Allergic diseases affect a significant proportion of people around the world. The striking increase in the prevalence of allergic diseases over the last several decades involves various factors including changes in environmental pollutants, lifestyles, sanitary conditions, and diet.1 Pollen is a factor for the development of Type 1 allergic diseases, including pollinosis – a common seasonal allergic disease characterised by symptoms such as rhinitis and conjunctivitis. Pollinosis that occurs during spring and autumn in Japan is related to the pollen from the trees of the Cypress family, including Japanese cedars. Most recently, cedar pollinosis has become prevalent in Japan not only among adults but also in children, and it has emerged as a major public health problem, due to its high prevalence and low natural recovery rate.2
Moreover, lines of epidemiological evidence indicate that exposure to pollen is also a risk factor for the development of allergic asthma. Although a grain of cedar pollen itself is too large to penetrate the lower airways, fine particles released from ruptured cedar pollen in contact with water are small enough to reach the lower airways and induce bronchial asthma.3 In other studies, cedar pollen contaminated by air pollutants such as diesel exhaust particulates, yellow sand, and acid gases causes more persistent antigen-specific responses and airway inflammation.4
To date, five major and minor allergens (Cry j 1, Cry j 2, Cry j 3, CJP-4, and CJP-6) from Japanese cedar pollen have been identified and characterised.5 Several studies have reported that specific IgE antibodies to Cry j 1 and Cry j 2 are detected in up to 90% of the patients suffering from cedar pollinosis, and both allergens are recognised as major potential allergens for cedar pollinosis.5 Thus Cry j 1 and Cry j 2 are considered to be responsible for allergic airway inflammation through adaptive immune responses such as inducing specific IgE. However, the underlying pathological mechanisms at cellular and molecular levels remain to be elucidated, whereas other allergens such as house dust mite allergens, an indoor inhaled allergen, have been well studied both in vitro and in vivo.6,7
Antigen-presenting cells (APCs), including dendritic cells (DCs) and macrophages, play important roles in the immune system. APCs in the airways take up antigens and migrate to lymphoid organs such as local lymph nodes, where they present antigen-derived peptides on their MHC molecules after maturation. There, antigen-specific T cells differentiate into effector T cells via an interaction with APCs. Therefore, APCs and lymphocytes play crucial roles in the immune system and in the mechanisms of allergic airway inflammation.
To elucidate the precise cellular and molecular events that are induced by Cry j 1 and Cry j 2 contained in cedar pollen extract, we investigated the effects of cedar pollen extract on the responses of bone marrow-derived cells and splenocytes from atopic prone NC/NgaTendCrlj male mice (Charles River Japan, Osaka, Japan) in vitro, and we determined which cells and biomarkers are more appropriate and useful for evaluations of the allergic effects of cedar pollen. The procedures for all animal studies were approved by the Animal Research Committee at Kyoto University.
Bone marrow cells were differentiated using a modified protocol of Lutz et al.8 In brief, they were cultured in R10 medium containing 20ng/mL GM-CSF (Sigma, St. Louis, MO, USA). The bone marrow-derived cells were exposed to cedar pollen extract (Cosmo Bio Co., Tokyo, Japan) at 0, 1, or 5μg/mL as the dose of Cry j 1+Cry j 2 for 24h. The DEC205, CD86, and CD14 protein expressions on the cell surface were evaluated by fluorescence-activated cell sorting (FACS), using Ab for CD86, CD14 (BD Biosciences PharMingen, San Diego, CA, USA) and DEC205 (Milteny Biotec, Gladbach, Germany). DEC205 is an endocytic receptor and CD86 is critical for T lymphocyte activation and differentiation of T helper subsets. CD14 binds to a variety of microbial products. Splenocytes were exposed to cedar pollen extract at the same dose for 72h. The release of IL-2 and IL-4 in the culture supernatants was evaluated by enzyme-linked immunosorbent assay (ELISA) (R&D Systems, Minneapolis, USA).
