The diagnosis of IgE-mediated cow's milk allergy (CMA) is often based on clinical history and on specific IgE levels and/or skin-prick tests (SPT), both of which are sensitive but not specific. The gold standard, oral food challenge (OFC), is expensive and time-consuming and involves a risk of severe allergic reactions. This study aimed to determine the value of specific IgEs, ratios of specific IgEs for cow's milk and its components to total IgE, and wheal size on SPT for predicting a positive OFC for CMA.
Material and methodsWe retrospectively studied 72 patients [median age, four years; age range 0.75–15 years] sensitized to cow's milk who underwent OFCs to milk. predictive variables between patients with positive and negative OFCs were compared. Receiver operator characteristic (ROC) curves were uses to assess variables’ discriminatory capacity and Youden's index to determine the best cut-offs for predicting CMA.
ResultsThe OFC was positive in 39 (54%) patients. Wheal size on SPT and all specific IgEs and specific-to-total IgE ratios were significantly different between patients with positive OFCs and those with negative OFCs (p<0.001). The variable with the greatest area under the ROC curve was casein-specific IgE (0.98), followed by β-lactoglobulin-specific IgE (0.923), casein-specific-to-total-IgE ratio (0.919), and α-lactalbumin-specific IgE (0.908). Casein-specific IgE ≥0.95kU/L yielded 88.9% sensitivity and 90.9% specificity.
ConclusionsIn our center, casein-specific IgE >0.95kU/L can obviate an OFC to cow's milk for the diagnosis of CMA in patients sensitized to cow's milk with a compatible history.
Cow's milk allergy (CMA) is among the most common food allergies in infants and young children; its prevalence ranges from 2% to 5%.1 CMA can even occur in neonates.2 According to the immunological reaction to milk proteins, CMA is classified as immunoglobulin E (IgE)-mediated or non-IgE-mediated, although both reactions can occur together.3,4 The wide spectrum of manifestations of CMA ranges from mild symptoms to life-threatening anaphylaxis.5 Most allergic reactions to cow's milk are IgE-mediated, appearing within minutes to up to two hours after ingestion; these immediate reactions can affect one or more organs and can even result in systemic reactions such as anaphylaxis.6 Non-IgE-mediated reactions are typically delayed, occurring several hours after cow's milk ingestion and mainly affect the gastrointestinal system.4,7
IgE-mediated food allergies are often diagnosed through a combination of clinical history and skin-prick tests (SPT) or laboratory investigations to detect specific IgEs for the allergen triggering the reaction; both SPTs and IgE detection are sensitive but not specific7 and often yield false-positive results, especially in children, who have a higher rate of sensitization without true allergy.8 Thus, oral food challenges (OFC) remain the gold standard for diagnosing these allergies, although OFCs are expensive, time-consuming, and sometimes cause severe allergic reactions.9–13 The probability of patients’ reacting to an OFC increases with increasing serum levels of food-specific IgE and increasing size of wheals induced in SPTs.11 Thus, various researchers have sought to establish cut-offs for specific IgEs and SPTs for cow's milk and its components (casein, α-lactalbumin, and β-lactoglobulin) that could predict whether a patient would react to an OFC; however, the cut-offs reported in different studies vary according to patient characteristics, such as age or concomitant allergies.11–22 Moreover, some studies have evaluated the usefulness of SPTs,23–25 specific IgEs,12,17,23,24 and/or the ratios of these IgEs to total IgE26,27 for predicting the result of the OFC for decreasing the risk of a positive OFC. Recent systematic reviews have reported the heterogeneity in these cut-offs.28
This study aimed to determine the value of specific IgEs for cow's milk and its components (casein, α-lactalbumin and β-lactoglobulin), of the ratios of these specific IgEs to total IgE, and of SPTs (whole cow's milk, casein, α-lactalbumin, and β-lactoglobulin, and prick-prick) in the prediction of CMA and to identify possible cut-offs that might be useful in our clinical practice.
Material and methodsStudy population and designThis retrospective real-life study analyzed findings from children and adolescents who underwent an OFC to cow's milk in our department between January 1, 2017 and December 31, 2018. Inclusion criteria were age ≤15 years, a history suggestive of CMA (those with previous anaphylaxis to cow's milk were not excluded), and sensitization to cow's milk (specific IgE >0.35kU/L and/or SPT >3mm). Patients in whom CMA was strongly suspected who underwent an OFC to determine the optimal dose of milk from which to start desensitization were also included. Exclusion criteria were suspected non-IgE-mediated milk allergies or current treatment with omalizumab (as an adjunctive to oral immunotherapy or for severe asthma).
