Eosinophilic esophagitis (EoE) is a chronic, local immune-mediated esophageal disease that has been on the increase lately. There is currently enough evidence to conclude that EoE is an allergic disorder triggered by food allergens, with cow’s milk (CM) being the most frequent. Dietary intervention is the first-line approach. This study aimed to assess the clinical characteristics, the diagnostic method, and the prognosis of patients whose culprit food was CM, as opposed to other triggers.
MethodsChildren with EoE evaluated in our pediatric Allergy Department were retrospectively studied from 2004 to 2017. We collected clinical variables, diagnostic protocol, treatment, and follow-up data. We compared patients whose culprit food was CM and patients with EoE due to other causative agents.
ResultsWe analyzed 31 children with EoE and found the causative food to be cow’s milk in 14 (45%). Clinical characteristics were similar in patients with EoE due to milk or any other cause. Eight of 14 patients with milk-induced EoE (57.14%) presented positive skin prick test results against cow’s milk. All patients had positive IgE against cow’s milk. None of the patients had any other food as the trigger. The median follow-up was 2.68 years (6 months to 9 years) with initial remission of 100%.
ConclusionTesting-based elimination diets effectively treated all of the patients with milk-induced EoE. The advantage of this diagnostic protocol is that it required a mean of only two foods to be tested, significantly smaller number than in empiric diets.
Eosinophilic esophagitis (EoE) is a chronic, local immune-mediated esophageal disease that has increased in frequency during the last few decades, with an estimated incidence of four per 100,000 persons.1 It is a pathologic condition that is likely immune or antigen-driven and is characterized clinically by symptoms of esophageal dysfunction, and histologically by the presence of ≥15 eosinophils per high-power field (eos/hpf).1,2
Its pathophysiology is complex and constitutes an active research area. The implication of IgE in this disease remains unknown. EoE is caused by an adaptative immune response to patient-specific antigens, primarily but not exclusively foods.3 The most frequent finding is the presence of Th2 lymphocytes with altered esophageal barrier function. The many culprit cytokines and chemokines involved in the recruitment and remodeling of eosinophils include thymic stromal lymphopoietin, interleukin-13, CCL26/eotaxin-3, and transforming growth factor-b.1 The IgE implication in this disease is still unknown. Recently, high levels of milk protein serum IgG4 have been associated with EoE,4 but the relationship with its pathogenesis has yet to be clarified.5
New diagnostic criteria are accepted and consist of the following: (a) symptoms of esophageal dysfunction; (b) eosinophilic esophageal inflammation, with 15 eos/hpf, affecting the esophagus alone; and (c) exclusion of other causes of esophageal eosinophilia.2 The management of EoE includes dietary, pharmacologic, and endoscopic interventions. The main pharmacologic intervention is high-dose proton-pump inhibitors (PPI). If monotherapy fails, a combination of therapies is acceptable. Based on the most recent guidelines, dietary intervention can be used as first-line therapy.6
Data regarding the natural history of EoE are limited, although available evidence suggests that the disease can persist into adulthood7; however, the proportion of patients with progressive disease remains unknown.8
Cow’s milk (CM) is the most frequent trigger of EoE in children,9–16 and some studies11,12 propose a CM elimination diet as the first step in treatment, with remission being achieved in 64% of patients. EoE should therefore be considered an important aspect of the CM allergy spectrum both in children and in adults.15
The aim of this article was to describe the characteristics of a population of patients with CM-induced EoE, the diagnostic protocol used, and follow-up after diagnosis. We also compared this profile of EoE patients with other culprit or unknown causative agents in order to identify differences. Our data provide a useful picture of the follow-up of EoE pediatric patients, especially CM-induced EoE, which was diagnosed in our department over a 14 year-period.
