The variety of foods and methods of preparation are part of the cultural identity of each population, and thus the main foods that cause symptoms vary among different regions. Due to their increasing frequency, Adverse Reactions to Food (AFR) have been the subject of extensive study, especially in North America and Europe but few studies have been conducted in other areas, especially in populations located in the tropics and subtropics. In this article, we review available information on the epidemiology of food sensitization and food allergies in tropical regions and explore the different epidemiological data considering the major food involved, the underlying immune mechanism and clinical symptoms partners. In addition, we identify the possible limitations and questions that arise from studies conducted in tropical countries, which helps to generate objectives for future research.
The variety of foods and preparation methods are part of the cultural identity of each population. Food allergy is the consequence of maladaptive immune responses to common and otherwise innocuous food antigens. Because of their increasing frequency, adverse reactions to foods (AFR) have been the subject of extensive study, especially in North America and Europe.1 AFR is a non-specific term meaning any undesirable reaction after ingestion (or contact) with food.2 It is estimated that 25% of the world's population have had at least one episode of AFR.3 AFR by intoxication are usually transient, self-limited and the patient can usually return to consume the suspected food if it is prepared under appropriate conditions. Intoxication represents 80% of the AFR; usually the patient's symptoms are gastrointestinal and the patient usually does not consult a health service unless very severe symptoms appear, so the diagnosis can be underestimated.
Among other AFR mechanisms is Th2 hypersensitivity. Food allergy (FA) occurs frequently in children under three years of age (3–15%), but also in older people (6–8%).4 FA can be mediated by immunoglobulin E or cellular mechanisms and can be presented with a wide variety of symptoms in the skin, respiratory and gastrointestinal tract.5 The human immune response encompasses mechanisms that allow the development of antibodies to any protein; thus, all proteins and many other molecules are potentially immunogenic and allergenic. Allergenicity is defined as the ability to stimulate the production of IgE antibodies that will provoke a clinical reaction. Individuals with food allergies react adversely to the ingestion of foods and food ingredients that most consumers can safely ingest. Although any food can potentially cause allergic symptoms, studies in North America and Europe suggest that more than 90% of reactions occur by a small group of foods: common reactions in children are to egg and to cow's milk, while in adults fish and shrimp are more frequent. Soy, wheat, tree nuts and peanuts are also common in these populations.6 Nevertheless, recent studies in tropical regions in Asia and Latin America suggest that other foods have an equal or higher frequency of sensitizations than previously described.7,8
