Tonsils are part of Waldeyer's ring and play important immunological roles, including mucosal antigen capture and presentation to lymphocytes. A healthy immune response to allergens and successful allergen-specific immunotherapy both require the generation of allergen-specific cells for induction of peripheral T-cell tolerance.1 Functional allergen-specific T-regulatory cells have been identified in lingual and palatine tonsils with percentages approximately three times higher than those in peripheral blood, indicating their role in controlling allergen-specific T-cell responses in human tonsils.2 To date, nothing is known about the possible effect of tonsillectomy on the efficacy of sublingual immunotherapy (SLIT).
We aimed to assess the effect of tonsillectomy on clinical and immunological responses before and after three months of SLIT. The study was conducted at Ain Shams University Hospital, Cairo, Egypt, from January 2011 to August 2011, and included 26 adult patients with bronchial asthma and/or allergic rhinitis, of whom 13 had undergone tonsillectomy during childhood. All patients had a positive skin prick test (SPT) reaction to house dust mite. An informed consent was obtained from all study participants, and the study was approved by the Research Ethics Committee of the Faculty of Medicine, Ain Shams University. Clinical and immunological assessments were performed at baseline and after three months of SLIT with a house dust mite extract. Clinical parameters included the presence of cough, wheezing, sneezing and itchy nose, rhinorrhea, and nasal obstruction. Immunological parameters included SPT reactivity, serum levels of total IgE, house dust mite-specific IgE, total IgA and total IgG.
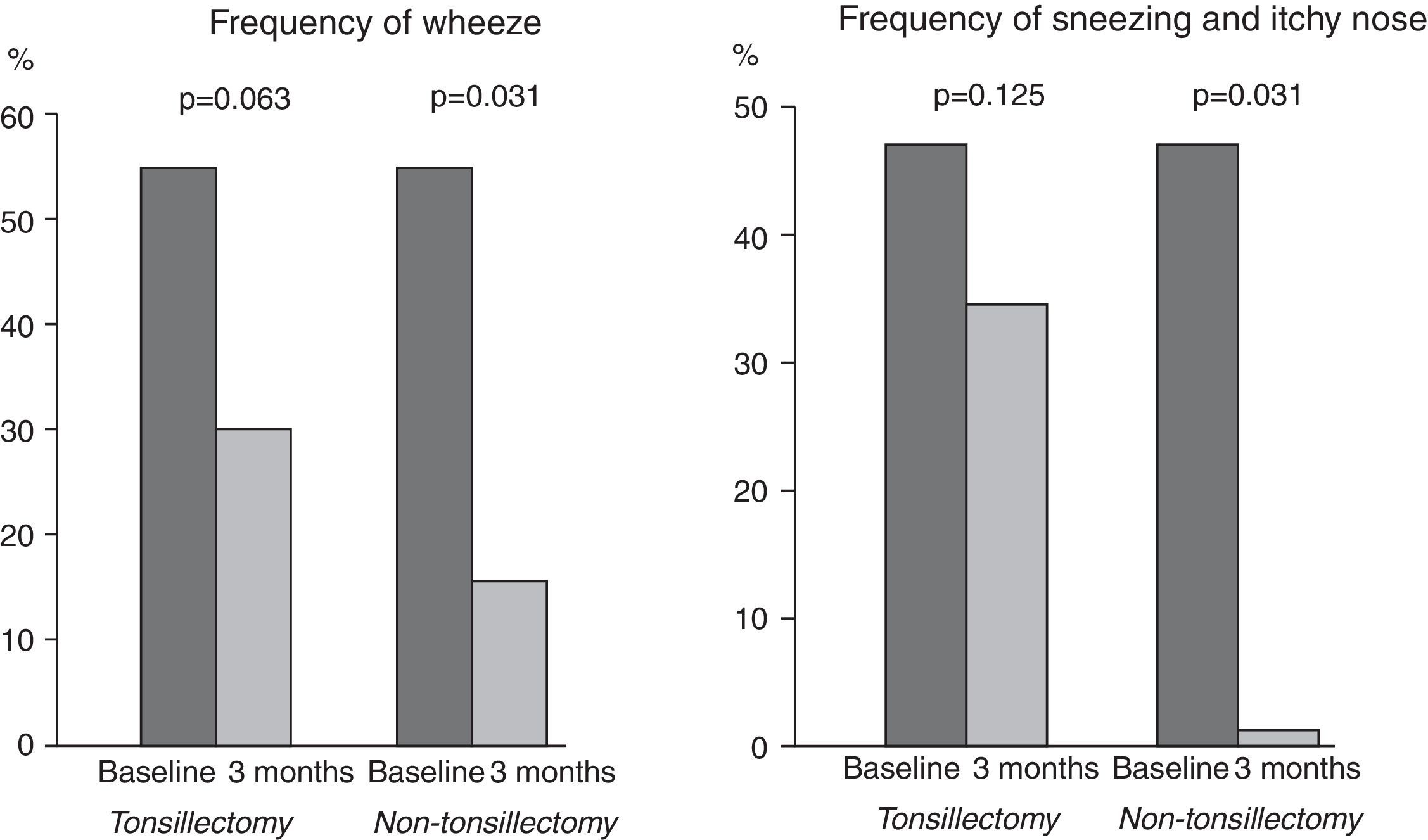
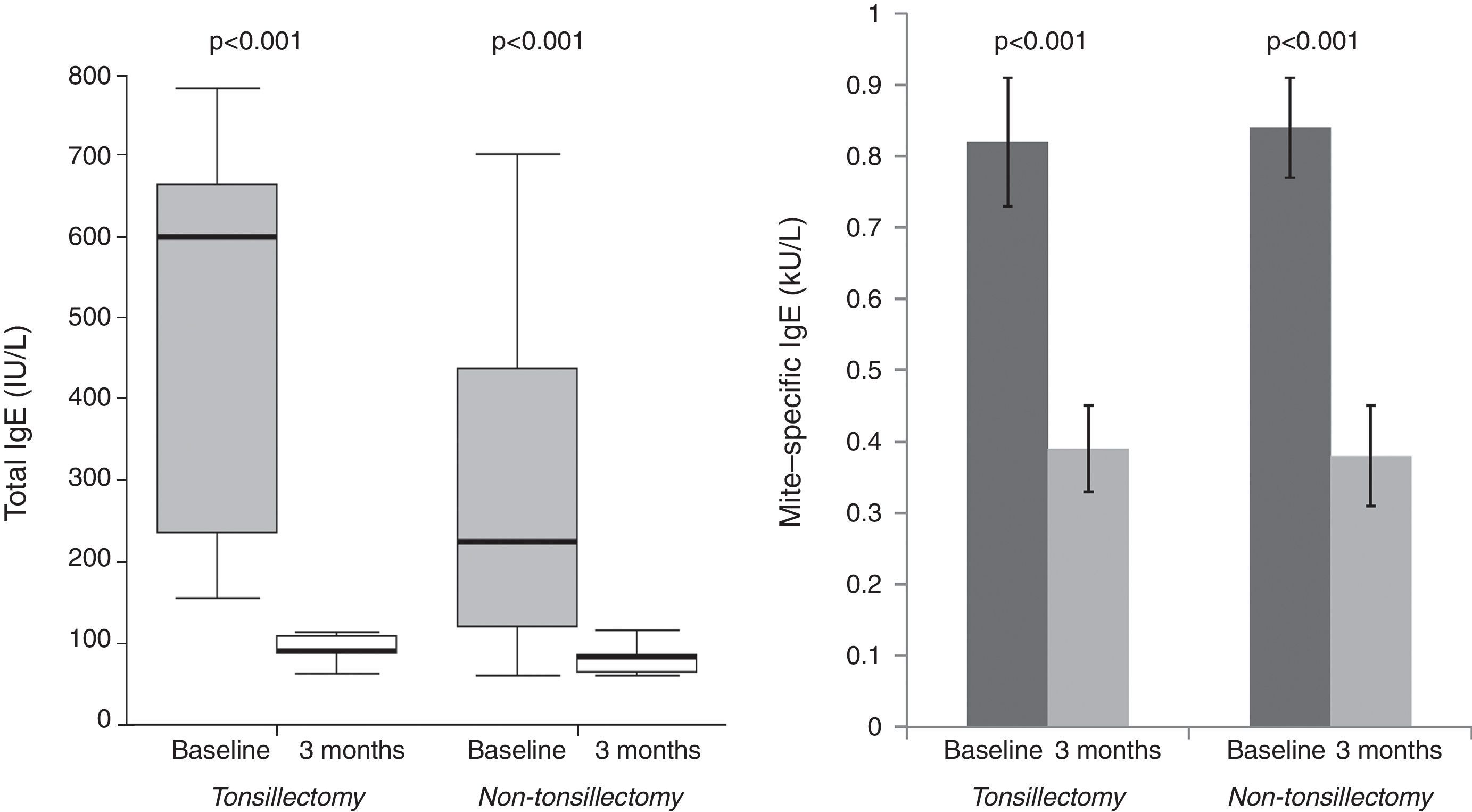
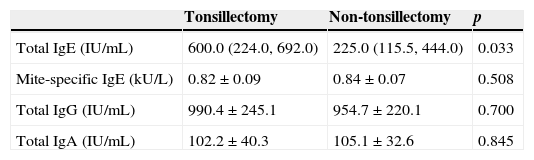
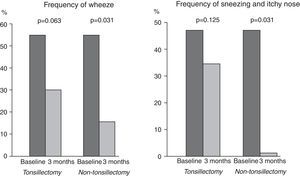
Tonsillectomy and non-tonsillectomy groups were comparable at baseline in age (21±11.5 years vs. 26.5±8.1 years, respectively, p=0.172), gender [4 males (30.8%) vs. 6 males (46.2%), respectively, p=0.420], serum levels of mite-specific IgE, total IgA and total IgG, whereas total IgE levels were significantly higher in the tonsillectomy group (Table 1). After three months, SPT reactivity to house dust mite decreased significantly in both groups. The number of patients with a positive SPT to house dust mite was significantly reduced to four (30.8%), p=0.004 in the tonsillectomy group, and three (23.1%), p=0.002 in the non-tonsillectomy group. Furthermore, a highly