoon dogs,7 but the intermediate hosts for COVID-19 transmission within bats and humans are still unknown. All highly pathogenic SARS coronavirus (MERS-CoV, SARS-CoV, and SARS-CoV2) are related to the bat coronavirus genus compared to low pathogenic coronavirus (HCoVHKU1, HCoV-OC43, HCoV-NL63, and HCoV-229E). There is no curative therapy or vaccine for all types of coronaviruses to date, although a few vaccines have been developed and registered in clinical trials against the SARS-Cov-2 virus.8 COVID-19 enters into the host cell by using their transmembrane spike (S) proteins. Spike proteins are glycoproteins that bind with host cells ACE-2 cell membrane receptors.9 Current data is emphasizing that the available vaccines prevent viral infections by activation of the antiviral immune response, such as BCG. According to the literature available, BCG activates the human immune system against several types of viruses such as human Respiratory Syncytial Virus (hRSV), and human papillomavirus (HPV).10 This review deals with the importance of BCG in the prevention of COVID-19 expansion and its severity. Literature and surveys exhibiting the COVID-19 spread and severity are much greater in those countries which did not have any BCG vaccination regimen.
Different countries implemented different policies for BCG immunization because of their undefined efficacy.11 Various countries, such as India, Japan, etc., are having a universal BCG immunization program, whereas other countries such as Canada, USA, Italy, Spain, etc. implemented for the high-risk community. BCG immunization procedures differ from one country to another in favor of age, administration route, and doses of the vaccine. Most of the countries previously used three booster doses of BCG vaccine but nowadays only a single dose is used by an intradermal route at an early age, around the first year of life in newborns.12 No scientific evidence is available for booster doses or revaccination of BCG13 so the World Health Organization (WHO) Global Programme on Tuberculosis and Vaccines in 1995 did not recommend repeat BCG schemes. The WHO recommends that one dose of the BCG vaccine should be administered in all neonates of countries with a high incidence of TB.14 Immunization policies are revised or changed country-wise from time to time, depending on health policies, variation in evidence, community perception, the difference in TB, and comorbid incidence (HIV).11 The meta-analysis found the variation in BCG vaccine efficacy reduced the TB risk by 50% in controlled trials and the duration of the vaccine susceptibility remains unknown.15,16 One study reported that the TB mortality attributed to vaccination in a 20-year BCG and placebo-controlled trial fell by 82%.17,18 In that clinical trial, vaccination started from 1935 to 1938, and prospective TB cases finding by 1947.18 Another controlled trial stated the efficacy of the BCG vaccine with long term protection, approximately 60 years of age after vaccination.19
Why BCG vaccine onlyBCG vaccination provides a wide range of safety against bacterial and viral infections but there is no evidence regarding BCG, whether it directly reduced the COVID-19 infection or not.10 A study has shown the correlation between BCG vaccination and COVID-19 infection, and studies have also shown fewer COVID-19 cases in universally implemented countries. The universal use of the BCG vaccine for the community might decrease the spread of COVID-19, and it can help to stop the transmission of the disease.20 Randomized controlled trials are needed to determine the role of BCG vaccination in immune activation against COVID-19. Nevertheless, BCG has shown a number of side effects (blood in urine, joint pain, nausea, vomiting, painful urination, etc.) in immune-compromised people and pregnant women.21 The BCG vaccine may boost the immune system’s ability to fight off pathogens, including the deadly coronavirus. Various investigations showed that the BCG vaccine also defends against viral infections affecting the respiratory tract in humans and mice. BCG protects against bacterial infection and also protects against respiratory viral infections.10,22 In this study, mice who have BCG vaccination before infection have low Influenza A load in their blood with less damage to the lungs.23,24 Several studies have stated that the BCG vaccine stimulates the resistance against viral infection in animals by inducing the epigenetic modifications in macrophages, monocytes, dendritic cells, and other immune cells. These immune cells enhance the production of pro-inflammatory cytokines such as INF-γ, TNF-α, and IL-1b, and develop the resistance for herpes type 1 and 2 viruses.24,25 These studies provide an idea that BCG vaccination might activate the immune system against viral infection. Thus, there is a path by which vaccine provides protection and reduces the risk of severely infectious diseases. Further studies also revealed that the BCG vaccine increases resistance in laboratory animals against other viruses, and ensure that it can be uses as a method of COVID-19 treatment. COVID-19 spread extensively in those countries which did not implement BCG vaccination, such as the USA, Italy, Spain, France, Germany, South Korea, Iran, etc. whereas those countries that have implemented BCG vaccination earlier showed a slower spread and low severity of COVID-19. Italy implemented the BCG vaccination. Four clinical trials are recruited in clinicaltrial.gov with BCG vaccination to prevent or reduce the severity of COVID-19 in the elderly population and Health Care Workers.26 To manage the COVID-19 infection, the whole world is busy with developing the vaccine against this pandemic based on proteins, RNA, DNA, and viral vectors technology. Few of them are registered in clinicaltrials.gov, such as the Minigene vaccine, Adenovirus type 5 vector recombinant vaccine, Pathogen-specific aAPC vaccine, ChAdOx1 nCoV-19/MenACWY/COV001, bacTRL spike vaccine, and mRNA-1273 and immunize the population against the COVID-19 infection (clinicaltrials.gov).