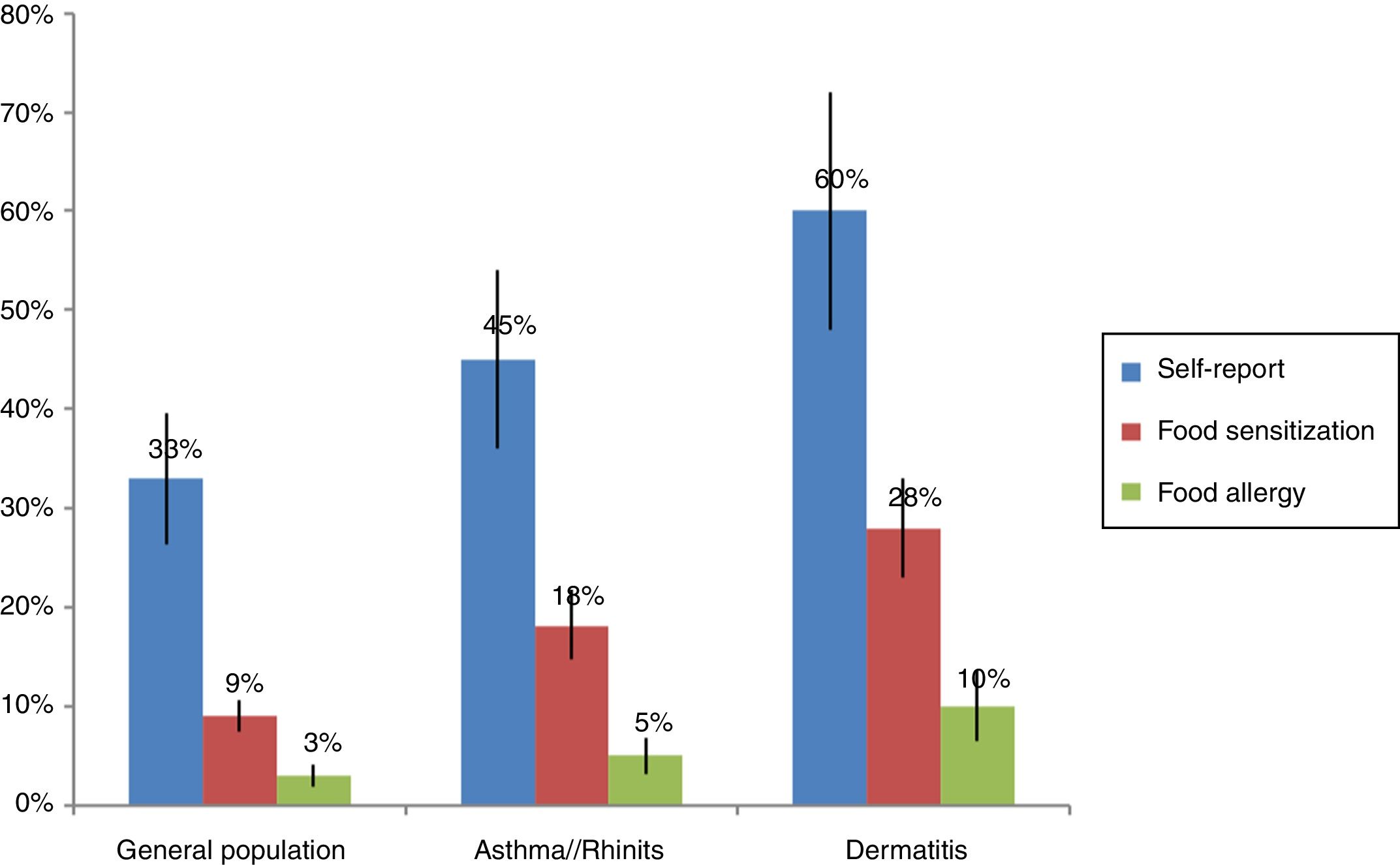
The prevalence of food allergies varies widely between studies: FA is more frequent in children and in people with atopic diseases such as asthma (6–8%) and atopic dermatitis (30–50%).9,10 Higher frequencies are found (10–25%) in self-report studies in comparison to objective studies with food oral provocation (1–3%).11 In the studies that have evaluated food sensitization by skin tests or serum IgE, it was found that 3–40% of the general population is sensitized to at least one food. The wide variation in the prevalence depends especially on the age group studied and the study design. Another difficulty to determine the true prevalence of FA is that the mechanism is not always by IgE hypersensitivity and currently methods for assessment of cellular mechanisms such as food patch test are not adequately standardized.12
Foods implicated in food allergies vary with dietary practices and dietary practices are closely linked to the availability of food, so it would be expected that in the tropics the frequency of sensitization is different from that reported in subtropical areas. While allergy to peanuts and tree nuts is high in the U.S.A., the most common reactions in the Mediterranean area of Spain and Portugal are to fish and shellfish, in Israel to sesame and in France to mustard.12,13 Recent studies describing the pattern of anaphylaxis show that food is an important cause of severe allergic reactions in Asia14 and in Latin America,15 with fruits being primarily involved in tropical countries like Singapore, Mexico, and South of China, over milk or egg in North America. The tropical climate promotes the growth of a high number of fruits and vegetables in Latin America, and the large number of people who eat them increases the possibility of reactions. The most frequently reported were chili, tomato, banana, guava and mango.7
Recently, J Boye8 carried out a review on the epidemiology of food allergy in developing countries, and noted that with the current data it was inadequate to perform a systematic review or meta-analysis. We find the same difficulty with a systematic review conducted in Latin America.7 In this article, we review the available information about the epidemiology of sensitization to foods and food allergy in tropical region and explore the different epidemiology data within this region, taking into account the main foods involved, the underlying immune mechanism, and the clinical symptoms associated with allergic reactions.
