Plant lipid transfer proteins (LTPs) are widespread plant food allergens, highly resistant to food processing and to the gastrointestinal environment, which have been described as the most common food allergens in the Mediterranean area. LTP allergy is widely described in adults, but it represents an emerging allergen also in the pediatric population. Little is known about the real prevalence and the clinical features of this allergy in children and it still often remains underdiagnosed in these patients.
An early identification and a deeper knowledge of this allergy in childhood can avoid severe systemic reactions and improve the child's quality of life. Pediatricians should always consider the possibility of LTP involvement in cases of plant-derived food allergy.
Lipid transfer proteins (LTPs) are a ubiquitous panallergen widely distributed through the plant Kingdom. They represent the main cause of food allergy in adults living in the Mediterranean Basin and are the main cause of primary food allergy in Italian adults.1,2
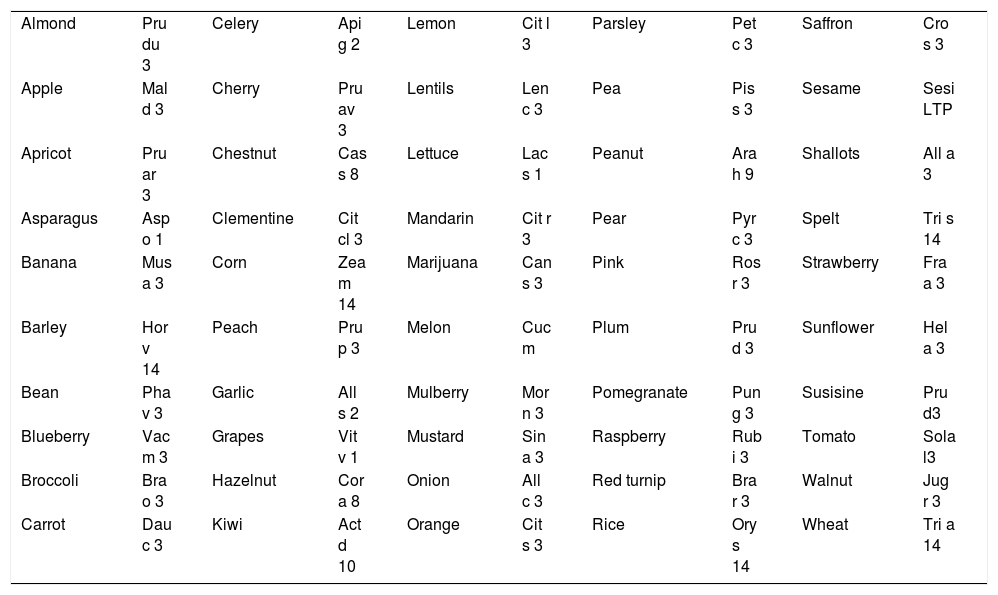
This protein is the major allergen in the Rosaceae family for patients not sensitized to birch pollen, but it has also been detected and characterized in a large number of other plant-derived foods, including dried fruits, rice, corn, grape, asparagus, beer, spelt, wheat, citrus fruits, lettuce, and cabbage (Table 1 shows the main food LTPs currently classified). Besides, relevant allergens from Parietaria, Olea and Artemisia pollen are also members of the LTP family.
Food LTPs currently classified.
