The storage mite, Tyrophagus putrescentiae, detected in the samples collected from stored products and house dust, is one of the major causes of allergic disorders.
ObjectiveThe purpose of this study was to ameliorate the T. putrescentiae faeces allergic immunological disorder by garlic.
MethodsAlbino experimental rats were classified into control, inhaled and treated groups. Mass rearing of T. putrescentiae on different diets, and ELISA of some cytokines and IgE techniques were used.
ResultsThe results obtained showed the highest population of T. putrescentiae reared in four from thirteen tested diets. In addition, significantly higher serum levels of INF-γ and IgE were found in rats treated with faeces than the other groups; especially the garlic-treated group. In contrast, IL-4 was lower in faeces-treated rats than the others; however, the control group had the highest level of IL-4. Statistical analysis of data showed a significant difference between the garlic-treated group and either control or faeces-treated groups (P<0.05).
ConclusionsThe population of T. putrescentiae mites peaked in four from thirteen tested diets. The immunological disorder caused by repeated exposure to T. putrescentiae faeces might be modulated by garlic.
Medicinal plants have been used to cure human illness since ancient times. Certain types of these plants are believed to promote positive health and maintain organism resistance against infection by re-establishing body equilibrium and conditioning the body tissues. Among these plants, garlic (Allium sativum) a traditional dietary and medicinal applications as an anti-microbial agent1 is a common food spice widely distributed and used in all parts of the world as a spice and herbal medicine for the prevention and treatment of a variety of diseases, ranging from infections to heart diseases.2 Garlic is thought to have various pharmacological properties and medical applications. It is mainly consumed as a condiment in various prepared foods.3 This prompted us to propose garlic as an asthmatic modulator medicinal plant.
Understanding the nature of environmental triggers is fundamental in attempts to prevent/reduce allergic diseases. As previously noted, exposure to common aeroallergens, especially perennial inhalable allergens such as mite, is associated with a significantly increased risk for asthma.4 Mite infestation is associated with negative effects on man and his resources. Storage mites consume stored grain and oil seeds, transfer toxicogenic microorganisms and produce allergens, thereby causing occupational allergies and endangering food safety.5 The storage mite, Tyrophagus putrescentiae is found in stored products such as dried eggs, ham, herring meal, cheese, and different kinds of nuts.6 Storage mites are the cause of occupational allergic diseases of bakers7 and food industrial workers8,9 and among residents in agricultural environments.10 En-Chih et al. had also mentioned that both T. putrescentiae and Dermatophagoides pteronyssinus are causative factors for the development of airway hypersensitivity.11 The mass rearing processes were also reported elsewhere.12,13
The storage mite T. putrescentiae causes allergic response and IgE production in animals.14 The asthma symptoms are caused by inhalation of live mites and fragments of dead mites, or their excretory pellets.15
Bronchial asthma is a chronic inflammatory disease of the airway.16 Chronic exposure to allergens triggers a distinct array of immunobiological and biochemical reactions that directly stimulate and induce abnormalities of air-way structure resulting in the development of clinical symptoms.17 Allergic disorder symptoms are associated with high levels of serum allergen-specific IgE and eosinophilia.18 In addition, both allergen exposure protocols result in immune-mediated airway inflammation defined by elevated levels of IgE, the T-helper cell 2 (TH2) cytokines IL-4 and eosinophils.19 The pro-inflammatory cytokine, IFN-γ, promotes T-helper type-1 (Th1) responses, which down-regulate the Th2-like immune responses that are hallmarks of allergic diseases, including asthma.20
The objective of the present investigation was to develop mass rearing techniques of this species by 13 different natural foods and to examine their immunological disorders in male albino rats. Modulation of these disorders was tested by garlic.
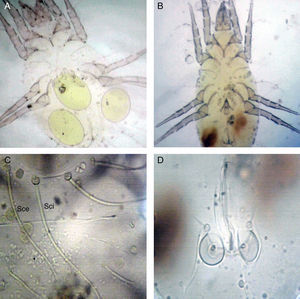
Materials and methodsMass rearing of Tyrophagus putrescentiaeThe choice of the effective diets or feeding media is necessary for mass rearing of allergic mites, because the antigen preparation needs a very big numbers of allergic mites. Stored product samples, collected from El-Minia Governorate, were used for establishing a stock culture of T. putrescentiae. Live mites were isolated by a modified Berlese funnel with a wire screening21 covered with muslin. Each sample was placed on the muslin and spread. Mites which escaped from light were received in a Petri dish. The bottom of the Petri dish is filled with a paste made of a mixture of charcoal and gypsum (2:8 parts) to construct a porous medium.22 The rim of the dish is coated with a phaslene. Mites were sorted out, preserved and mounted on microscope slides for species identification.23,24T. putrescentiae figures were captured by light microscopy at 400× and 600× magnifications.