Methodology for literature searchWe did a systematic search of all articles about food allergy in tropical regions registered in PUBMED and LILACS. Original articles in English, Spanish or Portuguese performed with Latin American, African or Asian populations and published in scientific journals before March 2018 were included. For the search, the following keywords were used: allergy, food, asthma, dermatitis, eczema, atopy, rhinitis, anaphylaxis. 1786 articles were found, although only 62 met the selection criteria.
Limitations to select data for revision of food allergy in the tropicsSome studies suggest an increase in the prevalence of food allergies,16,17 but this evidence may not always be convincing. Different methodologies to evaluate the prevalence of food allergies and sensitization include: collecting participants’ allergic vs. non-allergic, self-report by questionnaire, telephone survey, or clinical charts, IgE evaluation by skin prick test, serum allergen-specific IgE and food challenge. The unselective use of these variables and/or technologies yields wildly different frequencies between studies. Well-defined guidelines and standardized protocols and algorithms must be developed in order to conduct meaningful epidemiological studies on food allergy in a large population-base, with reliable data for comparison between geographical regions. Some of these initiatives have been done in developed countries18 with a clear methodology design that allows comparisons of epidemiology data using, in most of the cases, the definitions proposed by the World Allergy Association (WAO).19 As an example, the European Community Respiratory Health Survey (EuroPrevall) analysis included 14 countries (13 European and the U.S.A.)20 and found that the most frequent foods sensitizations were to cow's milk, hen's egg, peanut, fish and shellfish. Comparing the European results there were significant differences in the prevalence between the countries, ranging from just over 4% in Spain to 18% in Sweden, which is similar to the Australian results of 19%.21,22 There were little differences for egg and cow sensitization among countries, but other foods like mustard and fruits had a frequency two times more frequent in tropical locations (2.5% and 3.6%). Such studies have helped to raise awareness to food allergies, which has resulted in national and international legislation and recommendation for the identification of priority allergens when they are present in processed food products.
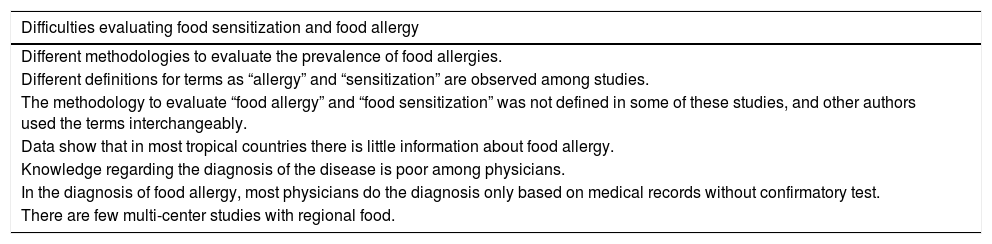
In tropical countries from Latin America, South Asia and in Africa, the original literature about the topic is scarce and different definitions for terms such as “allergy” and “sensitization” are observed among studies. In a systematic review of original articles in Latin America, without a limit of time or language, only 41 articles were related to original epidemiological investigations about FA, and most of them were from Mexico (58%).7 The methodology to evaluate “food allergy” and “food sensitization” was not defined in some of those studies, and other authors used the terms interchangeably. One study in Brazil assessed pediatricians’ knowledge about food allergy23: 895 pediatricians completed the questionnaire focused on questions about the clinical symptoms of patients and how to manage FA. In the diagnosis of FA, the study concludes that pediatricians have poor information, since most of them only do the diagnosis based on medical records (97%) and do not use confirmatory tests, performing aggressive management as extensive restriction diets are usually not needed and they do not avoid foods with cross-reactivity. These data shown that in most tropical countries there is little information about food allergy, and knowledge regarding the diagnosis of the disease is poor among physicians. Another similar study conducted by Cortez et al. was also conducted in Brazil, finding similar results.24 The most important limitations described are collected in Table 1.