The data recorded were: total IgE and specific IgEs (for whole cow's milk, casein, α-lactalbumin, and β-lactoglobulin); ratios of specific IgE to total IgE; wheal size on SPTs (for whole cow's milk, casein, α-lactalbumin, β-lactoglobulin, and prick-prick with fresh cow's milk); and demographic data (age, sex, comorbidities, types of allergic reaction, and family history of atopy).
The institutional review board approved the study and patients’ parents or legal guardians provided written informed consent. Patient data were recorded, stored, and analyzed in accordance with confidentiality regulations (EU 2016/679).
IgE determinations and SPTsTotal IgE and specific IgEs (cow's milk, casein, α-lactalbumin, and β-lactoglobulin) were determined in serum using sandwich assays (ImmunoCAP®, Thermofisher Scientific, Uppsala, Sweden) according to the manufacturer's instructions.29 For SPTs, standardized extracts of cow's milk, casein, α-lactalbumin, and β-lactoglobulin, as well as fresh cow's milk (prick-prick) were applied on the volar surface of the forearm or on the back with a 1mm lancet. Both negative (saline) and positive (histamine 1%) controls were used after the application of the allergen solution. Wheal diameter was measured after 20min. Children were advised to avoid antihistamines for at least 72h prior to the SPT.
Laboratory processing truncated values of specific IgEs >100kU/L to 100. SPTs or blood tests for total IgE and specific IgE collected >12 months before the OFC or collected after the OFC were excluded. If a subject had more than one value for a specific IgE or SPT, the value obtained most recently was used.
Oral food challengesOFCs were performed in our pediatric day hospital, which is near the emergency department and intensive care unit. Full emergency equipment was on hand (including adrenaline, glucocorticoids, antihistamines, supplemental oxygen, β-agonists, and intravenous fluids). All OFCs were performed with an open protocol in routine clinical practice. Children had to be free of acute symptoms of asthma or allergic rhinoconjunctivitis. In patients with atopic dermatitis, challenges were started only when a stable clinical condition was reached. Patients ingested fresh pasteurized cow's milk, starting with 1ml, followed every half hour with increasing doses (2ml, 5ml, 10ml, 25ml, 50ml, and 100ml), except patients considered at risk of reacting with minimal doses, who received lower doses (0.1ml, followed by 0.2ml and 0.5ml). Throughout the OFC, vital signs were checked, and pertinent physical examinations were repeated at least every 30min at the clinician's discretion. All patients remained under observation for at least two hours after the last dose of milk. If a clinical reaction appeared, the OFC was discontinued, treatment was administered, and the test was considered positive. If no clinical reaction appeared after a cumulative dose of at least 192ml cow's milk, the test was considered negative.
The type of each dose-related allergic adverse event was recorded, and its severity was classified as mild, moderate, or severe, according to modified Bock's criteria.30
Before discharge, families were instructed to record any symptoms that the child might have during the following week and to contact the center by telephone if necessary.
Statistical analysisCategorical variables are expressed as frequencies and percentages. Continuous variables are expressed as means or medians and ranges. To compare the groups of patients with positive versus negative OFCs, we used the chi-square test for categorical variables and the Mann–Whitney U test for continuous variables.
Receiver operating characteristic (ROC) curve analysis was used to assess each variable's ability to discriminate between positive and negative OFCs. The predictive value was considered excellent when the area under the curve (AUC) was ≥0.97, very good when 0.9 ≤AUC <0.97, good when 0.75≤AUC <0.9, fair when 0.6 ≤AUC <0.75, and poor when AUC <0.6.31 We used the Youden Index32,33 to calculate the optimal cut-offs for the variables associated with the diagnosis of CMA. Statistical significance was set at p<0.05. We used SPSS 25.0 for Windows (IBM, Armonk, NY, USA) for all analyses.