The following culture media obtained from local market were compared: (1) wheat germ, (2) roomy cheese, (3) nedo, (4) egg yolk, (5) potatoes, (6) fish meal, (7) copra, (8) egg albumin, (9) chicken bouillon, (10) pollens, (11) powdered bean, (12) human hair and scales, and (13) yeast extract.
Five males and five females in copulation state were caged with the tested diet in certain plastic vials (5mm diam.×20mm length), cigarette paper held in place by a snap-on plastic ring confined the mites and allowed gas exchange between the culture environment and a humidity chamber environment. The bottom of each vial was filled with the last described paste. Each combination of food and species of mites was replicated five times within a test. The Petri dishes of the stock culture and the plastic vials of the tested diets were carried out at 25±1°C and 75% relative humidity (RH). The temperature was adjusted at 25°C by placing the entire system inside an incubator. The relative humidity inside the desiccators was maintained at 75% by the concentration of the KOH (22.25g of KOH per 100ml dis. water). The concentration is also sufficient to absorb the CO2 as soon as it is released.25 Every two months the stock cultures were transplanted into fresh media to prevent outgrowth and a consequent breakdown of the population. Contamination with fungi was controlled by: (1) sterilising the tested diets and containers using dry heat (roughly 90°C for 30min). (2) Adding diets in small crumbled amounts. (3) Using of 75% RH instead of 80%, the deal RH needed for T. putrescentiae culture. The procedure of mite counts was as mentioned by Ree and Lee.26
Crude extractsWhen the population density was sufficiently high, T. putrescentiae crude faeces were isolated by the Berlese–Tullgren method by photonegative live mites escaping. Five grams from these faecal pellets were used for each inhalation treatment. Garlic cloves were purchased from the local market in El-Minia Governorate, Egypt. They were then cleaned of any adhered dried material. Each animal was allowed free access to food 50mg of seeds and cloves daily during the course of the treatment, after fasting for about 12h.
Experimental animalsMale albino rats, Rattus norvegicus (6–8 weeks old, weight 100–120g) were purchased from the Biological Supply Center, Theodore Bilharz Research Institute, TBRI, Cairo, Egypt and housed under specific pathogen-free conditions and maintained on a 12-h light-dark cycle, with food and water ad libitum. The experiments were conducted according to the ethical norm approved by the Institutional Animal Ethics Committee (IAEC). Animals were classified into four groups (10 animals each). The first group (control) was untreated. The second group was allowed free access to food 50mg of garlic daily during the course of the treatment, after fasting for about 12h. The third group was intranasal inhaled with T. putrescentiae faeces extract (5g) daily for 10 consecutive days. The fourth group inhaled as in the third group and allowed free access to food as in the second group.
Cytokine and immunoglobulin in serum, 72h after the last treatment, ten animals from each group were sacrificed under chloroform anaesthesia. Blood samples were taken and centrifuged at 3000rpm for 30min. Sera were removed and kept at −20°C for the estimation of the cytokines (IFN-γ and IL-4) and IgE antibody levels by ELISA using kits purchased from R&D Systems (Minneapolis, MN, USA).
Statistical analysisData were analysed using SPSS program version 13.0. Statistical analysis of the obtained data was performed using one way analysis of variance (ANOVA) test followed by least square differences (LSD) analysis for comparison between means. Results were expressed as mean±standard error (SE). Values of P<0.05 were considered statistically significant, while values of P>0.05 were considered statistically non-significant.