Difficulties found for food sensitization and food allergy study interpretation in tropical regions.
| Difficulties evaluating food sensitization and food allergy |
|---|
| Different methodologies to evaluate the prevalence of food allergies. |
| Different definitions for terms as “allergy” and “sensitization” are observed among studies. |
| The methodology to evaluate “food allergy” and “food sensitization” was not defined in some of these studies, and other authors used the terms interchangeably. |
| Data show that in most tropical countries there is little information about food allergy. |
| Knowledge regarding the diagnosis of the disease is poor among physicians. |
| In the diagnosis of food allergy, most physicians do the diagnosis only based on medical records without confirmatory test. |
| There are few multi-center studies with regional food. |
The most common way to evaluate IgE sensitization to food is by measuring serum IgE specific or by skin prick test. Although both tests are frequently used, the specificity and sensitivity may vary between them. In a recently meta-analysis in Europe,25 the prevalence of sensitization to at least one food as assessed by specific IgE was 10.1% (95% CI: 9.4–10.8) and skin prick test 2.7% (95% CI: 2.4–3.0). Ling et al. in a retrospective analysis of medical records, observed that the agreement between SPT and sIgE results on adults is moderate (k=0.530–0.874), and it changes according to the tested food.26 These observations make it difficult to compare estimates when different diagnostic tests are used. Another difficulty when evaluating sensitization among food is that the main allergenic protein of many of them has not been identified so it cannot be adequately represented in the extracts used.20
These and other limitations make it difficult to compare results across studies, however in this review we present results considering these differences and evaluating the common points.
Sensitization studies in patients with suspect of allergic diseasesFood sensitization studies in populations with asthma, rhinitis, atopic dermatitis or conjunctivitis usually present higher frequency than in the general population (Fig. 1). Mexico has tropical and subtropical climates. Throughout its territory, several studies have been conducted to determine the prevalence of food sensitization. Avila et al.,27 in Mexico City, evaluated food sensitization between 1319 patients with suspicion of FA. They observed a food sensitization in 442 (31%) patients tested by skin test read by the method of Aas – an intradermal test evaluating the size of the wheal in relation to the percentage of the negative control; >25% will be considered positive. They also found that the age group with highest sensitization was children aged between 1 and 7 years (73%). The most frequent foods were fish (12%), milk (7.7%), and seafood (6.5%). It is important to note that in this study there was no sensitization to egg, and other high-consumed food in Mexican population such as tomato, onion or orange, which are frequent sources of allergens. Similar results were observed by Ortega et al.,28 with 356 patients with suspected allergic asthma, rhinitis or dermatitis. However, Ortega observed a concordance of less than 10% between the food suspect by the patient and food sensitization. The results found in Mexico29–32 show that, in general, sensitization to egg and peanuts is less than that reported in the U.S.A. and Europe, where these sources account for almost 80% of FA.5,6 It is also important to note that other sources infrequently described in European and U.S.A. populations, such as corn, beans and chili have a proportionally high prevalence in the Mexican population, perhaps because they are highly present in their diet. Nevertheless the method used for skin test interpretation (Aas method) has a high specificity but a very low sensitivity33 and it is difficult to compare frequencies in Mexico with trials in other countries because this methodology is infrequently used.9,34 Similar to these results, sensitization to egg and milk was less frequent than shrimp and fruits in other tropical countries from Latin America but in the subtropical area milk and egg were more frequent.35,36 something curious happens in the case of shrimp in that the frequency of sensitization in children sometimes exceeds their consumption. This sensitization can be by cross reactivity to panallergens such as tropomyosin present also in mites that are the main source of sensitization in the tropics.