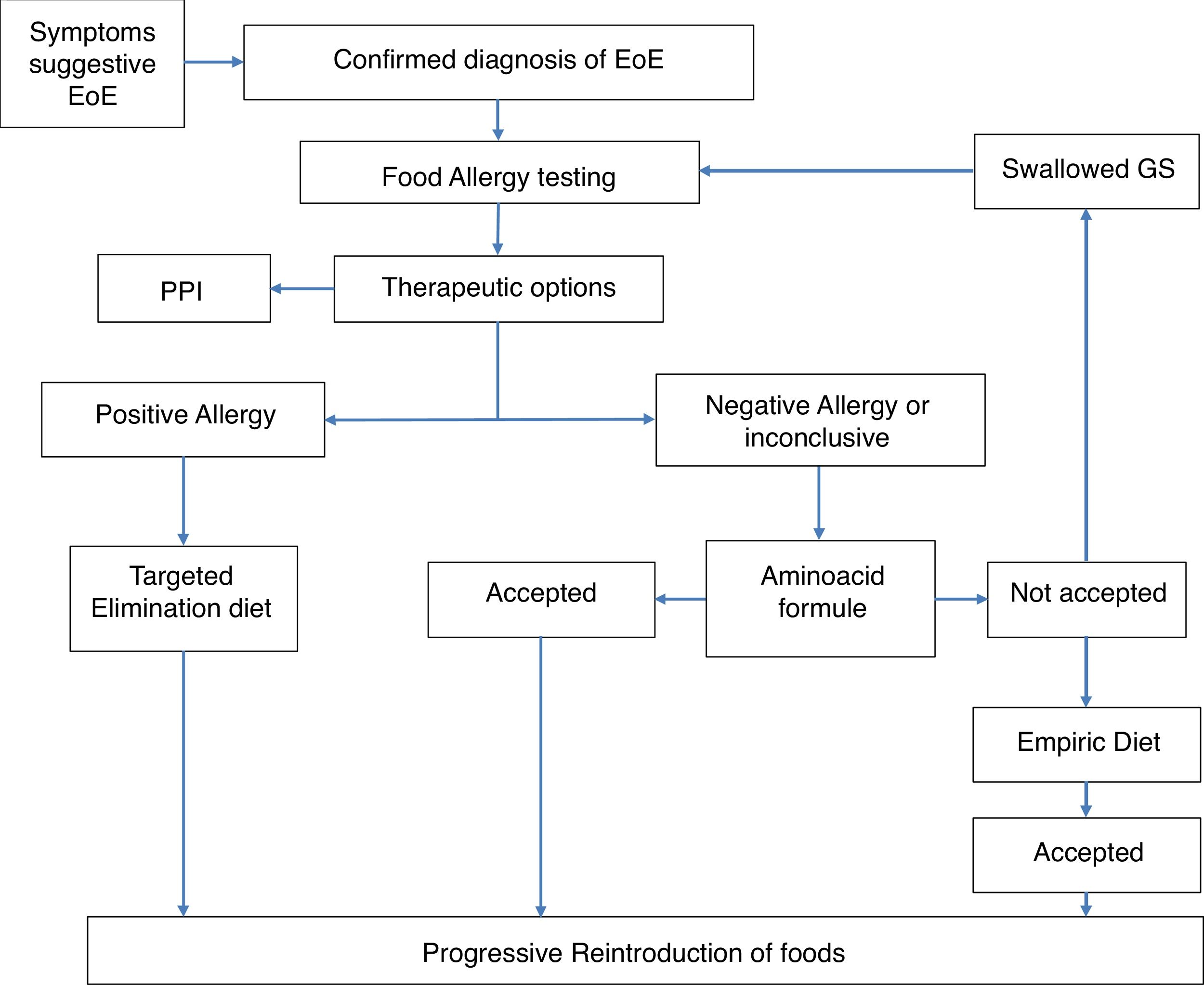
MethodsThis is a retrospective study performed in the Department of Allergy of the Hospital Ramón y Cajal, Madrid, Spain, from 2004 to 2017. The clinical records of patients who followed an established protocol for the diagnosis and treatment of EoE in the pediatric population were recruited. The protocol was agreed upon by the departments of Allergy and Pediatric Gastroenterology. The protocol has been published and is briefly described below and summarized in Fig. 1.17 All patients diagnosed with EoE, confirmed by histology (The eos/hpf), underwent allergy examination by skin prick tests (SPT) with commercially available food allergens and determination of specific IgE to milk (cow milk, alphalactoalbumin (ALA), betalactoblogulin (BLG) and casein), wheat, egg (white egg and yolk), legumes, (lentil, chick pea, bean and soya), meat (veal, pork) and fish. Positive SPT were considered 3 mm upper saline and specific IgE (sIgE) with a value >0.1 kU/L.