significant reduction in serum levels of total and mite-specific IgE was observed in both groups after three months (Fig. 2). However, no significant differences were observed after three months of SLIT in the serum levels of total IgG (943.2±204.7IU/mL, p=0.133 for the tonsillectomy group; 909.5±253.1IU/mL, p=0.133 for the non-tonsillectomy group) or total IgA (101.2±37.7IU/mL, p=0.814 for the tonsillectomy group; 107.7±29.1IU/mL, p=0.401 for the non-tonsillectomy group). As regards the studied clinical parameters, a significant reduction in the frequency of wheezing, sneezing and itchy nose was observed in the non-tonsillectomy group but not in the tonsillectomy group (Fig. 1). However, the frequency of cough, rhinorrhea, and nasal obstruction did not change significantly in either group (data not shown).
Baseline serum levels of immunological markers in both groups.
| Tonsillectomy | Non-tonsillectomy | p | |
|---|---|---|---|
| Total IgE (IU/mL) | 600.0 (224.0, 692.0) | 225.0 (115.5, 444.0) | 0.033 |
| Mite-specific IgE (kU/L) | 0.82±0.09 | 0.84±0.07 | 0.508 |
| Total IgG (IU/mL) | 990.4±245.1 | 954.7±220.1 | 0.700 |
| Total IgA (IU/mL) | 102.2±40.3 | 105.1±32.6 | 0.845 |
All values are presented as mean±SD except total IgE as median (interquartile range).