Sensitization in general populationHill et al. found a similar food allergy prevalence in young children in Australia and several tropical countries in South Asia (4–9%) (Hong Kong, China, Taiwan, Indonesia, Philippines, Malaysia, Singapore, Japan, Thailand).37 The major difference between these populations was the culprit allergenic foods: prevalence rates of food hypersensitivity in Australian infants and children were 3.2% for egg, 2% for cow's milk, 1.9% for peanut, 1.2% for tree nuts and sesame and 0.1% for fish. In Asia, hypersensitivity to shellfish and fish (3%) was more common than for nuts (0.1%), peanut (0.8%) and milk (1.2%), As in the Mexico and Colombia studies presented before,27,35 shellfish sensitization was determined to be higher in children with allergic rhinitis.
Using skin prick tests, Levin et al., reported 5% rate of food sensitization in a cross-sectional study of 211 urban high school black children of Xhosa ethnicity in South Africa.38 The principal foods causing sensitization were egg (3.3%), peanut (1.9%) and milk (1.9%) but wheat, soy and fish have also been reported as common allergens in other studies in this zone.39 In Ghana, a study of food allergy in 1407 school children found 11% of children reporting AFC but only 50% of these children showed a positive SPT reaction, mostly directed against peanut and pineapple.40
Gadeirab et al.,41 in a study of 217 patients suffering from asthma, rhinitis and urticaria in Riyadh, Saudi Arabia found 17.5% to have specific IgE antibodies to various foods. The most common sensitivities were to peanut (23%), egg (15%) and cow's milk (13%). Similar results were found in other studies in the Middle East42 but in Israel, the prevalence of clinically relevant IgE-mediated food allergic reaction in 9070 infants and young children was 1.2%, with egg, cow's milk and sesame being the most common food allergens identified.43 Other studies in Colombia, Costa Rica and other Latin America cities show that fruits could be a higher source of allergens than milk or egg.44–47
Patients with atopic dermatitis in tropical and subtropical regions seems to have a higher frequency of sensitization to foods, but the involved food in some cases is different. Medina et al.,48 evaluated the frequency of food sensitization in 119 atopic dermatitis patients and they observed a sensitization by skin tests to egg (66%), milk (10%), corn (6%), chicken (8%), pork (4%), wheat (4%) and potatoes (4%). In Sao Paulo (Brasil), Tassi et al.,49 studied sensitization to food in 110 patients (1–66 years old) with atopic dermatitis. A total of 46 (42%) patients were sensitized to at least one food but only 26 (23.6%) associated exacerbation of symptoms after the consumption of one food. From the 26 patients with AFR, 11 (46%) were sensitized with the suspect food. Due to the high frequency of sensitization among patients with no history of AFR (41%), this study suggests that sensitivity (42%) and specificity (58%) of the skin test and serum specific IgE are low in patients with atopic dermatitis and provocation tests are necessary. Moreover, these and other similar results50 highlight the trouble for patients with atopic dermatitis to identify the foods involved in cutaneous exacerbations, in part due to the severity of the underlying disease and also because multiple ingredients are involved in the preparation of food products, thus hindering the correct identification of the causal food.
T cell sensitizationPatch test is a simple and secure method to confirm or rule out the suspicion of a cell-mediated reaction, especially in patients with atopic dermatitis where late reactions are common. However, there is an active debate about the utility of the patch test due to the high frequency of false positives and lack of universal standardization of the test. In tropical countries, studies evaluating FA cells mediated by patch tests are scarce and most of them have different methodologies. Levy et al.,51 in Brazil, observed a good correlation between skin test, specific IgE levels and patch testing in a group of 72 children with suspected FA between 2 and 12 years of age, who were divided between those who had asthma or rhinitis and those who had atopic dermatitis, and were compared with a control group. Among 32 patients with dermatitis, 40% had a positive patch test, 34% to milk, 37% to egg, 28% to soy and 28% to wheat. In the group of 26 patients with asthma and/or rhinitis 19% were positive to cow's milk, 11.5% to eggs, 15% to soy and 11.5% to wheat. They observed that readings at 72h had higher sensitivity and specificity. These results agree with those published in Europe which show that patients with dermatitis are at increased risk of cell-mediated reactions to food, however it is important to note that the high frequency of reactions in patients with asthma and rhinitis found in Brazil suggests a possible role of food sensitization in respiratory symptoms.