| Almond | Pru du 3 | Celery | Api g 2 | Lemon | Cit l 3 | Parsley | Pet c 3 | Saffron | Cro s 3 |
| Apple | Mal d 3 | Cherry | Pru av 3 | Lentils | Len c 3 | Pea | Pis s 3 | Sesame | Sesi LTP |
| Apricot | Pru ar 3 | Chestnut | Cas s 8 | Lettuce | Lac s 1 | Peanut | Ara h 9 | Shallots | All a 3 |
| Asparagus | Asp o 1 | Clementine | Cit cl 3 | Mandarin | Cit r 3 | Pear | Pyr c 3 | Spelt | Tri s 14 |
| Banana | Mus a 3 | Corn | Zea m 14 | Marijuana | Can s 3 | Pink | Ros r 3 | Strawberry | Fra a 3 |
| Barley | Hor v 14 | Peach | Pru p 3 | Melon | Cuc m | Plum | Pru d 3 | Sunflower | Hel a 3 |
| Bean | Pha v 3 | Garlic | All s 2 | Mulberry | Mor n 3 | Pomegranate | Pun g 3 | Susisine | Pru d3 |
| Blueberry | Vac m 3 | Grapes | Vit v 1 | Mustard | Sin a 3 | Raspberry | Rub i 3 | Tomato | Sola l3 |
| Broccoli | Bra o 3 | Hazelnut | Cor a 8 | Onion | All c 3 | Red turnip | Bra r 3 | Walnut | Jug r 3 |
| Carrot | Dau c 3 | Kiwi | Act d 10 | Orange | Cit s 3 | Rice | Ory s 14 | Wheat | Tri a 14 |
The wide range of involved foods may be explained by their cross-reactivity between the LTPs present in botanically related and unrelated foods due to their molecular structure similarity. In contrast, LTP pollen allergens of Parietaria (Par j 1 and Par j 2) and olive (Ole e 7) do not cross-react with allergenic LTPs from plant foods nor between themselves.3
LTPs are a family of proteins stable to the heat, gastric digestion and food preservation methods.4–6 For these reasons, they may determine reactions after oral ingestion of fresh or processed food. The sensitization is induced by the oral route and in the LTP allergic patients the clinical manifestations can range from mild symptoms to severe anaphylaxis.
Currently, the LTP allergy is well described in adults, but less is known about the epidemiology and clinical features of this disease in pediatric patients. In this review article, we tried to summarize the current knowledge about this allergy in children population.
The molecular featuresThe LTPs are small and soluble proteins characterized by six to eight cysteine residues; on the basis of their molecular weight, it is possible to classify them into two classes: nsLTP1 (9kDa) and nsLTP2 (7kDa). Almost all LTPs belong to the nsLTP1 family (www.allergen.org).
Lipid transfer proteins are constituted by a stable and compact secondary structure consisting of a hydrophobic cavity able to accommodate ligands surrounded by four α-helices stabilized by highly conserved disulfide bridges. This structure gives them a high resistance to gastrointestinal proteolysis, to pH and high temperatures. For these reasons, sensitization to LTPs is associated with a high risk of systemic reactions, even severe (angioedema, anaphylaxis), following the intake of foods that are contained, either in the form of raw foods, cooked or preserved ones.
LTPs were originally identified for their ability to catalyze intracellular lipidic exchanges, but it has also been recently proposed that they may have an important defensive role against fungal and bacterial pathogens of plants.7
In view of their defensive function, LTPs are essentially localized in the pericarp of the fruits and they could directly trigger contact reactions in sensitized people.8 Indeed, the presence of LTPs in the peel is seven times higher than in the pulp.8,9 Borges et al.10 have demonstrated with immunolocalization that LTPs are primarily located in the cytosol but are subsequently excreted and finally accumulate at the plasmalemma–cell wall interface and in the cell wall. They therefore recommended a consumption of peeled-off fruits to reduce the risk of severe allergic reactions (anaphylactic shock) in individuals sensitized to Rosaceae fruits.
Although LTPs belong to the protamine group, they behave like pathogenesis-related proteins that could be stimulated by biotic and abiotic plants stress; for these reasons they are counted as PR-14.11
Clinical issuesThe manifestation and severity of LTP hypersensitivity are extremely variable. Many patients are sensitized although completely asymptomatic, others may show exclusively local reactions, such as contact urticaria or oral allergic syndrome (SOA), whilst others may present more important symptoms such as vomiting, abdominal pains, urticaria-angioedema, asthma and systemic reactions up to anaphylactic shock.
Anaphylaxis is defined as a serious, generalized or systemic allergic reaction that is unpredictable, rapid in onset and may cause death.
Patients and their families therefore need to receive good education on how to manage potential anaphylactic reactions with training in the use of adrenaline auto-injectors and personalized emergency management plans (see below).