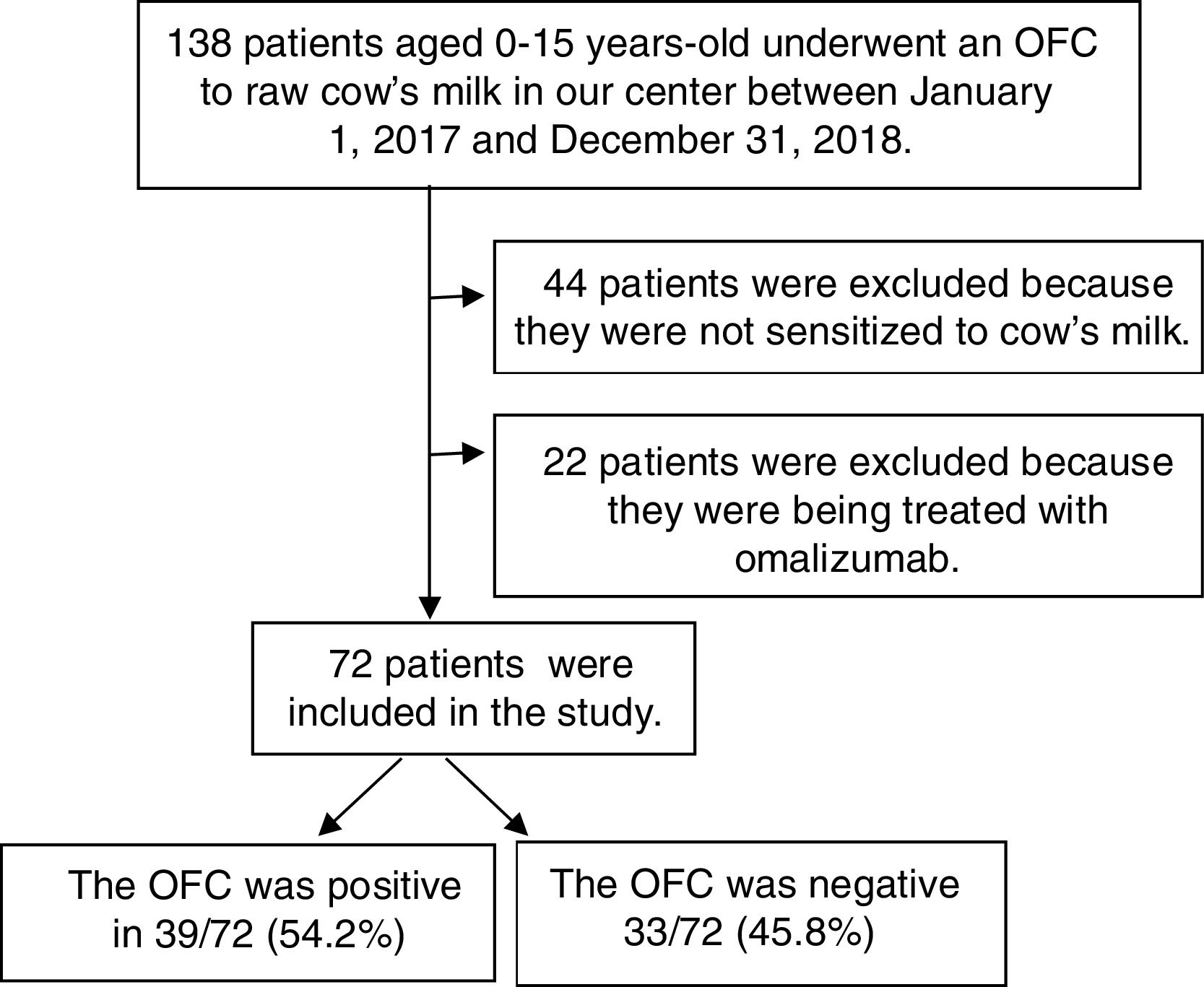
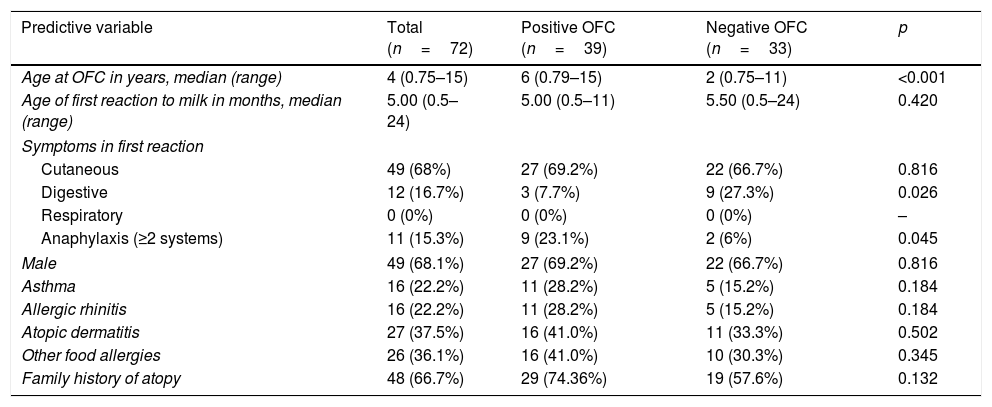
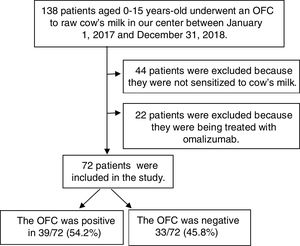
ResultsPopulation characteristicsThe patients involved in the study are shown in a flowchart in Fig. 1. A total of 138 children underwent OFCs to cow's milk during the study period; 66 were excluded (44 because they were not sensitized to cow's milk and 22 because they were being treated with omalizumab). Thus, we analyzed 72 patients [49 (68.1%) male; median age at testing, four years, range 0.75–15 years]. Table 1 reports these patients’ characteristics. In 55 (76.4%) of them, symptoms appeared before six months of age. The symptoms of the first reaction consisted of cutaneous manifestations (hyperemia, urticaria, and/or angioedema) in 49 (68%), gastrointestinal symptoms (vomiting and/or diarrhea) in 12 (16.7%), and anaphylaxis (symptoms in two systems or more) in 11 (15.2%). None had respiratory symptoms (rhinorrhea, sneezing, laryngeal stridor, hoarseness, coughing, or dyspnea). A total of 69 (95.8%) had allergy-related comorbidities: 16 (22.2%) had asthma, 16 (22.2%) allergic rhinitis, 27 (37.5%) atopic dermatitis, 26 (36.1%) other food allergies, and 48 (66.7%) a family history of atopy. Compared with the group of patients with negative OFCs, those with positive OFCs were older (p<0.001), less likely to debut with gastrointestinal symptoms (p<0.05), and more likely to debut with anaphylaxis in the first reaction (p<0.05).