In a study in Mexico, Estrada et al.,52 compared sensitization with patch tests (milk, egg, wheat and soy) in 28 children under three years who had atopic dermatitis and 28 children without dermatitis and also, they performed an intradermal test with milk, egg, wheat and soy in all patients. Sensitization evaluated by patch test was higher in patients with dermatitis, especially to egg (43% vs. 18%) followed by milk (42% vs. 18%), soy (35.7% vs. 18%) and wheat (28% vs. 18%). Due to the high frequency of positive results in the control group, this test was not enough to predict the risk of food allergy in dermatitis, but they observed that the patch test together with the intradermal test were able to predict an increased food allergy risk in dermatitis (OR=4.2, 95% CI 1.3–13.4) which was not achieved with each of these tests independently.
Food allergic reactionsIt is known that self-report studies tend to over-estimated frequencies, and do not measure the frequency of food sensitization or FA.18 Nevertheless, it could be useful to study the frequency of AFR and the principal suspect foods. A study by Leung et al.,53 with 3677 pre-school children aged 2–7 years living in Hong Kong showed prevalence rates of parent-reported AFR and doctor diagnosed AFR to be 8.1% and 4.6%, respectively. The six leading causes of AFR were shellfish (15.8%), egg (9.1%), peanut (8.1%), beef (6.4%), cow's milk (5.7%), and tree nuts (5.0%). In Cartagena (Colombia), Marrugo et al.,54 evaluated AFR by self-report in a total of 3099 individuals with an age range of one to 83 years. The prevalence of self-reported AFR was 14.9%. Fruits and vegetables (41.8%), seafood (26.6%) and beef (20.8%) were the principal suspects. Interestingly, cow's milk and egg as suspects were reported in fewer than 10% of the cases. Subjects with suspected allergic diseases such as asthma, rhinitis or dermatitis most often reported AFR (62.9% vs. 29.6% in control group p<0.001), showing a possible link between these diseases and atopy but it may also be due to an incidental association by patients. In another study, self-reported food allergy in Maputo, Mozambique was 19.1%; with seafood (54.8%), meat (13.0%), fruits and vegetables (13.0%) being the most frequently reported.55 Other exotic foods such as the mopane worm, a high protein product consumed in some regions in Africa, have been reported to cause AFR but this has not been contrasted with sensitization studies or provocation tests.39,56
Nwaru et al.,25 reported in a meta-analysis that the overall pooled prevalence of self-reported FA in Europe was 17.3% (95% CI: 17.0–17.6); this was highest in Eastern Europe (>20%) and lowest in a tropical area like Southern Europe (<12%). High prevalence was also reported in other subtropical areas like Western and Northern Europe. However, even after stratification by age and region, there was a high heterogeneity between the studies.
The oral challenge test and the elimination diet are confirmatory tests for FA. However, because the challenge test is not recommended for severe cases (e.g. risk of anaphylaxis) and elimination diet without prior confirmation is difficult to maintain, both are in the last step of the studies for FA. Relatively few epidemiological studies have utilized oral food challenge (OFC) and less the gold standard of FA diagnosis, the double-blind, placebo-controlled food challenge (DBPCFC). Most of these studies are from Europe.4,57,58 The overall pooled prevalence of food challenge (OFC or DBPCFC) in Europe was 0.9% (95% CI: 0.8–1.1)25 and was similar among children and adults, but highest in Western Europe, and was higher in Northern Europe than in Southern Europe, suggesting that food allergy is less frequent in the tropical area of Europe. By food, the most common were 0% to 3% for milk, 0% to 1.7% for egg and 1% to 10.8% for any other food. In Canada, Ben-Shoshan et al.12 reported prevalence rates of 0.9%, 1.1%, 0.5%, 1.4% and 0.1% to peanut, tree nut, fish, shellfish and sesame. Similar results have been observed in Australia and Japan.37
The most frequent atopic diseases associated with FA is dermatitis, but the clinical impact of food sensitization is controversial. Navarro et al.50 compared 56 patients with atopic dermatitis and 53 controls to determine the relationship of atopic dermatitis, food allergy and gastrointestinal symptoms. Among the patients with dermatitis, 45 (80%) were sensitized to one food, with cow's milk being the most frequent (48%). Gastrointestinal symptoms were associated with sensitization (p<0.05). In this study, FA diagnosis was confirmed when patients’ symptoms improved after elimination diet.