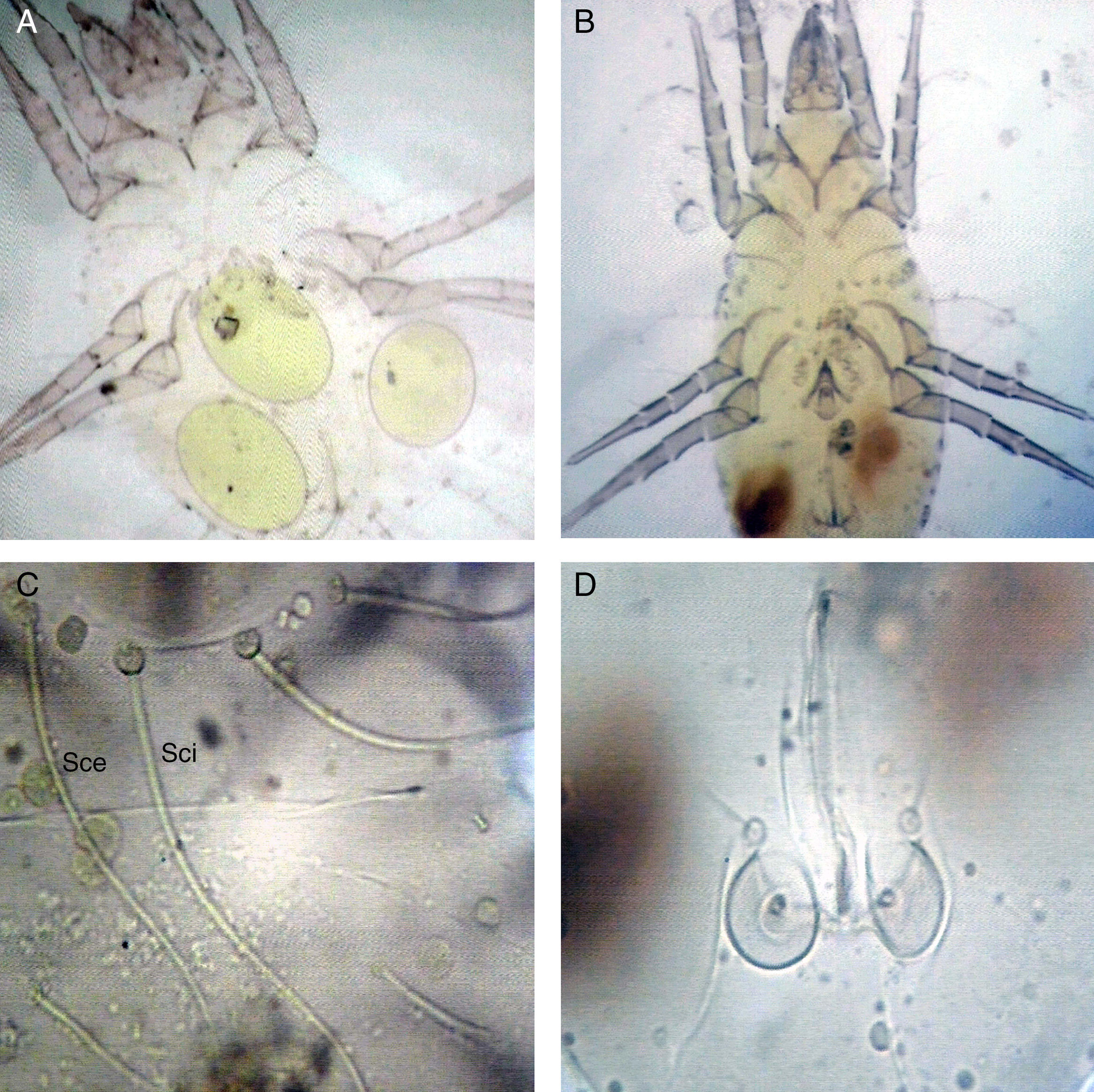
ResultsMass rearing of Tyrophagus putrescentiaeThis species belongs to the family Acaridae. Its dorsal Setae Sci is longer than Sce. In addition, the femur of male forelegs is not enlarged and without a ventral conical process. Females do not have anal suckers that are present in males (Fig. 1).
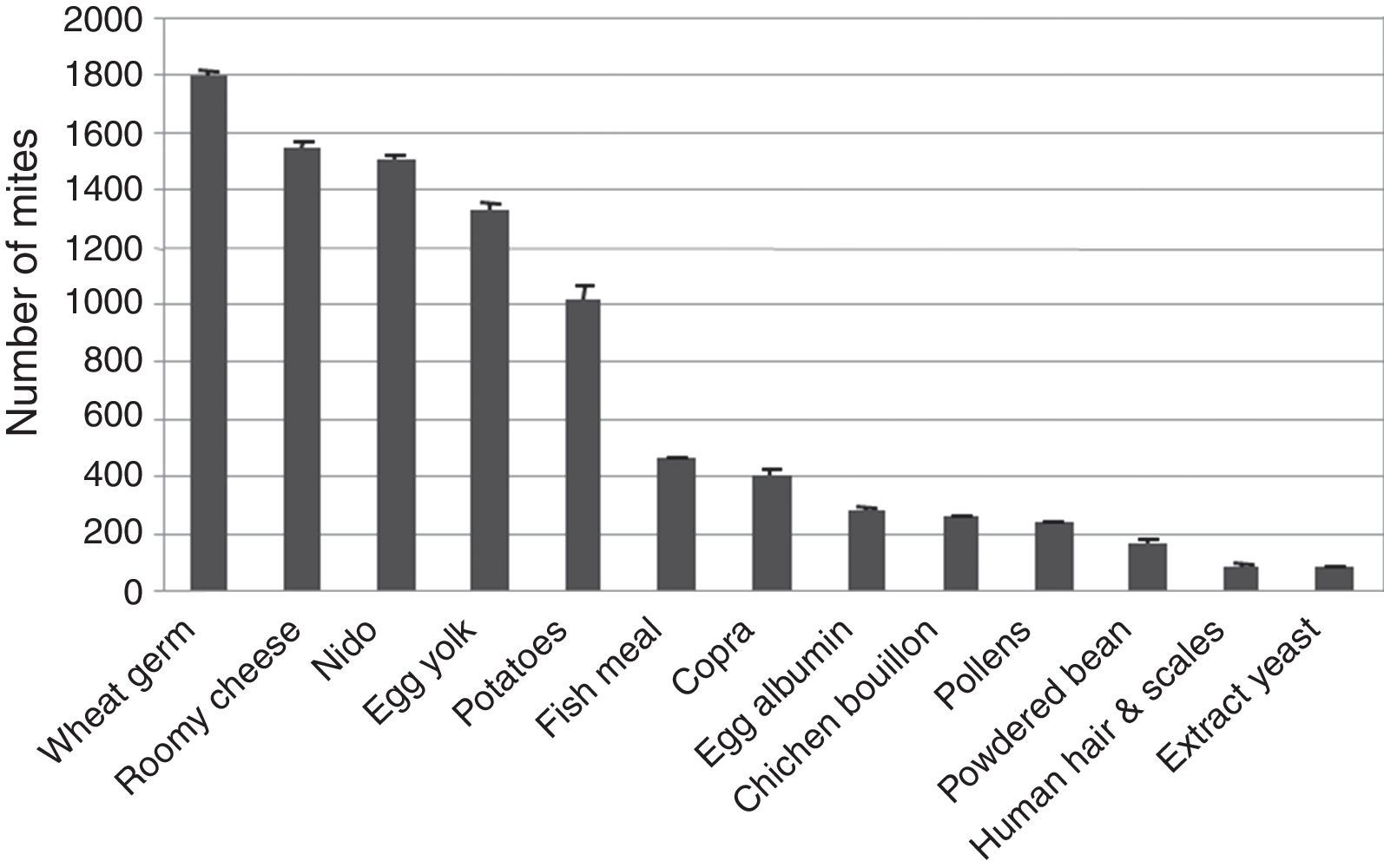
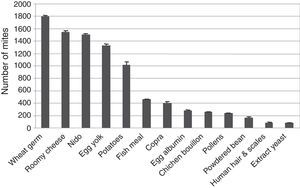
Among 13 different media tested for mass rearing of T. putrescentiae mites, statistical analysis of the obtained data showed a significant difference (P<0.05) in mite population recorded by wheat germ, roomy