The minigene and Pathogen aPAC vaccines are synthetic vaccines developed by using the conserved domains of COVID-19’s polyprotein protease, and structural proteins. The COVID-19 virus interacts with ACE-2 receptors of host cells by using the Spike protein. Viral replication inside the host cell depends on the molecular mechanisms of viral proteins. This clinical trial aims to develop and examine the COVID-19 minigenes vaccine, based on multiple viral genes. For the expression of viral genes and immunomodulatory genes a powerful lentivirus (NHP/TYF) is used as a vector, which might activate T cells and modify the dendritic cells and antigen presenting cell (aAPC).27,28
Adenovirus type 5 vector recombinant vaccine trial is planned to estimate the potential to activate the immune system and safety of Ad5-nCoV, full-length spike (S) protein encodes for SARS-CoV-2.29
bacTRL spike vaccine contains live Bifidobacterium longum as colony-forming-units (CFU), which is designed to deliver synthetic DNA with spike Proteins of SARS-CoV-2 containing plasmids.30
mRNA-1273 vaccine trial is planned to evaluate the immunogenicity, reactogenicity, and safety of the mRNA vaccine constructed by ModernaTX, Inc. It is encapsulated by a novel lipid nanoparticle (LNP) that encodes SARS-CoV-2′s prefusion stabilized spike (S) protein.31 mRNA vaccines are essential for generating the specific immune response against infections by immune system activation with quickly exposing the immune cells to the antigen. mRNA vaccine development is very critical and problematic because the efficacy of the mRNA vaccine could be altered at the time of manufacturing and can cause side effects.
Proteins encoded by synthetic mRNA of interest are used as a cellular mRNA to the immediate translation of the antigen genes.32 The efficacy of mRNA vaccines can be improved by choosing or developing appropriate methods. Developers faced several technical problems at the time of mRNA vaccine production and might overcome this by verifying whether the vaccine works accurately or not.33 Unintentional properties of the mRNA vaccine can produce an unwanted immune response. To overcome this problem, requires designing the mRNA vaccine sequences and confirming that they should mimic those mRNAs transcribed by mammalian cells. Successful delivery of the vaccine into the cell is a major challenge because free RNA quickly degrades in the body. For successful delivery of the RNA, the vaccine RNA strands should be incorporated with a bigger molecule that provides stability into nanoparticles or liposomes. Several mRNA vaccines have to be frozen or refrigerated like conventional vaccines.33
Role of BCG in activation of immune system against the virusesAfter BCG vaccination, BCG initiates the body’s immune response against the foreigner BCG antigen. At the site of vaccine administration, local immune cells (Dendritic cells, neutrophils, and macrophages) get activated and interact with the bacterial colony.34,35 Immune cells recognize the pathogen through the different types of pathogen-associated molecular patterns (PAMPs) and pathogen recognition receptors (PRRs), which preserved molecular signatures of bacteria and viruses. PAMPs like peptidoglycans, cell wall proteins, lipopolysaccharides, mycolic acids, glycoproteins, etc. bind with PRRs that present on immune cells. Toll-like receptors such as TLR2 and TLR4 are associated with BCG recognition.34 TLRs perform an essential function in pathogen recognition for a different variety of PAMPs. It is known that six represent a subclass of TLRs that recognize the ligands of viruses.36 TLR2 and TLR4 receptors are present on the cell surface activated by viral glycoproteins or by other foreigner proteins produced by extracellular milieu. Antiviral innate immune activation depends on the particular type of TLR signaling mechanism that is stimulated through the particular type of pathogen.36–40 Studies have shown that BCG expressed different proteins that activate TLRs and activate macrophages and dendritic cells. After the activation of these cells, they produce pro-inflammatory cytokines.41 DC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) is a C-type lectin that interacts with bacterial wall constituents, and helps to recognize and internalize the process of BCG.42 Dendritic cells get activated after interactions with the pathogen and initiate dendritic cell migration and maturation, which is described by the upregulation of CD40, CD80, CD83, and CD86 co-stimulatory molecules.43 Antigen 85 expresses on the M.TB surface also present on the BCG surface, which induced the secretion of tumor necrosis factor (TNF-α), interleukin-6 (IL-6), and, interleukin- 1beta (IL-1β).44,45 It could activate immune cells by generating pro-inflammatory cytokines.43 Adaptive immune response initiates by antigen presentation when an antigen-presenting cell presents an antigen peptide with major histocompatible complex (MHC) molecules to naive T cells, found spleen to be the most affected organ or any secondary lymphoid tissues.46 In vitroandin vivo studies have reported that the skin dendritic cells having BCG inside migrate to the lymph nodes and activate both types of T cells CD4+ and CD8+T cells by the secretion of TNF-α, IL-6, and IL-12.47–50 Surprisingly, it has been stated that the stimulation of antigen-specific T cell responses by the BCG infected dendritic cells is induced by infected neutrophils.51
After BCG vaccination, adaptive immune cells (CD4+ and CD8+T cells) become activated, initiate the immune response against the BCG antigens46,52 and increase the secretion of IFN-γ. IFN-γ improves the potential against mycobacteria of the macrophages,45,46 and it also activates against viruses. IFN-γ, the specific cell type of cytokine that involved in B cells activation and differentiation, B-cells differentiated into plasma B cells, and memory B cells where plasma B cells produced antibodies against the particular antigen. Activated CD8+ T cells proliferate into specific CD8+ T cells against BCG antigen and persist for ten weeks in peripheral blood.53 Specific CD8+T cells against an antigen released IFN-γ, and also express the perforins and granzymes to the cytotoxic activity of CD8+T cells.53,54 CD4+ and CD8+ T cells specific for BCG antigen converted into effector memory T cells with their functional features of IFN-γ secretion.55,56 One study has reported the strong lymphoproliferative activity of effector memory T cells, sustained for many months, against the TB antigens in mice.56
BCG can be a game changer for SARS-CoV-2 infectionSeveral clinical trials started to treat the SARS-CoV-2 using the BCG vaccination. A study has been published by the New York Institute of Technology (NYIT) exploring that the BCG vaccine could be a game-changer in the fight against SARS-CoV-2.20 The BCG vaccine is used all over the world (except the USA, Germany, Spain, Italy, etc.) to defeat TB infection. The researchers observed that the countries without universal BCG vaccination policies, are having ten-times more severe COVID-19 infections and high mortality.20 Five clinical trials have started in different countries using the BCG vaccine as a preventive treatment for the COVID-19 in Health Care Workers and the elderly population.26 According to the available literature, the BCG vaccine might help in reducing the incidence of COVID-19 infections with less morbidity and mortality; BCG vaccine might be a game-changer in preventing the spread of the COVID-19 pandemic.