According to these results, our protocol included an avoidance diet based on the allergological study. If the allergology study was negative, an empirical or elemental diet and the sequential introduction of a different food group was offered. Clinical, endoscopic and histological evaluations were performed to assess the response. Regular clinical assessments were made of all patients. A causative food was considered when after avoidance diet a histological remission was achieved and relapsed when the food was reintroduced into the diet.
Only those patients who completed the strategy were selected (see Fig. 1) and we were able to follow them up for at least six months. We excluded patients who responded to PPI as a first treatment.
We compared clinical and treatment variables between patients with EoE due to milk and EoE caused by other foods.
The study protocol was approved by the Ethics Committee for Clinical Investigation of Hospital Universitario Ramón y Cajal (Protocol number: 141/19).
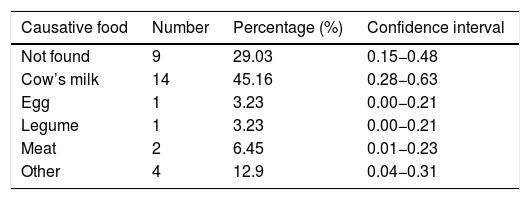
ResultsWe analyzed 31 children with EoE aged from 2.6 to 15.7 years. The culprit food was found in 22 patients (71%). No causative food was found in nine patients (29%). In 14 patients (45%) CM was the implicated food; the remaining causative foods identified were much less frequent (egg [3.23%], legumes [3.23%], and meat [6.45%]) (Table 1). 22 patients were managed from the beginning with avoidance diet after performing a complete allergology study. Two patients refused to undergo a diet approach and they were treated with corticosteroids.
Median follow-up time was 4.3 years (from 0.5 to 9 years). Remission was achieved in 29 out of the 31 patients (93.5%). Out of the seven patients following an elemental diet, three remitted after identifying the causative agent and four patients needed corticosteroids additionally. All the 22 patients managed with a diet according to the allergological study achieved remission. Two patients treated with corticosteroids as monotherapy failed to achieve remission. During the follow-up, clinical and histological relapses of EoE were assessed in five patients (16%), with unexplained etiology and the patients were treated with glucocorticoids or PPI. Most patients had a positive SPT result against aeroallergens (64.29%, 34–86%) and against any other food (64.29%, 34–86%). The median total IgE was 126 IU/L.
All patients had at least one symptom at the time of diagnosis. The most common symptoms were impaction (64.29%; 95%CI, 0.34−0.86), abdominal pain (50%; 95%CI, 0.23−0.76), vomiting (35.71%; 95%CI, 0.13−0.6), and dysphagia (14.28%; 95%CI, 0.02−0.48).