Patients’ characteristics.
| Predictive variable | Total (n=72) | Positive OFC (n=39) | Negative OFC (n=33) | p |
|---|---|---|---|---|
| Age at OFC in years, median (range) | 4 (0.75–15) | 6 (0.79–15) | 2 (0.75–11) | <0.001 |
| Age of first reaction to milk in months, median (range) | 5.00 (0.5–24) | 5.00 (0.5–11) | 5.50 (0.5–24) | 0.420 |
| Symptoms in first reaction | ||||
| Cutaneous | 49 (68%) | 27 (69.2%) | 22 (66.7%) | 0.816 |
| Digestive | 12 (16.7%) | 3 (7.7%) | 9 (27.3%) | 0.026 |
| Respiratory | 0 (0%) | 0 (0%) | 0 (0%) | – |
| Anaphylaxis (≥2 systems) | 11 (15.3%) | 9 (23.1%) | 2 (6%) | 0.045 |
| Male | 49 (68.1%) | 27 (69.2%) | 22 (66.7%) | 0.816 |
| Asthma | 16 (22.2%) | 11 (28.2%) | 5 (15.2%) | 0.184 |
| Allergic rhinitis | 16 (22.2%) | 11 (28.2%) | 5 (15.2%) | 0.184 |
| Atopic dermatitis | 27 (37.5%) | 16 (41.0%) | 11 (33.3%) | 0.502 |
| Other food allergies | 26 (36.1%) | 16 (41.0%) | 10 (30.3%) | 0.345 |
| Family history of atopy | 48 (66.7%) | 29 (74.36%) | 19 (57.6%) | 0.132 |
OFC: oral food challenge.
The allergic reactions observed in the positive OFCs were cutaneous manifestations in 18 (46.1%) patients, anaphylaxis in eight (20.5%), gastrointestinal symptoms in eight (20.5%), and respiratory symptoms in five (12.8%). The mean dose of cow's milk after which the reaction occurred was 14.5ml (range: 0.3–61.1). Reaction severity was classified as mild in 16 (41%) patients, moderate in 12 (30.8%), and severe in nine (23%).
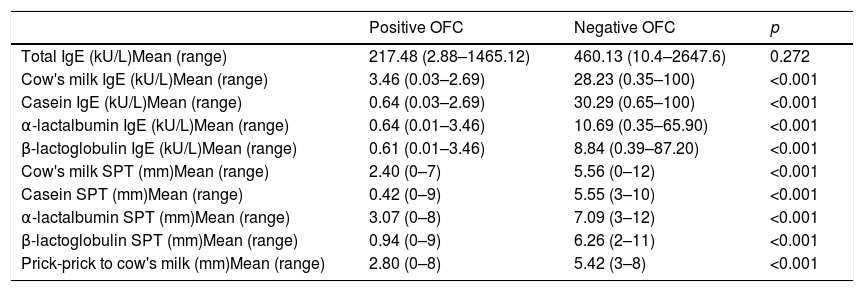
Logistic ROC for clinical thresholdsTotal IgE, specific IgEs, and wheal size on SPTs were significantly different between patients with positive and those with negative OFCs (p<0.001) (Table 2).