Safety of revaccinationRevaccination of BCG did not provide any extra protection against TB.13 Control and prevention of tuberculosis provided guidelines that people who work in hospital settings regardless of age, those unvaccinated earlier, and those having Heaf grade 1 or negative on tuberculin testing, might be vaccinated for the BCG vaccine. The HCW were directly dealing with TB patients and did not have a BCG scar so revaccination might be recommended.57,58 The BCG vaccine causes swelling at the site of vaccination. However, cross-reactions of BCG may occur in people with a compromised immune system and pregnant women, so extra protection could be provided to pregnant women and immune-compromised people before BCG vaccination.13 A study has shown that after revaccination in students, the relative risk of adverse reactions with the scar was twice, as compared to without scar.59 The researchers reported that the second dose of BCG or revaccination did not generally cause any adverse reactions - sometimes it can cause adverse reactions but these are very rare. The study reporting in American Indians and Alaskan natives BCG vaccination provided that the long-lasting potency and it has shown that a dose of BCG provides safety for 50–60 years.60 The clinical trials also observed the same efficacy of the BCG vaccine in observational case-control studies, but unknown in the elderly population.59 Another study performed on elderly guinea pigs revealed that the revaccination of the BCG-Tokyo vaccine against the infection reduced the replication of bacteria in the lungs, spleen, and alveolar lymph nodes.61
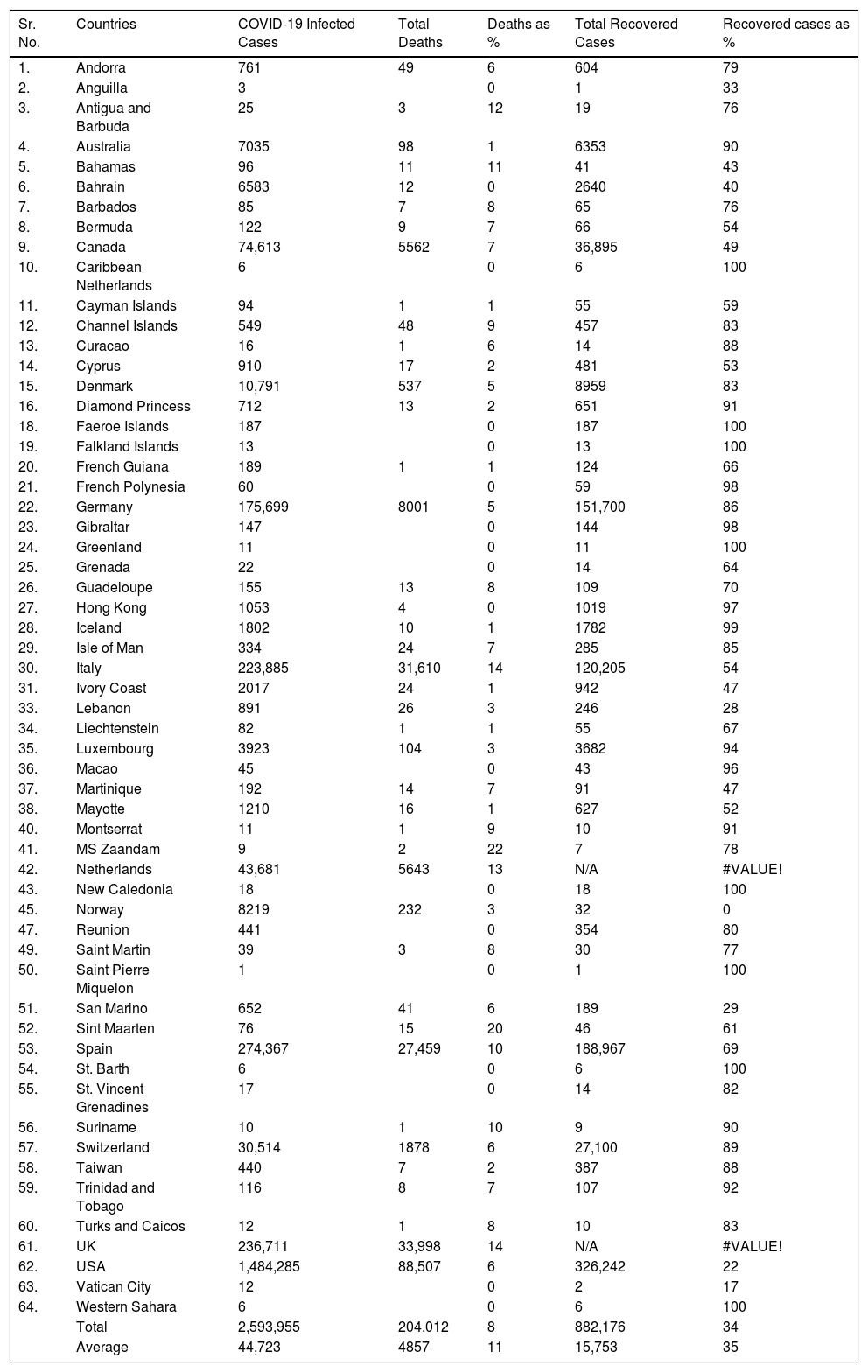
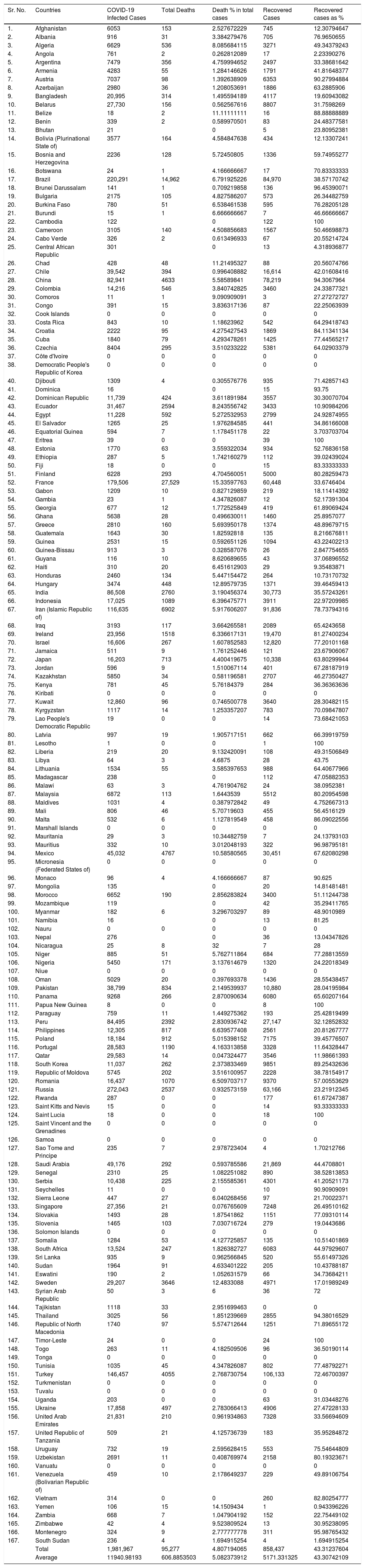
COVID-19 status in BCG implemented and non-implemented countries (May 16, 2020)The preliminary studies have observed a correlation between countries which have universal policies of BCG vaccination for their citizens, showing fewer COVID-19 confirmed cases with a very low mortality rate.10 Estimation of the correlation of BCG with the spread of COVID-19 infection in different countries started the clinical trials to determine whether the BCG vaccine provides any protection against the COVID-19 pandemic. Status of the coronavirus infection is shown in the tables with or without a universal BCG immunization program. Data of COVID-19 collected from the worldometer (https://www.worldometers.info/coronavirus/) and converted into the death percentage, percentage of recovered cases, and total infected cases in the form of BCG implemented countries status and non-implemented countries status. BCG vaccination data was collected country-wise from the BCG World Atlas Database site (Tables 1 and 2).
SARS CoV-2 infection in non-BCG implemented countries.
| Sr. No. | Countries | COVID-19 Infected Cases | Total Deaths | Deaths as % | Total Recovered Cases | Recovered cases as % |
|---|---|---|---|---|---|---|
| 1. | Andorra | 761 | 49 | 6 | 604 | 79 |
| 2. | Anguilla | 3 | 0 | 1 | 33 | |
| 3. | Antigua and Barbuda | 25 | 3 | 12 | 19 | 76 |
| 4. | Australia | 7035 | 98 | 1 | 6353 | 90 |