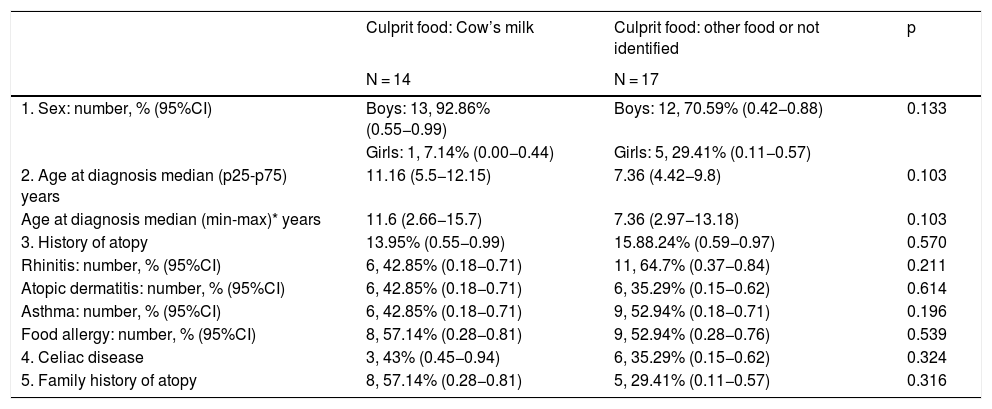
A comparative analysis was made between the group of children with EoE caused by CM and other foods, as well as with unidentified causative foods, although no statistically significant differences were found for any of the variables studied (Table 2).
General clinical characteristics of the patients.
| Culprit food: Cow’s milk | Culprit food: other food or not identified | p | |
|---|---|---|---|
| N = 14 | N = 17 | ||
| 1. Sex: number, % (95%CI) | Boys: 13, 92.86% (0.55−0.99) | Boys: 12, 70.59% (0.42−0.88) | 0.133 |
| Girls: 1, 7.14% (0.00−0.44) | Girls: 5, 29.41% (0.11−0.57) | ||
| 2. Age at diagnosis median (p25-p75) years | 11.16 (5.5−12.15) | 7.36 (4.42−9.8) | 0.103 |
| Age at diagnosis median (min-max)* years | 11.6 (2.66−15.7) | 7.36 (2.97−13.18) | 0.103 |
| 3. History of atopy | 13.95% (0.55−0.99) | 15.88.24% (0.59−0.97) | 0.570 |
| Rhinitis: number, % (95%CI) | 6, 42.85% (0.18−0.71) | 11, 64.7% (0.37−0.84) | 0.211 |
| Atopic dermatitis: number, % (95%CI) | 6, 42.85% (0.18−0.71) | 6, 35.29% (0.15−0.62) | 0.614 |
| Asthma: number, % (95%CI) | 6, 42.85% (0.18−0.71) | 9, 52.94% (0.18−0.71) | 0.196 |
| Food allergy: number, % (95%CI) | 8, 57.14% (0.28−0.81) | 9, 52.94% (0.28−0.76) | 0.539 |
| 4. Celiac disease | 3, 43% (0.45−0.94) | 6, 35.29% (0.15−0.62) | 0.324 |
| 5. Family history of atopy | 8, 57.14% (0.28−0.81) | 5, 29.41% (0.11−0.57) | 0.316 |
A descriptive analysis of the 14 patients with milk-induced EoE revealed that eight of the 14 patients (57.14%) presented a positive skin prick test result against CM.
All the patients had positive IgE against cow’s milk with a median of 0.66 UI/L (from 0.11 to 2.46 UI/L). In 10 patients out of 14 we had data about their sensitization profiles, the median IgE against ALA was 0.23 UI/L) (0.09–1.03 UI/L; against BLG median was 0.09UI/L (0.08−0.67 UI/L) and against CM casein was 0.22UI/L (0.06−0.40 UI/L). None of the patients had any additional food as a trigger.
A total of 52 endoscopies were performed in 14 patients, with an average of four endoscopies per patient (minimum two, maximum nine). An average of two forbidden foods were necessary to determine the causative food. All patients with milk induced-EoE achieved initial remission (100% of patients). Thereafter, the median number of years of follow-up was 2.68 years (from 6 months to 9 years),
During follow-up, only one patient experienced a relapse. The patient was a boy who was diagnosed at 11 years of age suffering chest pain and impaction. The diagnostic protocol revealed CM to be the trigger, and EoE resolved. One year after a dairy and milk–free diet, he began to have esophageal symptoms again, and the biopsy was positive for EoE. Oral fluticasone was subsequently prescribed.