cheese, nido, egg yolk and potatoes media compared to those recorded by fish meal, copra, egg albumin, chicken stocks, pollens, bean, human hair and scales and yeast extract (Fig. 2).
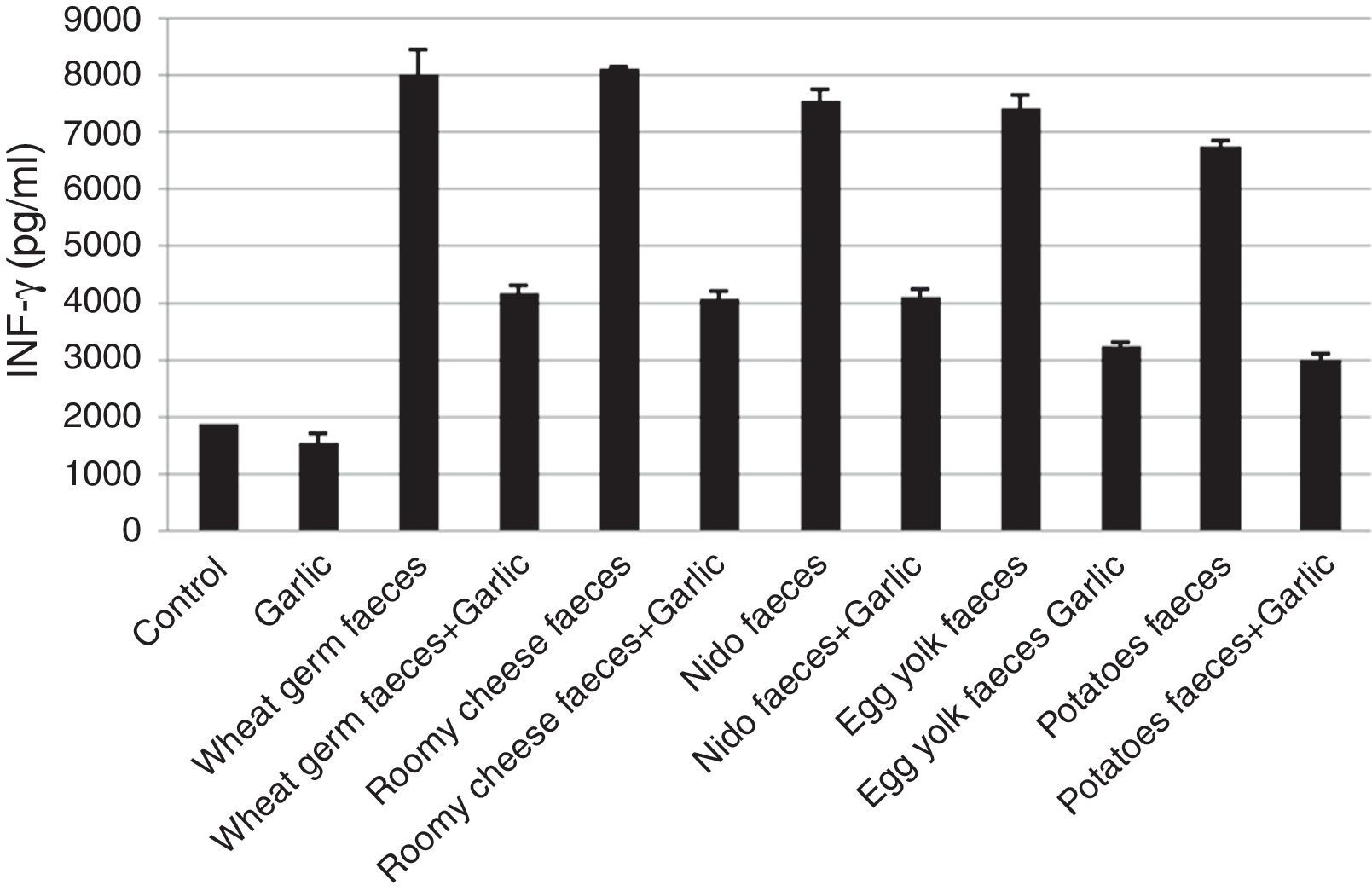
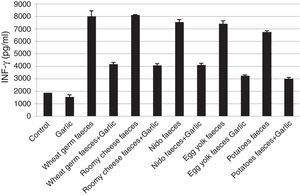
Quantification of INF-γThe INF-γ ELISA value demonstrated that the garlic-treated group (1556.47±179.452pg/ml) was lower than the control group (1885.88±003.867pg/ml). The groups treated with T. putrescentiae faeces produced by wheat germ (8030.29±422.930pg/ml), roomy cheese (8113.06±036.869pg/ml), nido (7554.91±186.849pg/ml), egg yolk (7430.97±214.261pg/ml) and potatoes (6748.63±102.913pg/ml) were higher than the groups treated with garlic in addition to the above five diets (4201.97±141.100pg/ml, 4101.43±110.254pg/ml, 4129.77±147.799pg/ml, 3275.37±38.531pg/ml, 3022.40±105.782pg/ml, respectively) (Fig. 3). This study also showed that there is a significant difference in INF-γ level between garlic-treated group and either the native or faeces-treated group (P<0.05).
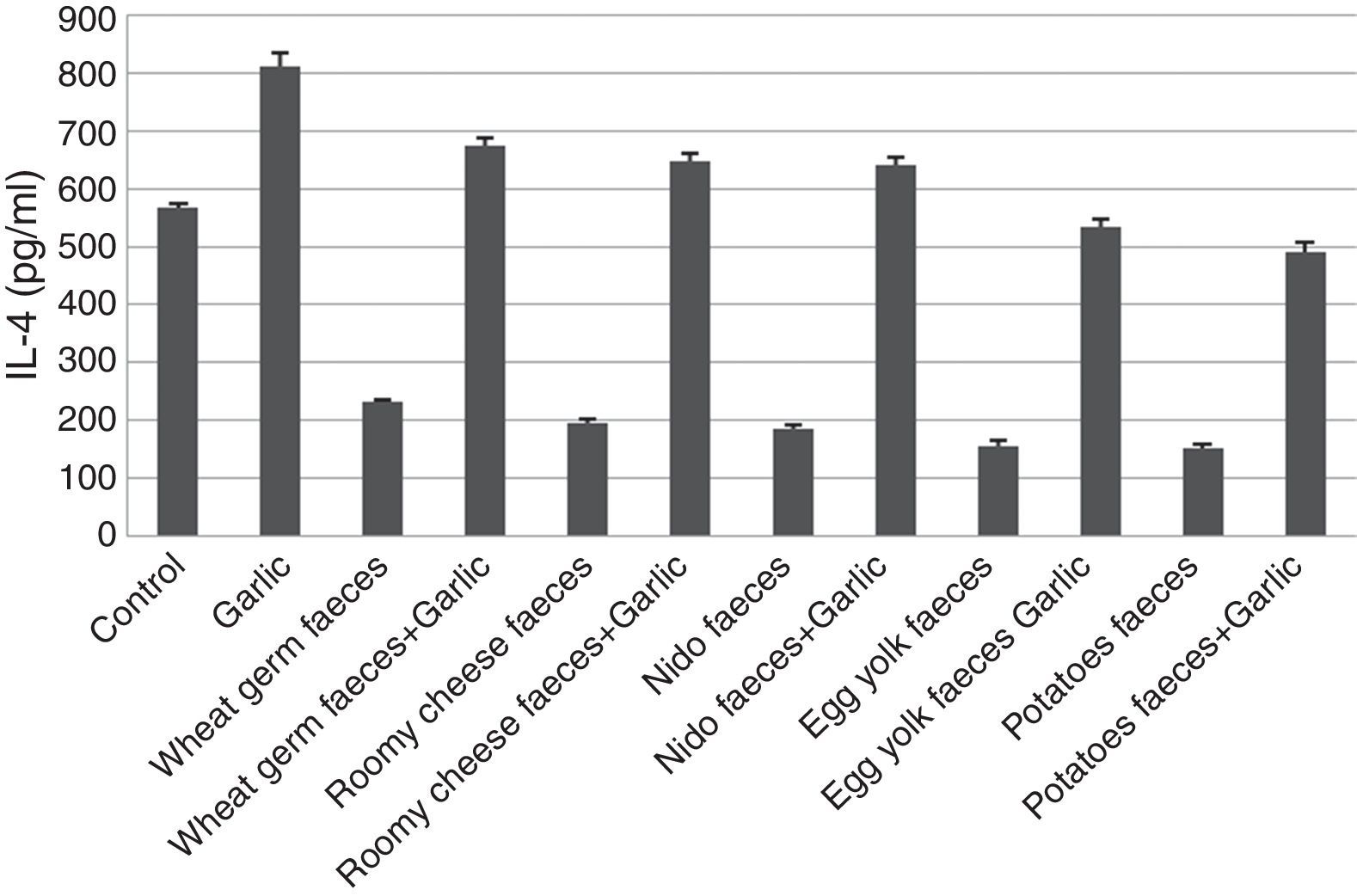
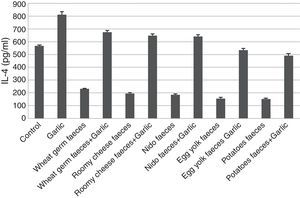
Quantification of IL-4Results of ELISA in Fig. 4 showed that IL-4 recorded 567.07±07.929pg/ml in control group. The inhaled group with T. putrescentiae faeces counted high level of IL-4 (812.51±22.897pg/ml). In contrast, the groups treated with T. putrescentiae faeces produced by wheat germ (231.12±03.448pg/ml), roomy cheese (196.90±05.448pg/ml), nido (187.43±05.339pg/ml), egg yolk (154.51±10.097pg/ml) and potatoes (150.80±08.091pg/ml) are lower than the groups treated with garlic in addition to the above five diets (674.22±13.834pg/ml, 645.90±15.277pg/ml, 640.54±13.643pg/ml, 532.48±15.371pg/ml, 489.12±17.354pg/ml, respectively) (Fig. 4). Statistical analysis showed a significant difference in IL-4 between garlic-treated group and either control or faeces-treated group, P<0.05.