| 5. | Bahamas | 96 | 11 | 11 | 41 | 43 |
| 6. | Bahrain | 6583 | 12 | 0 | 2640 | 40 |
| 7. | Barbados | 85 | 7 | 8 | 65 | 76 |
| 8. | Bermuda | 122 | 9 | 7 | 66 | 54 |
| 9. | Canada | 74,613 | 5562 | 7 | 36,895 | 49 |
| 10. | Caribbean Netherlands | 6 | 0 | 6 | 100 | |
| 11. | Cayman Islands | 94 | 1 | 1 | 55 | 59 |
| 12. | Channel Islands | 549 | 48 | 9 | 457 | 83 |
| 13. | Curacao | 16 | 1 | 6 | 14 | 88 |
| 14. | Cyprus | 910 | 17 | 2 | 481 | 53 |
| 15. | Denmark | 10,791 | 537 | 5 | 8959 | 83 |
| 16. | Diamond Princess | 712 | 13 | 2 | 651 | 91 |
| 18. | Faeroe Islands | 187 | 0 | 187 | 100 | |
| 19. | Falkland Islands | 13 | 0 | 13 | 100 | |
| 20. | French Guiana | 189 | 1 | 1 | 124 | 66 |
| 21. | French Polynesia | 60 | 0 | 59 | 98 | |
| 22. | Germany | 175,699 | 8001 | 5 | 151,700 | 86 |
| 23. | Gibraltar | 147 | 0 | 144 | 98 | |
| 24. | Greenland | 11 | 0 | 11 | 100 | |
| 25. | Grenada | 22 | 0 | 14 | 64 | |
| 26. | Guadeloupe | 155 | 13 | 8 | 109 | 70 |
| 27. | Hong Kong | 1053 | 4 | 0 | 1019 | 97 |
| 28. | Iceland | 1802 | 10 | 1 | 1782 | 99 |
| 29. | Isle of Man | 334 | 24 | 7 | 285 | 85 |
| 30. | Italy | 223,885 | 31,610 | 14 | 120,205 | 54 |
| 31. | Ivory Coast | 2017 | 24 | 1 | 942 | 47 |
| 33. | Lebanon | 891 | 26 | 3 | 246 | 28 |
| 34. | Liechtenstein | 82 | 1 | 1 | 55 | 67 |
| 35. | Luxembourg | 3923 | 104 | 3 | 3682 | 94 |
| 36. | Macao | 45 | 0 | 43 | 96 | |
| 37. | Martinique | 192 | 14 | 7 | 91 | 47 |
| 38. | Mayotte | 1210 | 16 | 1 | 627 | 52 |
| 40. | Montserrat | 11 | 1 | 9 | 10 | 91 |
| 41. | MS Zaandam | 9 | 2 | 22 | 7 | 78 |
| 42. | Netherlands | 43,681 | 5643 | 13 | N/A | #VALUE! |
| 43. | New Caledonia | 18 | 0 | 18 | 100 | |
| 45. | Norway | 8219 | 232 | 3 | 32 | 0 |
| 47. | Reunion | 441 | 0 | 354 | 80 | |
| 49. | Saint Martin | 39 | 3 | 8 | 30 | 77 |
| 50. | Saint Pierre Miquelon | 1 | 0 | 1 | 100 | |
| 51. | San Marino | 652 | 41 | 6 | 189 | 29 |
| 52. | Sint Maarten | 76 | 15 | 20 | 46 | 61 |
| 53. | Spain | 274,367 | 27,459 | 10 | 188,967 | 69 |
| 54. | St. Barth | 6 | 0 | 6 | 100 | |
| 55. | St. Vincent Grenadines | 17 | 0 | 14 | 82 | |
| 56. | Suriname | 10 | 1 | 10 | 9 | 90 |
| 57. | Switzerland | 30,514 | 1878 | 6 | 27,100 | 89 |
| 58. | Taiwan | 440 | 7 | 2 | 387 | 88 |
| 59. | Trinidad and Tobago | 116 | 8 | 7 | 107 | 92 |
| 60. | Turks and Caicos | 12 | 1 | 8 | 10 | 83 |
| 61. | UK | 236,711 | 33,998 | 14 | N/A | #VALUE! |
| 62. | USA | 1,484,285 | 88,507 | 6 | 326,242 | 22 |
| 63. | Vatican City | 12 | 0 | 2 | 17 | |
| 64. | Western Sahara | 6 | 0 | 6 | 100 | |
| Total | 2,593,955 | 204,012 | 8 | 882,176 | 34 | |
| Average | 44,723 | 4857 | 11 | 15,753 | 35 |
SARS-CoV-2 infection in BCG implemented countries.
| Sr. No. | Countries | COVID-19 Infected Cases | Total Deaths | Death % in total cases | Recovered Cases | Recovered cases as % |
|---|---|---|---|---|---|---|
| 1. | Afghanistan | 6053 | 153 | 2.527672229 | 745 | 12.30794647 |
| 2. | Albania | 916 | 31 | 3.384279476 | 705 | 76.9650655 |
| 3. | Algeria | 6629 | 536 | 8.085684115 | 3271 | 49.34379243 |
| 4. | Angola | 761 | 2 | 0.262812089 | 17 | 2.23390276 |