One CM-induced EoE patient was diagnosed at age two years experienced cough and impaction after eating solid foods, the allergological studies revealed a positive SPT and IgE result against wheat and CM. His biopsy results, which were normal when he undertook a milk- and gluten-free diet, became positive after the introduction of milk. After three years of milk avoidance diet, the skin test and IgE results turned negative, so milk was reintroduced, and no relapse was recorded. The patient was followed up for five years and remained asymptomatic.
DiscussionCM-induced EoE was the most frequent presentation in our series of patients with EoE. All patients in whom milk was confirmed as the causative agent had positive sIgE and/or positive skin tests to cow's milk. Their biopsies were normalized after the removal of dietary milk proteins and clinical relapse was observed after the reintroduction of milk into the diet. Milk was not the culprit agent in any patient with negative SPT and or sIgE to milk proteins. Therefore, through an allergological study we have correctly identified the patients with milk-induced EoE.
The clinical characteristics of CM-induced EoE were similar to those of the other patients diagnosed with EoE with other culprit foods or unknown causative agents. Therefore, at least in our series of patients, EoE induced by milk does not seem to have a different phenotype than that caused by other allergens.
At the beginning, when we started to diagnose and treat patients with EoE (2004) we built a protocol of diagnosis and a treatment flowchart (Fig. 1) based on the allergological study. At that time food elimination was the main treatment17 but progressively PPI and topical steroids have been added to the treatment armamentarium. These drugs are not etiological approaches. The demonstration of the specific causal food allows the etiological treatment of the patient, of vital importance in the pediatric patient to establish a long-term therapeutic attitude.
Dietary intervention has proven to be an effective first-line approach in children with EoE. The three possible feeding strategies are elemental diet, empiric elimination diet, and allergy testing–based therapy. It is important to take into account a series of factors, such as nutritional deficiencies, consumption of resources,17,18 impaired quality of life, psychological impact, and the development of eating disorders, including the avoidant/restrictive food intake disorder in diet-treated children with EoE.19 Therefore, a thorough dietary assessment by a nutritionist is essential.
Evidence that food allergy causes EoE in children has been shown after an elemental diet (total resolution in 90–95% of cases). A six-food elimination diet for CM, egg, soy, wheat, peanut/tree nut, and fish/shellfish has shown consistent effectiveness in the treatment of EoE, with demonstrated histologic remission in 74%.20,21 The empiric Four Foods Elimination Diet (FFED), which eliminates the four most common food triggers (milk, wheat, egg, and legumes [including soy]) has proven effective in two prospective studies: the first in 54% of a group of adult Spanish patients10 and the second in 64% of a group of children from the USA.21
In our protocol of diagnosis and treatment, the intervention on the diet based on the allergological study has been successful, since remission was achieved in all patients after the elimination of the causative food (22 patients). In the case of milk, all patients achieved remission and only one patient relapsed. Our experience with elementary diets is less satisfactory. Only the food involved in half of the patients could be established, so we currently do not recommend them.
Substantially lower response rates have been observed for allergy testing–based diets in adults. Two studies carried out in adult patients showed response rates of only 26.6% and 35%, respectively.22,23 Nevertheless, a recent study in adults reported histological remission in 73% of patients undergoing allergy testing–based diets.24 Pediatric studies have reported response rates of 53–69%.21,25 Despite the fact that the new evidence-based guidelines for diagnosis and management of EoE do not recommend allergy testing–based food elimination,2 the use of allergy testing–based diets in the present study was effective in 71% of patients. Therefore, we can state that at least in this group of pediatric patients, the predictive value of the allergological study is high, even more so in the case of CM-specific IgE. Moreover, in patients treated with allergy testing–based diets, a mean of two foods had to be tried to find the trigger. This feature is important (see above) owing to issues related to nutritional disorders. Other studies12,24 also highlight the advantage of the allergological study, as fewer foods need to be removed.