Madrigal et al.,59 in Guadalajara (Mexico), evaluated the medical records of children under four years of age with a history of AFR and with a confirmation test (elimination diet and/or challenge test). Taking into account the characteristics of the episode, patients were classified according to the pretest probability that the reaction had been due to a FA. Only 22 of the 291 children assessed had a high suspicion of FA; with the provocative testing and/or the elimination diet only 11 (50%) of these 22 patients were positive, which represents 3.7% of all patients; five patients with milk, two with eggs, one carrot, one meats and two to preservatives in food. This study provides interesting facts: a good clinical history can rule out a large number of AFR but confirmatory tests are necessary because only 50% of patients with a high suspicion of FA were positive during provocative testing or elimination diet. These confirmatory tests do not provide information on the underlying mechanism of the reaction; so it should always be preceded by a skin prick test or serum IgE measurement.
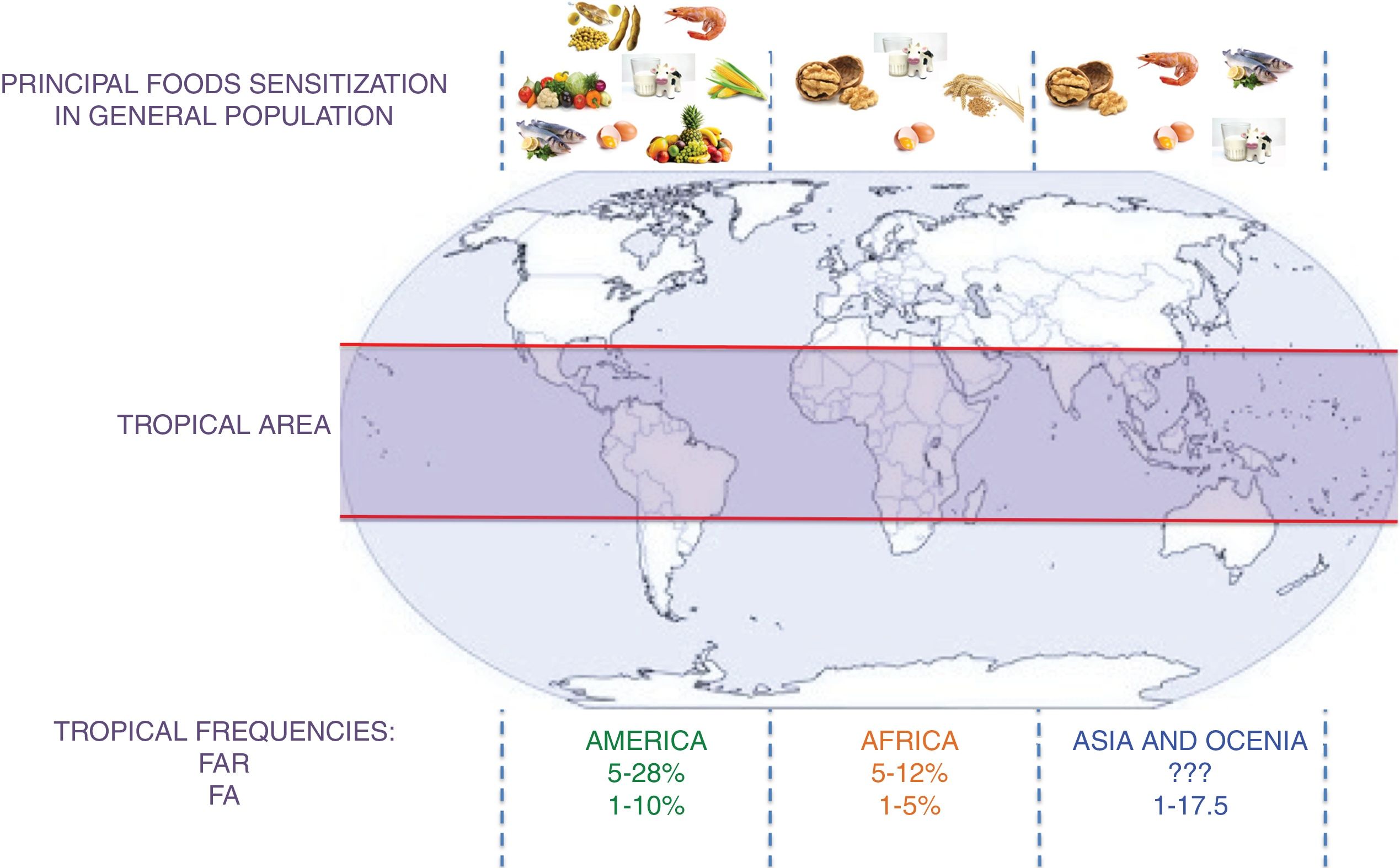
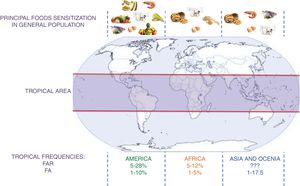
A schematic summary of the food sensitization, FAR, and FA in different tropical areas is presented in Fig. 2.
Principal foods in tropical areas by continent and frequency of FAR and FA. FAR: Food Adverse Reaction. FA: food allergy.?: no data. This figure summarizes the information contained in the articles cited in the section “IgE sensitization in the tropics” and “Food allergic reactions”.
Cohort studies with a prospective follow-up allow a better understanding of the characteristics of allergic diseases in the tropics and identify potential risk factors for food allergy and food sensitization.
Lopez et al., in a prospective study of 114 infants, assessed the clinical manifestations of allergy during the first year of life in Campinas Brazil.60 In this study during the first year of life only two patients were sensitized to cow's milk which means 0.01% of the total population. Due to the low frequency of food sensitization it was not possible to identify possible risk factors61 but in this study, breastfeeding for at least six months seems to be a protective factor for food allergy. This observation is supported by other birth cohorts conducted in Brazil (OR 0.09, 95% CI 0.01–0.51, p<0.01)62 