Short-term reductions in serum levels of immunoglobulins have been observed following tonsillectomy, followed by a return of these levels to normal within a few months.3,4 However, studies on the long-term immunological effects of tonsillectomy are scarce, and have been conducted mostly on children. Kaygusuz et al. recorded no difference in serum IgA, IgG or IgM levels among children 54 months after tonsillectomy, compared to that in non-tonsillectomised children.5 On the other hand, long-term reductions have been reported for IgA levels in serum among the tonsillectomised compared to the non-tonsillectomised children, while other immunological parameters, including IgE, IgG and IgM, were preserved.6 In the present study, serum levels of mite-specific IgE, total IgA and IgG were all comparable at baseline in both groups, which may be attributed to the long time since tonsillectomy. However, an interesting finding which warrants further investigation is the significantly higher total IgE levels in tonsillectomised adults at baseline, in comparison to levels in non-tonsillectomised adults.
Our results show that previous tonsillectomy does not affect the efficacy of SLIT in reducing SPT reactivity or in changing serum immunoglobulin levels. Both total and mite-specific IgE levels in serum were successfully reduced in both groups after SLIT. However, there was no change in serum levels of IgG in either group after treatment. Concerning this point, it may be more useful to measure the blocking activity of allergen-specific IgG or IgG subsets, including IgG1 and IgG4, instead of total IgG levels in sera.7 Similarly, IgA levels in our study did not change significantly in either group after three months. Data on serum IgA levels after SLIT are still conflicting, and it appears that in contrast to subcutaneous immunotherapy, SLIT elicits mucosal IgA responses, and shows IgA subclass selectivity.8 In line with our findings, an absence of early systemic immunological changes has been demonstrated for allergen-specific IgG1, IgG4, and IgA levels in plasma during the first eight weeks of SLIT in respiratory allergic children.9 A possible explanation for the similar trends in all measured immunological parameters in both of our study groups after three months of SLIT is that maybe during tonsillectomy, only palatine tonsils and occasionally adenoids are removed; the lingual and tubal tonsils are preserved and continue their lifelong role in the induction of immunological responses2; thus the effect of tonsillectomy might be limited and is of short duration.
Despite the similar changes in immunological parameters between the tonsillectomy and the non-tonsillectomy groups, the reduction in the frequency of wheezing, sneezing and itchy nose after SLIT was not statistically significant in the tonsillectomy group. This suggests that tonsillectomy might affect the efficacy of SLIT in improving local allergic symptoms. Since this finding depends on patient-reported outcomes, it could have been affected by the patients’ and the researchers’ knowledge of the study objective – namely, checking whether tonsillectomy negatively affects the efficacy of SLIT. To minimise this potential bias, triple-blinding should be performed for future studies (patients should be blinded to this specific objective of the study, and researchers and statisticians should be blinded to the tonsillectomy status of the subjects). In addition, large-scale randomised controlled studies may confirm these findings.
To our knowledge, this is the first study to report the effect of previous tonsillectomy on the clinical and immunological efficacy of SLIT. Further large-scale investigations should focus on the effect of tonsillectomy on local immunological changes following SLIT, and on similar findings for other allergens.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article. An informed consent was obtained from all study participants, and the study was approved by the Research Ethics Committee of the Faculty of Medicine, Ain Shams University.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors declare that no funding or grant was received for the study, and that they have no conflict of interest, financial or personal relationship related to the study.
The authors wish to thank Professor Al-Saied M. Abou-Gamrah, Professor Emeritus of Allergy and Clinical Immunology, Ain Shams University, Egypt, for his role in conceiving the study, and also Professor Cezmi A. Akdis, Director of the Swiss Institute of Allergy and Asthma Research, University of Zurich, Switzerland, for his valuable contribution in scientific revision of the article.