| 5. | Argentina | 7479 | 356 | 4.759994652 | 2497 | 33.38681642 |
| 6. | Armenia | 4283 | 55 | 1.284146626 | 1791 | 41.81648377 |
| 7. | Austria | 7037 | 98 | 1.392638909 | 6353 | 90.27994884 |
| 8. | Azerbaijan | 2980 | 36 | 1.208053691 | 1886 | 63.2885906 |
| 9. | Bangladesh | 20,995 | 314 | 1.495594189 | 4117 | 19.60943082 |
| 10. | Belarus | 27,730 | 156 | 0.562567616 | 8807 | 31.7598269 |
| 11. | Belize | 18 | 2 | 11.11111111 | 16 | 88.88888889 |
| 12. | Benin | 339 | 2 | 0.589970501 | 83 | 24.48377581 |
| 13. | Bhutan | 21 | 0 | 5 | 23.80952381 | |
| 14. | Bolivia (Plurinational State of) | 3577 | 164 | 4.584847638 | 434 | 12.13307241 |
| 15. | Bosnia and Herzegovina | 2236 | 128 | 5.72450805 | 1336 | 59.74955277 |
| 16. | Botswana | 24 | 1 | 4.166666667 | 17 | 70.83333333 |
| 17. | Brazil | 220,291 | 14,962 | 6.791925226 | 84,970 | 38.57170742 |
| 18. | Brunei Darussalam | 141 | 1 | 0.709219858 | 136 | 96.45390071 |
| 19. | Bulgaria | 2175 | 105 | 4.827586207 | 573 | 26.34482759 |
| 20. | Burkina Faso | 780 | 51 | 6.538461538 | 595 | 76.28205128 |
| 21. | Burundi | 15 | 1 | 6.666666667 | 7 | 46.66666667 |
| 22. | Cambodia | 122 | 0 | 122 | 100 | |
| 23. | Cameroon | 3105 | 140 | 4.508856683 | 1567 | 50.46698873 |
| 24. | Cabo Verde | 326 | 2 | 0.613496933 | 67 | 20.55214724 |
| 25. | Central African Republic | 301 | 0 | 13 | 4.318936877 | |
| 26. | Chad | 428 | 48 | 11.21495327 | 88 | 20.56074766 |
| 27. | Chile | 39,542 | 394 | 0.996408882 | 16,614 | 42.01608416 |
| 28. | China | 82,941 | 4633 | 5.58589841 | 78,219 | 94.3067964 |
| 29. | Colombia | 14,216 | 546 | 3.840742825 | 3460 | 24.33877321 |
| 30. | Comoros | 11 | 1 | 9.090909091 | 3 | 27.27272727 |
| 31. | Congo | 391 | 15 | 3.836317136 | 87 | 22.25063939 |
| 32. | Cook Islands | 0 | 0 | 0 | 0 | 0 |
| 33. | Costa Rica | 843 | 10 | 1.18623962 | 542 | 64.29418743 |
| 34. | Croatia | 2222 | 95 | 4.275427543 | 1869 | 84.11341134 |
| 35. | Cuba | 1840 | 79 | 4.293478261 | 1425 | 77.44565217 |
| 36. | Czechia | 8404 | 295 | 3.510233222 | 5381 | 64.02903379 |
| 37. | Côte d'Ivoire | 0 | 0 | 0 | 0 | 0 |
| 38. | Democratic People's Republic of Korea | 0 | 0 | 0 | 0 | 0 |
| 40. | Djibouti | 1309 | 4 | 0.305576776 | 935 | 71.42857143 |
| 41. | Dominica | 16 | 0 | 15 | 93.75 | |
| 42. | Dominican Republic | 11,739 | 424 | 3.611891984 | 3557 | 30.30070704 |
| 43. | Ecuador | 31,467 | 2594 | 8.243556742 | 3433 | 10.90984206 |
| 44. | Egypt | 11,228 | 592 | 5.272532953 | 2799 | 24.92874955 |
| 45. | El Salvador | 1265 | 25 | 1.976284585 | 441 | 34.86166008 |
| 46. | Equatorial Guinea | 594 | 7 | 1.178451178 | 22 | 3.703703704 |
| 47. | Eritrea | 39 | 0 | 0 | 39 | 100 |
| 48. | Estonia | 1770 | 63 | 3.559322034 | 934 | 52.76836158 |
| 49. | Ethiopia | 287 | 5 | 1.742160279 | 112 | 39.02439024 |
| 50. | Fiji | 18 | 0 | 0 | 15 | 83.33333333 |
| 51. | Finland | 6228 | 293 | 4.704560051 | 5000 | 80.28259473 |
| 52. | France | 179,506 | 27,529 | 15.33597763 | 60,448 | 33.6746404 |
| 53. | Gabon | 1209 | 10 | 0.827129859 | 219 | 18.11414392 |
| 54. | Gambia | 23 | 1 | 4.347826087 | 12 | 52.17391304 |