One of the studies cited above showed that sIgE effectively identifies CM as a food trigger in IgE-sensitized patients. The authors consider specific IgE >0.1 kU/L as the cut-off point for defining food sensitization and found a sensitivity of 66.7% and a specificity of 100% for milk.24 All of the patients with milk-induced EoE in the present study had a positive sIgE result to milk proteins, ranging from 0.11 to 2.46 kU/L, with a mean of 0.66 kU/L.
Taking the foods most often involved as causative agents of EoE in the Mediterranean area into account,9,17,18 we tested for milk, wheat, egg, legumes, and fish. Therefore, additional foods were not tested. This approach avoids confusion factors in patients with pollen allergy, who yield positive test results with vegetables, nuts, and fruits, with no symptoms caused by pan-allergens. Moreover, in a previous series in Spain, these foods frequently yielded positive test results, although they have been shown to be triggers in very few patients.9,10,16
There is a risk of developing EoE in patients undergoing oral immunotherapy for desensitization from IgE-mediated food allergy, which can increase to 2.72%.26 In our experience, up to 6.6% of patients receiving milk-based oral immunotherapy developed EoE (two out of 30 patients, unpublished data).
A thorough follow-up of these patients shows that they are all still asymptomatic, although one patient did experience a relapse, despite apparently adhering to a milk-free diet. As the patient was lost to follow-up, we were unable to reassess and identify the trigger or cause.
Follow-up studies comprising children and adults whose EoE has remitted are lacking. A series of 30 adult patients followed for a mean of 7.2 years showed that symptoms of dysphagia persisted in 29/30 patients and that EoE persisted in all of them.8 In addition, current data indicate that persistence of eosinophilic inflammation is associated with important complications, such as structural involvement, impaction, esophageal perforation, and, in children, nutrient deficiency and failure to thrive.
Even though spontaneous resolution is thought to be uncommon, in the case of children with milk-induced EoE, it seems reasonable to follow up the level of IgE; if this becomes undetectable, then an attempt should be made to introduce milk into the diet. This is the case of one of the children in the present study, who achieved complete resolution when his test results became negative, probably because he was diagnosed early. In a recent study the authors found that nine out of 1812 EoE patients reintroduced all foods excluded for management of EoE, that is, complete clinical tolerance was achieved in 0.5% of patients.27
The main limitation of this study is the reduced number of patients that we have been able to complete an accurate diagnosis (with the reintroduction of the food) and follow-up for at least six months.
Other studies published with similar therapeutic strategy also have a small sample size14,17,24 for similar reasons.
In conclusion, milk was the most frequent trigger of EoE in the patients we studied. Patients with EoE induced by milk show similar characteristics to patients with EoE induced by other foods or even caused by unknown foods. All but one, continue in remission of EoE with diet. One patient achieved complete resolution with milk tolerance.
Most of our patients were treated effectively by testing-based elimination diets. In patients with milk-induced EoE, the culprit food was identified in all cases using this approach. The advantage of this diagnostic protocol is that a mean of only two foods was necessary to identify the trigger, which is a significantly small number compared with those tested for empiric diets.
Funding sources for the studyThis study was partially supported by Ayudas de la Fundación de la SEAIC 2015 and by Ayudas Merck de Investigación 2014 and 2018.
Conflicts of interestThe authors have no conflict of interest to declare
Authors' contributionsST, CC, and BH assessed the patients. LV performed the statistical analysis.
ST, BH, LV, and DA wrote the draft and reviewed the manuscript. JM and LS reviewed the manuscript.
We thank Thomas O´Boyle for the English language review of the manuscript. LSR and JMB are investigators from FIBio-HRC, supported by Comunidad Autónoma de Madrid.
Part of this article has been presented as a Poster at the 2015 EAACI Congress. Villafana, L., Perez, N., Negrin, Y., Camarero, C., De la Hoz, B., Terrados, S. Álvarez-Cuesta, E. (2015). Management and follow up of paediatric eosinophilic esophagitis: 553. Allergy: European Journal of Allergy and Clinical Immunology, 70, 238–239.