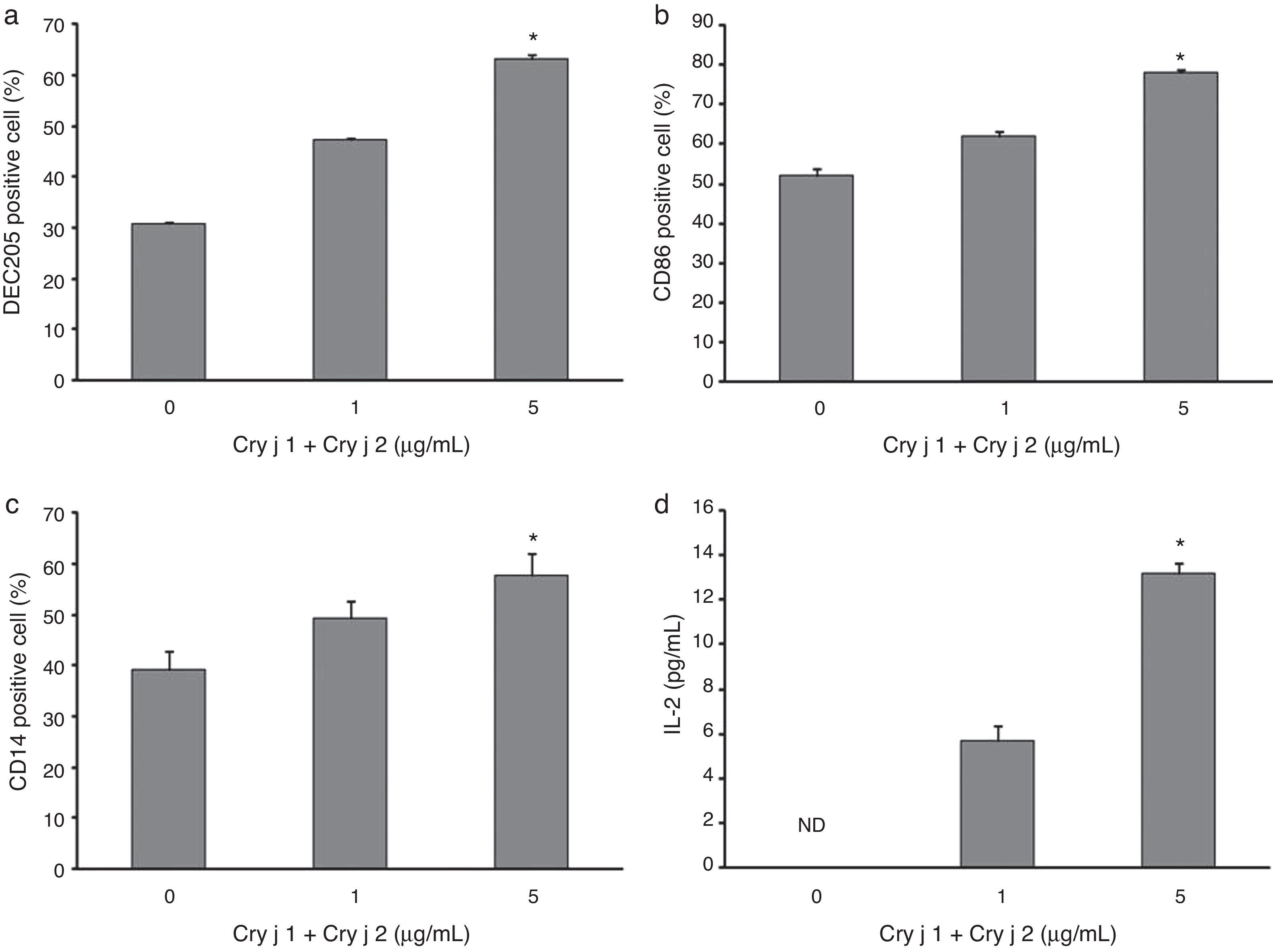
It is well established that APCs are crucial to the immune system. They do not only activate naïve T cells but also regulate T cell responses by various patterns of co-stimulatory signals and cytokine expression. We found that the cedar pollen extract significantly increased the percentages of DEC205, CD86, and CD14 expression on bone marrow-derived APCs in a dose-dependent manner (Fig. 1a–c). The levels of IL-2 from splenocytes tended to increase in a dose-dependent manner (Fig. 1d), whereas no significant trend was found in the production of IL-4 (data not shown). We also examined the expression of T cell receptor (TCR) and proliferation, but cedar pollen extract exposure did not increase the TCR positive cell percentage or the proliferation of splenocytes (data not shown).