| 55. | Georgia | 677 | 12 | 1.772525849 | 419 | 61.89069424 |
| 56. | Ghana | 5638 | 28 | 0.496630011 | 1460 | 25.8957077 |
| 57. | Greece | 2810 | 160 | 5.693950178 | 1374 | 48.89679715 |
| 58. | Guatemala | 1643 | 30 | 1.82592818 | 135 | 8.216676811 |
| 59. | Guinea | 2531 | 15 | 0.592651126 | 1094 | 43.22402213 |
| 60. | Guinea-Bissau | 913 | 3 | 0.328587076 | 26 | 2.847754655 |
| 61. | Guyana | 116 | 10 | 8.620689655 | 43 | 37.06896552 |
| 62. | Haiti | 310 | 20 | 6.451612903 | 29 | 9.35483871 |
| 63. | Honduras | 2460 | 134 | 5.447154472 | 264 | 10.73170732 |
| 64. | Hungary | 3474 | 448 | 12.89579735 | 1371 | 39.46459413 |
| 65. | India | 86,508 | 2760 | 3.190456374 | 30,773 | 35.57243261 |
| 66. | Indonesia | 17,025 | 1089 | 6.396475771 | 3911 | 22.97209985 |
| 67. | Iran (Islamic Republic of) | 116,635 | 6902 | 5.917606207 | 91,836 | 78.73794316 |
| 68. | Iraq | 3193 | 117 | 3.664265581 | 2089 | 65.4243658 |
| 69. | Ireland | 23,956 | 1518 | 6.336617131 | 19,470 | 81.27400234 |
| 70. | Israel | 16,606 | 267 | 1.607852583 | 12,820 | 77.20101168 |
| 71. | Jamaica | 511 | 9 | 1.761252446 | 121 | 23.67906067 |
| 72. | Japan | 16,203 | 713 | 4.400419675 | 10,338 | 63.80299944 |
| 73. | Jordan | 596 | 9 | 1.510067114 | 401 | 67.28187919 |
| 74. | Kazakhstan | 5850 | 34 | 0.581196581 | 2707 | 46.27350427 |
| 75. | Kenya | 781 | 45 | 5.76184379 | 284 | 36.36363636 |
| 76. | Kiribati | 0 | 0 | 0 | 0 | 0 |
| 77. | Kuwait | 12,860 | 96 | 0.746500778 | 3640 | 28.30482115 |
| 78. | Kyrgyzstan | 1117 | 14 | 1.253357207 | 783 | 70.09847807 |
| 79. | Lao People's Democratic Republic | 19 | 0 | 0 | 14 | 73.68421053 |
| 80. | Latvia | 997 | 19 | 1.905717151 | 662 | 66.39919759 |
| 81. | Lesotho | 1 | 0 | 0 | 1 | 100 |
| 82. | Liberia | 219 | 20 | 9.132420091 | 108 | 49.31506849 |
| 83. | Libya | 64 | 3 | 4.6875 | 28 | 43.75 |
| 84. | Lithuania | 1534 | 55 | 3.585397653 | 988 | 64.40677966 |
| 85. | Madagascar | 238 | 0 | 112 | 47.05882353 | |
| 86. | Malawi | 63 | 3 | 4.761904762 | 24 | 38.0952381 |
| 87. | Malaysia | 6872 | 113 | 1.6443539 | 5512 | 80.20954598 |
| 88. | Maldives | 1031 | 4 | 0.387972842 | 49 | 4.752667313 |
| 89. | Mali | 806 | 46 | 5.70719603 | 455 | 56.4516129 |
| 90. | Malta | 532 | 6 | 1.127819549 | 458 | 86.09022556 |
| 91. | Marshall Islands | 0 | 0 | 0 | 0 | 0 |
| 92. | Mauritania | 29 | 3 | 10.34482759 | 7 | 24.13793103 |
| 93. | Mauritius | 332 | 10 | 3.012048193 | 322 | 96.98795181 |
| 94. | Mexico | 45,032 | 4767 | 10.58580565 | 30,451 | 67.62080298 |
| 95. | Micronesia (Federated States of) | 0 | 0 | 0 | 0 | 0 |
| 96. | Monaco | 96 | 4 | 4.166666667 | 87 | 90.625 |
| 97. | Mongolia | 135 | 0 | 20 | 14.81481481 | |
| 98. | Morocco | 6652 | 190 | 2.856283824 | 3400 | 51.11244738 |
| 99. | Mozambique | 119 | 0 | 42 | 35.29411765 | |
| 100. | Myanmar | 182 | 6 | 3.296703297 | 89 | 48.9010989 |
| 101. | Namibia | 16 | 0 | 13 | 81.25 | |
| 102. | Nauru | 0 | 0 | 0 | 0 | 0 |
| 103. | Nepal | 276 | 0 | 36 | 13.04347826 | |
| 104. | Nicaragua | 25 | 8 | 32 | 7 | 28 |
| 105. | Niger | 885 | 51 | 5.762711864 | 684 | 77.28813559 |