Total IgE, specific IgE, and wheal size on skin prick test in children with positive versus negative oral food challenges to whole milk.
| Positive OFC | Negative OFC | p | |
|---|---|---|---|
| Total IgE (kU/L)Mean (range) | 217.48 (2.88–1465.12) | 460.13 (10.4–2647.6) | 0.272 |
| Cow's milk IgE (kU/L)Mean (range) | 3.46 (0.03–2.69) | 28.23 (0.35–100) | <0.001 |
| Casein IgE (kU/L)Mean (range) | 0.64 (0.03–2.69) | 30.29 (0.65–100) | <0.001 |
| α-lactalbumin IgE (kU/L)Mean (range) | 0.64 (0.01–3.46) | 10.69 (0.35–65.90) | <0.001 |
| β-lactoglobulin IgE (kU/L)Mean (range) | 0.61 (0.01–3.46) | 8.84 (0.39–87.20) | <0.001 |
| Cow's milk SPT (mm)Mean (range) | 2.40 (0–7) | 5.56 (0–12) | <0.001 |
| Casein SPT (mm)Mean (range) | 0.42 (0–9) | 5.55 (3–10) | <0.001 |
| α-lactalbumin SPT (mm)Mean (range) | 3.07 (0–8) | 7.09 (3–12) | <0.001 |
| β-lactoglobulin SPT (mm)Mean (range) | 0.94 (0–9) | 6.26 (2–11) | <0.001 |
| Prick-prick to cow's milk (mm)Mean (range) | 2.80 (0–8) | 5.42 (3–8) | <0.001 |
OFC: oral food challenge; SPT: skin prick test.
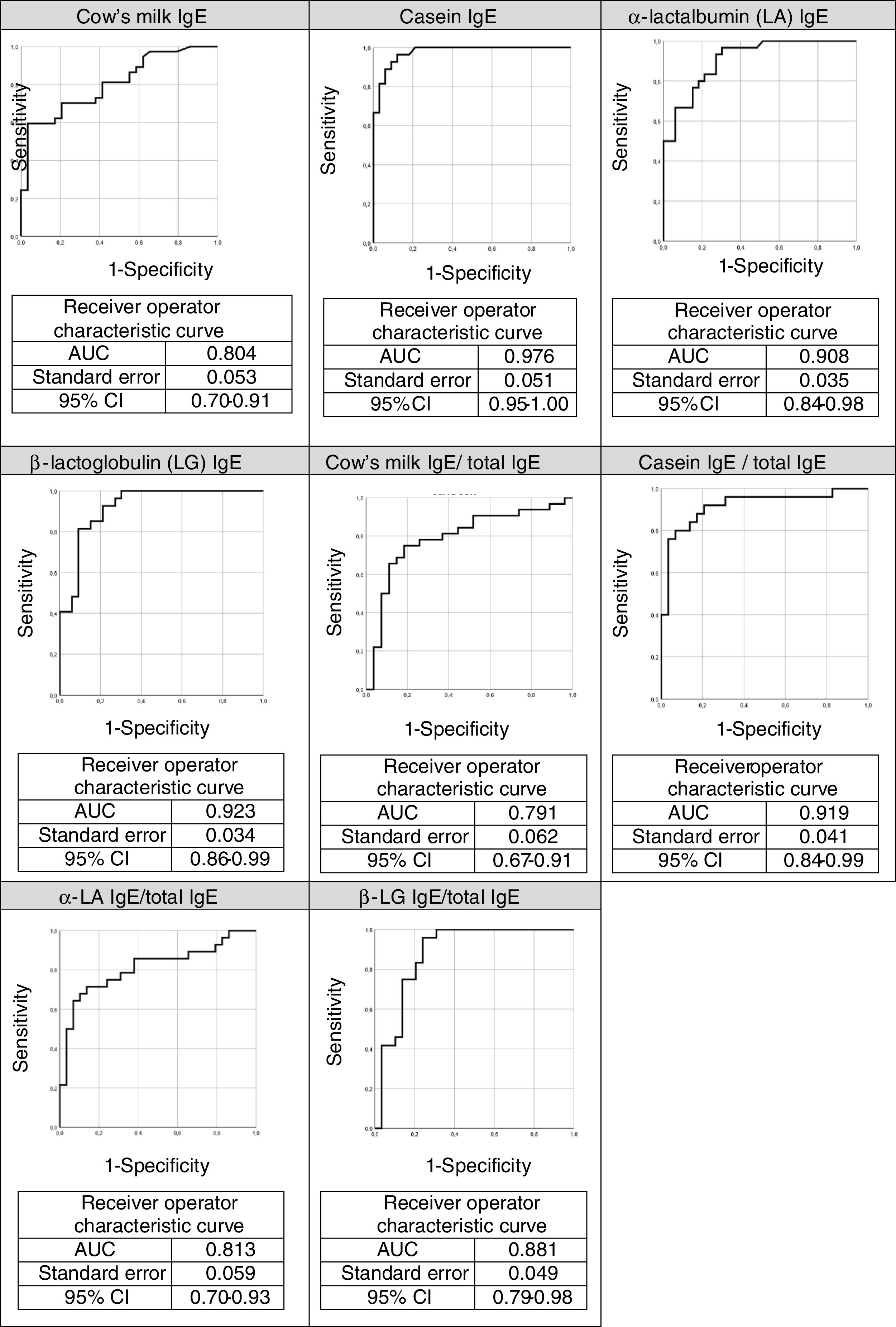
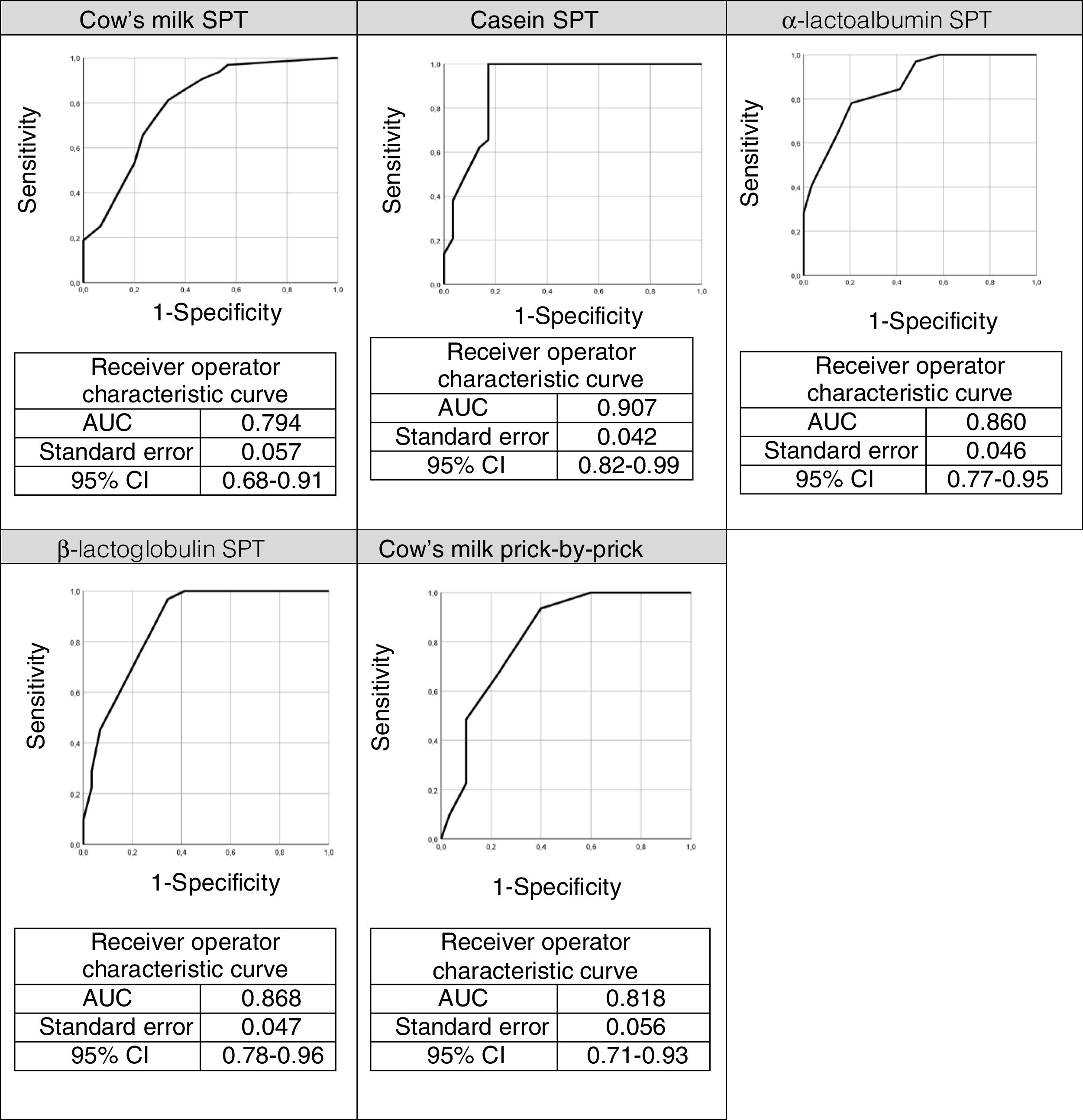