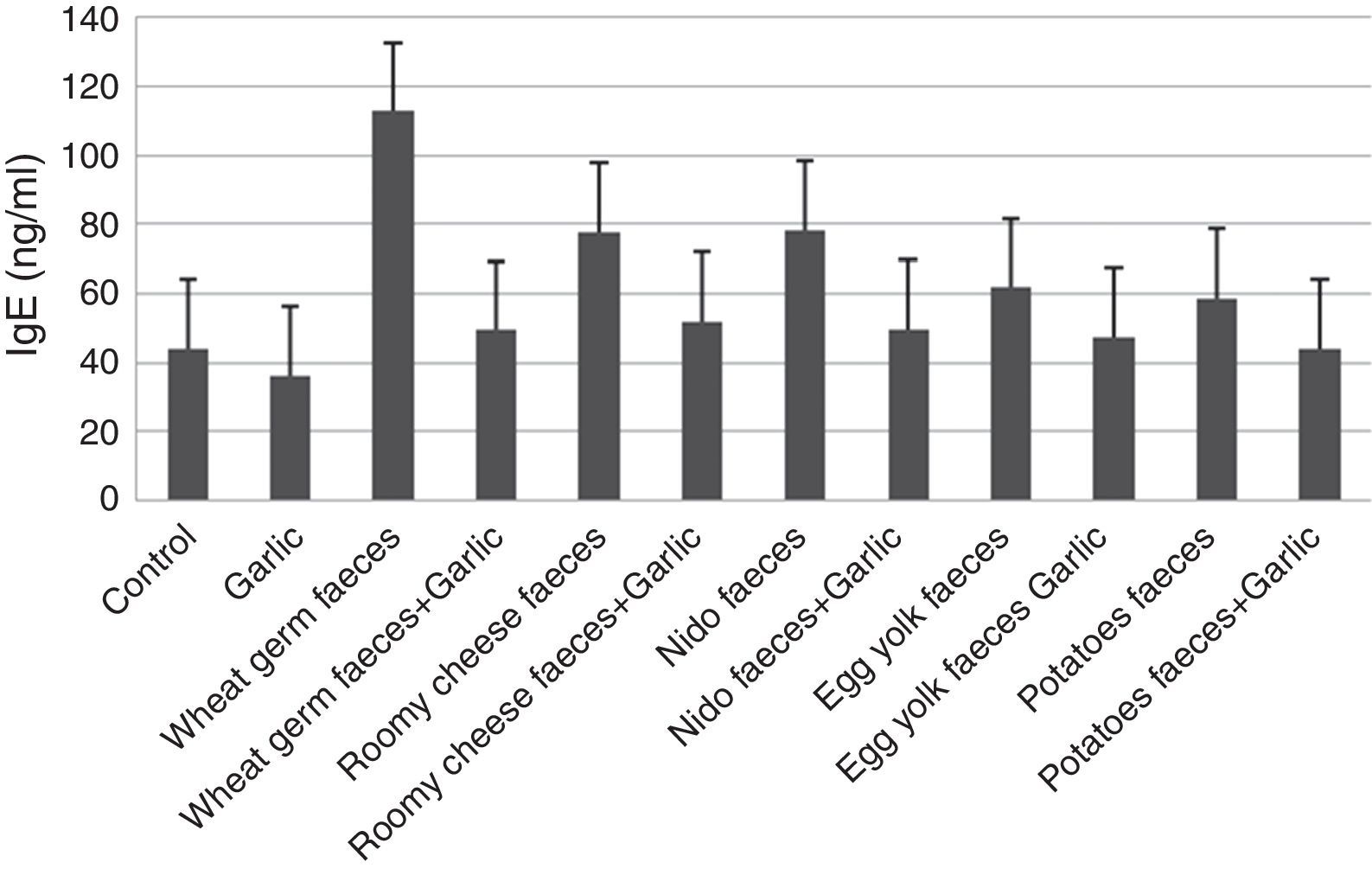
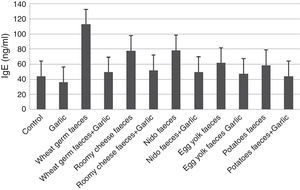
Quantification of IgEIn contrast to IL-4, ELISA tests of IgE showed that the garlic-treated group (36.30±0.427ng/ml) is lower than the control group (44.28±0.400) and the groups treated with T. putrescentiae faeces produced by wheat germ (112.87±2.798pg/ml), roomy cheese (77.71±7.646pg/ml), nido (78.46±5.449pg/ml), egg yolk (61.90±4.374pg/ml) and potatoes (58.84±3.213pg/ml) are higher than the groups treated with garlic in addition to the above five diets (49.51±3.642pg/ml, 51.91±1.954pg/ml, 49.87±1.691pg/ml, 47.65±1.241pg/ml, 44.19±1.525pg/ml, respectively) (Fig. 5). Statistical analysis showed that there is a significant difference between the garlic-treated group and either the control or faeces-treated group (P<0.05).
DiscussionT. putrescentae mites are known to cause hypersensitivity reactions and asthma.10 Selection of the culture media is one of the very important factors for successful mass rearing of house dust/storage mites.26 The mite problems described above can have severe effects on the health.15 The following points must be taken carefully to prepare a specific vaccine against house dust mite bronchial asthma, perennial rhinitis or allergic dermatitis. These are: (i) identification of the local house dust mites, (ii) determination of the sensitising mite allergen and this necessitates isolation of the identified house dust mites extracts, and (iii) identification of the specific house dust mite extract to which patients give positive reaction.
Mass rearing of T. putrescentiae was carried out, since this species has proven to be the second most important allergic mite in Egypt22 as well as in other places such as Sydney and New Guinea.27 It was also recorded in the three governorates with high numbers. In this study the best diet was the wheat germ by which 1881 mites were gained. The second effective diet (cake) gave 1575.9 mites. Roomy cheese gave 1539.7 mites. Also nido, egg yolk and egg albumin gave a number of mites greater than one thousand. On the other hand, potatoes, fish meal, copra, chicken bouillon, pollens, powdered bean, human hair and scales and yeast extract gave a number of mites lower than 500 mites.