| 106. | Nigeria | 5450 | 171 | 3.137614679 | 1320 | 24.22018349 |
| 107. | Niue | 0 | 0 | 0 | 0 | 0 |
| 108. | Oman | 5029 | 20 | 0.397693378 | 1436 | 28.55438457 |
| 109. | Pakistan | 38,799 | 834 | 2.149539937 | 10,880 | 28.04195984 |
| 110. | Panama | 9268 | 266 | 2.870090634 | 6080 | 65.60207164 |
| 111. | Papua New Guinea | 8 | 0 | 0 | 8 | 100 |
| 112. | Paraguay | 759 | 11 | 1.449275362 | 193 | 25.42819499 |
| 113. | Peru | 84,495 | 2392 | 2.830936742 | 27,147 | 32.12852832 |
| 114. | Philippines | 12,305 | 817 | 6.639577408 | 2561 | 20.81267777 |
| 115. | Poland | 18,184 | 912 | 5.015398152 | 7175 | 39.45776507 |
| 116. | Portugal | 28,583 | 1190 | 4.163313858 | 3328 | 11.64328447 |
| 117. | Qatar | 29,583 | 14 | 0.047324477 | 3546 | 11.98661393 |
| 118. | South Korea | 11,037 | 262 | 2.373833469 | 9851 | 89.25432636 |
| 119. | Republic of Moldova | 5745 | 202 | 3.516100957 | 2228 | 38.78154917 |
| 120. | Romania | 16,437 | 1070 | 6.509703717 | 9370 | 57.00553629 |
| 121. | Russia | 272,043 | 2537 | 0.932573159 | 63,166 | 23.21912345 |
| 122. | Rwanda | 287 | 0 | 0 | 177 | 61.67247387 |
| 123. | Saint Kitts and Nevis | 15 | 0 | 0 | 14 | 93.33333333 |
| 124. | Saint Lucia | 18 | 0 | 0 | 18 | 100 |
| 125. | Saint Vincent and the Grenadines | 0 | 0 | 0 | 0 | 0 |
| 126. | Samoa | 0 | 0 | 0 | 0 | 0 |
| 127. | Sao Tome and Principe | 235 | 7 | 2.978723404 | 4 | 1.70212766 |
| 128. | Saudi Arabia | 49,176 | 292 | 0.593785586 | 21,869 | 44.4708801 |
| 129. | Senegal | 2310 | 25 | 1.082251082 | 890 | 38.52813853 |
| 130. | Serbia | 10,438 | 225 | 2.155585361 | 4301 | 41.20521173 |
| 131. | Seychelles | 11 | 0 | 0 | 10 | 90.90909091 |
| 132. | Sierra Leone | 447 | 27 | 6.040268456 | 97 | 21.70022371 |
| 133. | Singapore | 27,356 | 21 | 0.076765609 | 7248 | 26.49510162 |
| 134. | Slovakia | 1493 | 28 | 1.87541862 | 1151 | 77.09310114 |
| 135. | Slovenia | 1465 | 103 | 7.030716724 | 279 | 19.0443686 |
| 136. | Solomon Islands | 0 | 0 | 0 | 0 | 0 |
| 137. | Somalia | 1284 | 53 | 4.127725857 | 135 | 10.51401869 |
| 138. | South Africa | 13,524 | 247 | 1.826382727 | 6083 | 44.97929607 |
| 139. | Sri Lanka | 935 | 9 | 0.962566845 | 520 | 55.61497326 |
| 140. | Sudan | 1964 | 91 | 4.633401222 | 205 | 10.43788187 |
| 141. | Eswatini | 190 | 2 | 1.052631579 | 66 | 34.73684211 |
| 142. | Sweden | 29,207 | 3646 | 12.4833088 | 4971 | 17.01989249 |
| 143. | Syrian Arab Republic | 50 | 3 | 6 | 36 | 72 |
| 144. | Tajikistan | 1118 | 33 | 2.951699463 | 0 | 0 |
| 145. | Thailand | 3025 | 56 | 1.851239669 | 2855 | 94.38016529 |
| 146. | Republic of North Macedonia | 1740 | 97 | 5.574712644 | 1251 | 71.89655172 |
| 147. | Timor-Leste | 24 | 0 | 0 | 24 | 100 |
| 148. | Togo | 263 | 11 | 4.182509506 | 96 | 36.50190114 |
| 149. | Tonga | 0 | 0 | 0 | 0 | 0 |
| 150. | Tunisia | 1035 | 45 | 4.347826087 | 802 | 77.48792271 |
| 151. | Turkey | 146,457 | 4055 | 2.768730754 | 106,133 | 72.46700397 |
| 152. | Turkmenistan | 0 | 0 | 0 | 0 | 0 |
| 153. | Tuvalu | 0 | 0 | 0 | 0 | 0 |
| 154. | Uganda | 203 | 0 | 0 | 63 | 31.03448276 |
| 155. | Ukraine | 17,858 | 497 | 2.783066413 | 4906 | 27.47228133 |