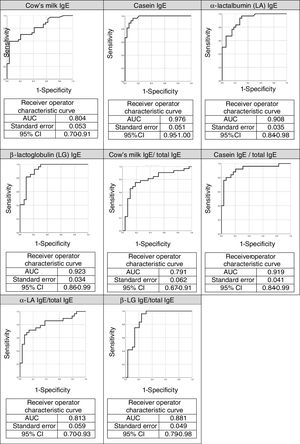
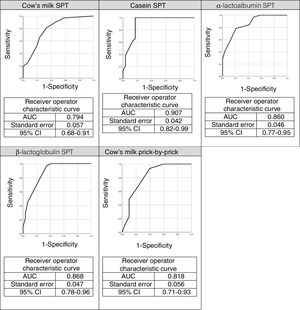
The ROC curves for the specific IgEs and their ratios to total IgE for cow's milk and its components are shown in Fig. 2, whilst Fig. 3 presents the ROC curves for SPTs to cow's milk and its components. The AUC was considered excellent for casein-specific IgE (0.976), very good for β-lactoglobulin-specific IgE (0.923), casein-specific IgE/total IgE (0.919), α-lactalbumin-specific IgE (0.908), and casein SPT (0.907), and good for β-lactoglobulin-specific IgE/total IgE (0.881), β-lactoglobulin SPT (0.868), α-lactalbumin SPT (0.86), prick-prick to cow's milk (0.818), α-lactalbumin-specific IgE/total IgE (0.813), cow's milk IgE (0.804), cow's milk SPT (0.794), and cow's milk-specific IgE/total IgE (0.791).
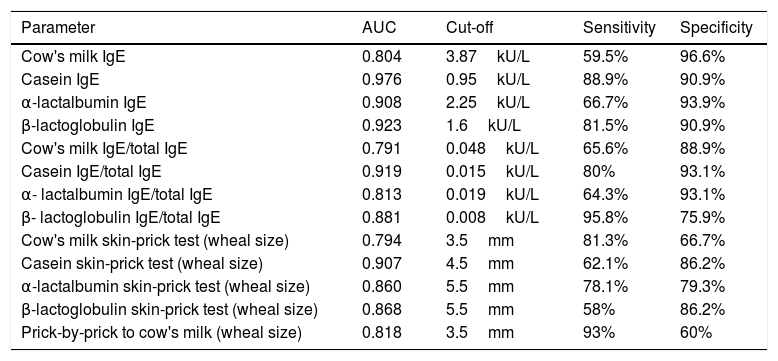
The AUC and the cutoffs (using Youden's index) for all variables, and their respective sensitivity and specificity are given in Table 3.
Area under the curve, cut-off points, sensitivity, and specificity for predictive variables for cow's milk allergy.