Effects of cedar pollen extract. The expressions of (a) DEC205, (b) CD86, and (c) CD14 on bone marrow-derived cells were analysed by FACS after cedar pollen exposure for 24h, and (d) IL-2 production from splenocytes were by ELISA after cedar pollen exposure for 72h. Data are represented as the mean±standard error of the mean (S.E.M.) for each experimental group (n=4). *A p-value <0.05 was considered to indicate a significant ordered increasing/decreasing trend by Jonckheere–Terpstra test. ND: not detected.
We found that cedar pollen extract could enhance immune activation on bone marrow-derived APCs from atopic prone mice. Cedar pollen extract significantly increased the surface expression of the molecules DEC205, CD86 and CD14 related to bone marrow-derived APCs maturation/activation. DEC205 is a member of the macrophage mannose receptor family and is a novel endocytic receptor that can be used to direct antigens captured from the extracellular space to a specialised antigen-processing compartment. This molecule is known to bind carbohydrates and mediate endocytosis for subsequent processing and presentation by DCs. CD86 is recognised as a family of receptors expressed on specialised APCs such as DCs, macrophages, activated T and B cells, and thymic epithelial cells, and it plays an important role in the differentiation of naïve T cells into the Th2 subsets that are involved in allergic inflammation including asthma. CD14 binds to a variety of microbial products such as lipopolysaccharide (LPS) derived from the outer membrane of Gram-negative bacteria, and it interacts with multiple Toll-like receptor (TLR) ligands, supporting the activation of TLRs. On the basis of the present status of our knowledge, the present study is the first report that pollen extract increases the surface expression of DEC205, CD86 and CD14 on bone marrow-derived APCs from atopic prone mice. Our findings suggest that cedar pollen extract exposure activates the differentiation of bone marrow-derived cells to BMDCs and monocytes/macrophages; in other words, it can enhance APCs function as evidenced by the results of the surface expression of DEC205, CD86, and CD14, which play fundamental roles in immune responses against foreign antigens. In addition, these molecules on bone marrow-derived cells can help elucidate the effects of exacerbating/reducing factors for allergic diseases such as air pollutants, and the effects of new drugs on immune cells at the cellular and molecular levels.
Conejero et al. have reported that olive pollen induces cytokine responses (IL-4, -5, -10, -13) in splenocytes.9 In another study, peripheral blood mononuclear cells from mice sensitised once with cedar pollen produce significant amounts of IL-4 and IgE antibodies.10 In contrast, our results showed that cedar pollen extract did not significantly activate splenocytes, especially T cells. Interaction with APCs may be needed to measure more clearly the effects of cedar pollen extract on splenocytes in vitro. Moreover, the variations in the protocol including sensitisation, the cell source, and/or the exposure method might contribute to the difference between these previous reports and our present results. Further investigations are needed to understand the interaction between cedar pollen extract and T cells.
In conclusion, the exposure of immune cells to cedar pollen extract activated the network of APCs and the subsequent immune response, at least via the surface expression of DEC205, CD86, and CD14 on bone marrow-derived APCs, and IL-2 production from splenocytes. Cedar pollen extract can contribute to the induction and/or exacerbation of allergic diseases. These biomarkers and cells should be appropriate and useful for a simple and fundamental method to screen the allergic effects of cedar pollen. Our screening method can help detect exacerbating/reducing factors for allergic diseases such as air pollutants at cellular and molecular levels. Additionally, it can be used to develop broader studies towards generating new drug treatments for pollinosis focusing on the immune system.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
This study was partially supported by Daikin Industries, Ltd., Osaka, Japan.