| 156. | United Arab Emirates | 21,831 | 210 | 0.961934863 | 7328 | 33.56694609 |
| 157. | United Republic of Tanzania | 509 | 21 | 4.125736739 | 183 | 35.95284872 |
| 158. | Uruguay | 732 | 19 | 2.595628415 | 553 | 75.54644809 |
| 159. | Uzbekistan | 2691 | 11 | 0.408769974 | 2158 | 80.19323671 |
| 160. | Vanuatu | 0 | 0 | 0 | 0 | 0 |
| 161. | Venezuela (Bolivarian Republic of) | 459 | 10 | 2.178649237 | 229 | 49.89106754 |
| 162. | Vietnam | 314 | 0 | 0 | 260 | 82.80254777 |
| 163. | Yemen | 106 | 15 | 14.1509434 | 1 | 0.943396226 |
| 164. | Zambia | 668 | 7 | 1.047904192 | 152 | 22.75449102 |
| 165. | Zimbabwe | 42 | 4 | 9.523809524 | 13 | 30.95238095 |
| 166. | Montenegro | 324 | 9 | 2.777777778 | 311 | 95.98765432 |
| 167. | South Sudan | 236 | 4 | 1.694915254 | 4 | 1.694915254 |
| Total | 1,981,967 | 95,277 | 4.807194065 | 858,437 | 43.31237604 | |
| Average | 11940.98193 | 606.8853503 | 5.082373912 | 5171.331325 | 43.30742109 |
The average of the total number of COVID-19 cases was 44,723 in without BCG implemented countries, whereas implemented countries have a very lower average of infected cases 11940.98. BCG implemented countries have fewer deaths percentage, around 5.08%, as compared to the 11% in without implemented countries. Moreover, the recovered cases percentage was also high in BCG implemented countries, around 43%, whereas in without implemented countries it was 35%.
ConclusionThe SARS-Cov-2 pandemic is spreading rapidly and the entire world under the grip of this severe pulmonary disease. The countries are fighting this pandemic with their ability but developed countries such as the USA, Italy, Spain, UK, etc., have been badly affected. People of these countries have a low immune response against any type of infection like COVID-19 because these countries have no universal immunization program or it was removed by the government at an earlier time. Other countries such as India, Afghanistan, Nepal, Bhutan, China, Pakistan, Bangladesh, etc. have universal immunization programs like the BCG vaccination program. The BCG vaccine has the potential to activate the immune response against the viral infection. The severity of the COVID-19 pandemic is very low with a slower spread in those countries that have the universal BCG immunization program. Australia, Germany, USA, etc., started the clinical trial of the BCG vaccine in Health Care Workers and the Elderly Population to prevent the infection of COVID-19. The correlation of BCG vaccination with COVID-19 has shown fewer confirmed cases with low mortality and a high recovered rate in universal BCG immunization countries. Table 1 contains the data of BCG unimplemented countries, and Table 2 contains the data of universal BCG implemented countries. Several new vaccines are being developed by different companies and clinical trials have started, until approval or success of any clinical trial for the specific vaccine of COVID-19 the BCG vaccine might be used as a preventive treatment for the COVID-19 pandemic.
Conflicts of interestThe authors declare that they have no conflict of interest.
Funding statementNone.