| Parameter | AUC | Cut-off | Sensitivity | Specificity |
|---|---|---|---|---|
| Cow's milk IgE | 0.804 | 3.87kU/L | 59.5% | 96.6% |
| Casein IgE | 0.976 | 0.95kU/L | 88.9% | 90.9% |
| α-lactalbumin IgE | 0.908 | 2.25kU/L | 66.7% | 93.9% |
| β-lactoglobulin IgE | 0.923 | 1.6kU/L | 81.5% | 90.9% |
| Cow's milk IgE/total IgE | 0.791 | 0.048kU/L | 65.6% | 88.9% |
| Casein IgE/total IgE | 0.919 | 0.015kU/L | 80% | 93.1% |
| α- lactalbumin IgE/total IgE | 0.813 | 0.019kU/L | 64.3% | 93.1% |
| β- lactoglobulin IgE/total IgE | 0.881 | 0.008kU/L | 95.8% | 75.9% |
| Cow's milk skin-prick test (wheal size) | 0.794 | 3.5mm | 81.3% | 66.7% |
| Casein skin-prick test (wheal size) | 0.907 | 4.5mm | 62.1% | 86.2% |
| α-lactalbumin skin-prick test (wheal size) | 0.860 | 5.5mm | 78.1% | 79.3% |
| β-lactoglobulin skin-prick test (wheal size) | 0.868 | 5.5mm | 58% | 86.2% |
| Prick-by-prick to cow's milk (wheal size) | 0.818 | 3.5mm | 93% | 60% |
Area under the curve (AUC) and cut-offs with their sensitivity and specificity for specific IgEs, ratios (specific IgE/total IgE) and skin prick test for cow's milk and its components.