Moreover, LTP-related symptoms can be variably associated with each other and may show a severity increasing for subsequent episodes. In most cases, systemic symptoms are preceded by SOA (itching and tingling of the lips, oral mucosa, tongue and pharynx) and it is probably the most frequent first clinical expression of LTP hypersensitivity.12
Furthermore, the sensitization to LTP could be included in the context of food-pollen syndrome triggered by cross-reactions between pollen and homologous plant allergens. So-called LTP syndrome can have three clinical patterns:
- -
primary LTP sensitization to a food without a concomitant pollen allergy (severe allergy with low total IgE);
- -
primary sensitization to a food allergen with a background of pollen allergy;
- -
primary allergic sensitization to pollen.
In all three cases, the patient may suffer from food allergies (either in primary form or as a result of cross-sensitization), but the symptoms may have different clinical severities, also depending on the avidity of the IgE antibodies involved.13
Indeed Pru p 3, the main primary sensitizer in the Mediterranean Basin, has been implicated in cross-reactivities, especially those involving other fruits, nuts and pollens such as those of mugwort and plane.14,15 On the other hand, Art v 3 can act as a primary sensitizer promoting secondary sensitization to peach and peanut, as demonstrated in the Chinese population.16,17
Moreover, it has also been shown that in patients sensitized to Pru p 3, if they are also sensitized to Art v 3, the number of plant food allergies is higher, which indicates that further awareness of Art v 3 may have extended the repertoire of the LTP epitope.18
The co-sensitization to artemisia and plane is correlated with respiratory symptoms, while the simultaneous sensitization to parietaria (Par j 2) is associated with less severe symptoms.19 Particularly Pla a 3 is associated with local and systemic food-induced reactions, but with lower past respiratory symptoms occurrence. Pla a 2 reactivity is correlated with respiratory symptoms but is inversely related to systemic reactions to food.20 The dosage of IgE vs. Pla a 3 and Art v 3 could be employed in clinical practice as a marker to identify allergic patients potentially at risk of LTP-mediated food reactions.21
Lipid transfer protein represents the main cause of food-induced anaphylaxis in Italian adults, although the proportion between anaphylactic episodes and number of sensitized patients is by far lower than that observed for other frequent allergens.2 There is still a lack of data referring to the pediatric population.
Serious systemic reactions are more likely in subjects sensitized to more than five nsLTP,19 however, simultaneous sensitization to other panallergens (profilins, PR10) represents a protective factor which is associated with a less severe clinical reactivity.22
The presence of co-factors as NSAIDs, alcohol and physical exercise is often necessary for eliciting the clinical expression of this allergy. Reactions in a lot of patients are triggered only in the presence of co-factors23 and these conditions are considered a risk factor for more severe systemic reactions. The mechanism by which co-factors act is not yet completely clear, however they seem to increase the permeability of the gastro-intestinal tract, so a greater concentration of allergens comes into contact with the gut mucosa.24
In Italian subjects, LTP allergy is the most frequently associated with food dependent exercise-induced anaphylaxis (FDEIA).25 Additionally, Pastorello et al. demonstrated that wheat LTP (Tri a 14) can play an important allergenic role in wheat-dependent exercise-induced anaphylaxis.26
Moreover, LTP of Cannabis sativa (Can s 3) represents an emergent allergen implicated in anaphylactic reactions widespread mostly among adolescent patients27 Cannabis-related symptoms could be elicited by smoke, touch, ingestion or pollen inhalation.28 For this diagnosis, Decuyper II et al.28 advise to start with a validated and standardized crude-extract based test, such as sIgE Can s 3 quantifications, where available. Future research should evaluate the true prevalence of cannabis allergy and underline the importance of other cannabis allergens in clinical practice.
DiagnosisAs with any food, diagnosis of LTP allergy is based on clinical history, supported by the skin prick test with the extracts or the fresh foods (prick-by-prick) and the demonstration of specific IgE for the culprit foods and confirmed by a positive oral challenge test for them. Regarding the fresh food prick-by-prick test, it may be useful in the case of LTP allergy to address our suspicions toward one group of panallergens rather than another. Given the high temperature resistance of LTPs and their high concentration in fruit peel, SPT performed with cooked foods and not peeled ones prove to be particularly helpful for the diagnosis simulating an in vivo molecular investigation.29
The direct diagnosis of LTP sensitization by skin tests is complicated by the limited presence on the market of purified and standardized extracts containing the LTPs. Only two extracts are currently available in Italy: the peach Pru p3 and the Mal d3 of the apple (Alk-Abellò).