and other studies in tropical regions.63–66 One study in the Federal District of Mexico by Lopez et al.67 assessed risk factors for allergies in a population of 4742 subjects between one and 98 years of whom 42% had allergic disease: 15% asthma, 20% rhinitis, 18% conjunctivitis, 19% atopic dermatitis and 4% urticaria. They did not find that the weaning period could be a protective or risk factor, but they observed that the family inheritance and early consumption of cow's milk, egg, fish, meat and legumes was associated with the onset of disease allergic. The results of this cohort suggest that rather than the weaning age, but the type of foods introduced early, would increase the risk of developing allergic disease. However, there may be a memory bias since these data were collected retrospectively. In short, the main risk factor for food allergy identified in birth cohorts in tropical areas were a family history of atopy and early weaning especially with cow's milk and egg. Because the risk and protective factors vary among populations, more birth cohort studies are needed.
ConclusionsGlobally, the existing studies about FA are difficult to compare due to variations in the definition of FA and differences in the food panel tested.18,68 The heterogeneity of the trials and the lack of uniformity in the foods tested within the same study, lead to significant errors when assigning the weight of each article.
According to the data obtained in this review, food allergy in the tropics seems to have points in common with studies in other regions but also distinctive features, especially with the type of food that causes sensitization. Studies with provocation tests are required to confirm the clinical relevance of these sensitizations in Latin America and Africa.38,39,44,45 Another urgent need in the study of food allergy in the tropics is to carry out multicenter studies using the same methodology and the same panel, including a large number of fruits, vegetables and other foods of each tropical region.
FundingWe declare that there was no external financing in the realization of this article.
Conflict of interestThe authors have no conflict of interest to declare
We thank the IPS university foundation and the University of Antioquia. This article was made as part of the interinstitutional call (IPS University / University of Antioquia 2015-2016).