Similar and other diets such as powdered animal food;28 (yeast, clover seed, ground nuts, linseed, maize germ, peas and rice);29 (mixture of ground and dried oil liver);30 a mixture of human skin scales and yeast, monosaccharides, disaccharides and polysaccharides;31 wheat germ;27 and a mixture of roomy cheese, dried egg yolk and yeast.22 Green and Woodcock mentioned that the type of food ingested can markedly affect the fecundity, duration of development, maturity, longevity and age structure of a T. putrescentiae population.27
In addition to the mite mass rearing, we have investigated whether the faecal antigen is important in the development of asthma or not, where mite faecal pellets break up into many small pieces and combine with dust particles to form an allergic dust.32
The potential of certain pollutants to elicit allergic airway disease has been established in both experimental models and in humans.33 Short exposure (10 days) to HDM alone or to HDM and OVA resulted in equivalent levels of airway inflammation due to a robust inflammatory response in the lung. Moreover, it has been recently shown that intranasal administration of HDM daily for 10 days induces allergic sensitisation and Th2 airway inflammation, both of which are substantially reduced by concurrent treatment with anti-M-CSF antibodies.34 This prompted us to expose the tested animals for about 10 days.
Allergic disorders have been rising in recent years. It has been demonstrated that IgE antibodies play an important role in mediating type I hypersensitivity in humans.35 In both food and inhalant allergy it is accepted that food- or HDM-specific IgE binds to high-affinity Fc¿RI on mast cells, basophiles, macrophages, and dendritic cells, as well as to low-affinity Fc¿RII on macrophages, monocytes, lymphocytes, eosinophils, and platelets.36 When mite allergens penetrate mucosal barriers of the gastrointestinal or respiratory tract and contact IgE antibodies bound to mast cells or basophiles, histamine and other mediators that induce symptoms of immediate hypersensitivity are released.37 Other studies have also suggested that cleavage of CD23 (low-affinity IgE receptor) may promote and enhance an IgE immune response.38
Another factor to consider is cytokine production. Indeed, it has been suggested that HDM may privilege the generation of a Th2-polarised response by modulating the balance between IL-4 and IFN-γ.39 In addition, various dust mite allergens act on bronchial epithelial cells in vitro to elicit the production of a number of cytokines.40
IL-4 is a key anti-inflammatory cytokine and plays an important role in the cytokine network and in the Th1/Th2 balance. It is generated by helper T cells, and possibly in the early adaptive immune response by mast cells and basophils.35 In this study, the low level of IL-4 in the faeces-treated group may be due to the negative feedback that is exerted by histamine itself to inhibit IL-4.37
Thus, this study tended to measure the levels of INF-γ, L-4 and IgE that are involved in allergic disease. Repeated exposure to T. putrescentiae allergic faeces caused an increase in both INF-γ and IgE, and a decrease in IL-4. The high level of both INF-γ and IgE, and the low level of IL-4 may participate in airway disorder. After garlic feeding, the increase of IL-4 and the decrease of both INF-γ and IgE were maintained, and this may play an important role in the airway remodelling processes. The immunomodulatory effects on INF-γ, IL-4 and IgE balance were tested by others.14,41
Garlic in small amounts applied as a food condiment does not usually pose an important risk, except for rare specific allergic reactions in hypersensitive people.42 In contrast, this study showed that some allergic disorder could be modulated by garlic.
In conclusion, these data demonstrated that wheat germ, roomy cheese, nido, egg yolk and potatoes media increase the mass rearing of T. putrescentiae compared to those recorded by fish meal, copra, egg albumin, chicken stocks, pollens, bean, human hair & scales and yeast extract. Repeated exposure to T. putrescentiae allergic faeces caused an increase in both the pro-inflammatory cytokine INF-γ and the main allergic immunoglobulin IgE, and a decrease in the anti-inflammatory cytokine IL-4. The high level of both INF-γ and IgE, and the low level of IL-4 may participate in airway disorder. After garlic treating, the increase of IL-4 and the decrease of both INF-γ and IgE were maintained, and this means that garlic may play an important role in the amelioration of the airway immune response.
Ethical disclosuresProtection of human and animal subjectsThe procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataNo patient data appears in this article.
Right to privacy and informed consentNo patient data appears in this article.
Conflict of interestThe author has no conflict of interest to declare.
We thank Prof. Dr. Mohamed H. I. (Zoology Department, Faculty of Science, El-Minia University, El-Minia, Egypt) for using his digital camera.