In our study population with a median age of four years, the outcome of OFCs for cow's milk correlated with all the specific IgEs, specific-to-total IgE ratios, and SPT to cow's milk and its component proteins. All these variables were good predictors of positive OFCs (AUC ≥0.79), but the best predictor of a positive OFC was casein-specific IgE (AUC=0.976). Using a cut-off of 0.95kU/L, this variable yielded 88.9% sensitivity and 90.9% specificity.
In studies with populations comprising children of similar ages to those in our study, Caubet et al.17 and D’Urbano et al.18 also found that casein-specific IgE was the best predictor of CMA. By contrast, in another study in younger patients (median age, 1.9 years), Castro et al.19 found that specific IgE for cow's milk was a better predictor than the specific IgEs for its components (casein, alpha-lactalbumin, beta-lactoglobulin). The best cut-off for casein-specific IgE in our study (0.95kU/L) was intermediate between those reported by Castro et al.19 (1.47kU/L) and Franco et al.24 (0.72kU/L).
Casein was also the best predictor of positive OFCs (AUC=0.907) in the STPs, where wheal size ≥4.5mm yielded 62.1% sensitivity and 86.2% specificity. The STP cut-offs in our study (≥3.5mm for whole milk and ≥4.5mm for casein) were similar to those reported by Castro et al.21 (≥4.5mm for whole milk and ≥3.0mm for casein) and by Franco et al.24 (≥3.5mm for whole milk and ≥3.0mm for casein). In our study, 79% of patients with ≥3.5mm in a cow's milk SPT had positive OFCs. Similarly, Sporik et al.25 reported that 75% of patients with wheals ≥3mm in a cow's milk SPT had positive OFCs.
The cut-offs reported in different studies are very heterogeneous,9–25 probably due to differences in epidemiological and demographic characteristics among studies. Most studies included many patients with atopic dermatitis, who have elevated IgE levels and high rates of sensitization; atopic dermatitis is associated with a high rate of false-positives during food allergy testing, especially in children.34–37 Although, like other studies, our study had few patients with atopic dermatitis,15,19,27 we found no differences in the outcome of the OFC between patients with atopic dermatitis and those without. Moreover, unlike Sindeher et al.,38 we found no association between other food allergies and the likelihood of a positive OFC.
We also explored other factors that might influence the outcome of OFCs. Children with positive OFCs were older [median age, six years versus two years in the group with negative OFCs (p<0.001)]. This difference might be due to differences in the indications for OFCs. Whereas older children usually undergo OFCs to confirm strong suspicion of CMA before starting oral immunotherapy, younger children usually undergo OFCs to determine whether they have achieved tolerance. Positive OFCs were less common in patients whose first reactions to cow's milk were digestive symptoms (p=0.026), and positive OFCs were more common in those whose first reaction was anaphylaxis (p=0.045).
Because total IgE concentrations can vary with factors such as age or atopic dermatitis, we assessed whether using the ratios of specific IgEs to total IgE could improve the capacity to predict the results of OFCs compared to specific IgE alone. However, although the specific-to-total IgE ratios for cow's milk and its components were also good predictors (especially casein-specific IgE/total IgE) of positive OFCs, the ratios were no better than casein-specific IgE alone. In previous studies, Mehl et al.26 and Michinena-Spera et al.27 found that ratios tended to predict symptomatic CMA better than specific IgE alone; nevertheless, they concluded that calculating specific IgE-to-total IgE ratios offered no real advantage.
Our study is one of very few to compare specific IgE, specific-to-total IgE ratios, and SPT results together in the same population. However, our study has some limitations. Being a real-life study in a single center, there might be a selection bias because patients who undergo OFCs to cow's milk in our hospital might be different from those doing the test in other contexts. On the other hand, our study has the advantage that it reflects routine clinical practice, with OFC protocols and postponement of OFCs when the clinical history strongly suggests active CMA. Another potential limitation is that our study was retrospective; however, but Sampson12 showed that validating retrospectively established decision points for the specific IgE level with prospectively analyzed data does not introduce a bias.
ConclusionsIn conclusion, our results show that the best predictor for diagnosing symptomatic CMA is casein-specific IgE. In patients with a history compatible with CMA, casein-specific IgE >0.95kU/L can replace an OFC to diagnose CMA in our center. Calculating the specific-to-total IgE ratios offers no advantage compared to specific IgE alone for diagnosing symptomatic CMA. Local studies should be done to determine the optimum specific IgE cut-offs for each population that enable the diagnosis of CMA without the need for OFCs in certain cases.
Funding sourceThis study won the award for the best poster at the 2018 SEICAP congress. In case, the study would be published, it would be rewarded with 500 euros from SEICAP.
Conflict of interestThe authors have no conflict of interest to declare.
We thank Joan Carles Oliva for assistance with the statistical analysis and John Giba for English language editing.