In recent years, component resolved diagnosis (CRD) has helped us to make LTP sensitization diagnosis, determining the specific IgE both with the single molecule dosage (Immuno-CAP) as well as with searching the different components simultaneously using the proteomic microarray (ISAC test) or the multiplex test (FABER test). Nowadays, we can evaluate the following LTPs with these methods: Pru p3, Mal d3, Ara h9, Cor a8, Jug r3, Tri a14, Tri a7k-LTP, Zea m4, Act d10, Pun g1, Sol l6.
Moreover, the performance of basophil activation test (BAT) in the diagnosis of panallergen-induced food allergies could be an important diagnosis tool in vitro.
Therapeutic managementElimination diet to trigger foods is today the only possible treatment for LTP-allergic patients. These patients often present a poly-sensitization to many vegetables and fruits and so they have a higher risk of increasing the spectrum of foods that can cause an allergic reaction; which is why this kind of diet is often difficult to be managed and can determine an unbalanced metabolism because of severe food restriction.
Asero et al.30 proposed a pragmatic approach based on immunological knowledge. The authors, in fact, suggested to the LTP-sensitized patients to continue eating the tolerated foods at least until evident symptoms appears. This therapeutic approach should aim at improving the patient's knowledge in distinguishing between tolerance and symptoms. So every patient should be educated on the basis of their personal immunological and allergic condition to recognize it.
Indeed, as suggested by Asero et al., to continue eating the tolerated food could determine a physiological “natural, attenuated oral immunotherapy”; while, avoiding so far tolerated food could cause an allergic reaction because of impaired immunological tolerance as a consequence of failed allergen exposure.
On the other hand in the case of polyallergic patients who have experienced moderate-severe multiple food reactions, a too-limited elimination diet could lead to nutritional deficits, an unbalanced metabolism and a delay of growth in the case of children; so their life style could compromise quality of life of the patients and/or their caregivers.
Similar to the other food allergy, the only method to solve these problems is immunotherapy. In the literature, few studies about immunotherapy in LTP-allergic patients have been carried out.
Fernandez- Rivas et al.31 attempted the first clinical trial involving 74 patients suffering from peach allergy that underwent a sublingual immunotherapy for six months. That study showed how a LTP-desensitization treatment could be a promising therapeutic option for this kind of patients.
Pereira C. et al.32 also confirmed the efficacy and the safety of immunotherapy to LTP publishing a case report concerning a woman with positive LTP-allergological evaluation who performed desensitization for one year.
Garrido-Fernández et al.33 performed a randomized double-blind, placebo-controlled trial of sublingual immunotherapy with a LTP-quantified peach extract, evaluating the clinical efficacy by double-blind placebo-controlled food challenge (DBPCFC) with peach and the immunological changes by BAT and the assay of specific Pru p 3 IgE and IgG4 after six months. That report demonstrated a clear increase of clinical tolerance and positive immunological modifications.
Likewise, Gomez et al.34 noticed that after one year, Pru p 3 SLIT induced both desensitization and immunological changes not only for peach but also for other food allergens relevant in the induction of severe reactions such as peanut.
Currently, these attempts should still be considered experimental treatment strategies to reduce the food allergic status and cannot be practiced on a routine basis in the clinic.
The pharmacological management of LTP adverse reactions includes adrenalin, antihistamines and steroids drugs. In the case of history of anaphylactic reactions in LTP allergic patients, adrenaline must be prescribed, while the role of the prescription of this drug is still debated in the literature for less important symptoms such as angioedema, urticarial, rhinitis etc.
LTP allergy/sensitization in the pediatric populationFood allergy affects 5% of children. Despite milk and egg proteins representing the main allergens involved in childhood reactions, fruits and vegetables are emergent allergens in children over five years of age.35 Given the possibility that LTP may cause serious systemic reactions it is important to focus on the young subjects suffering from this allergy.
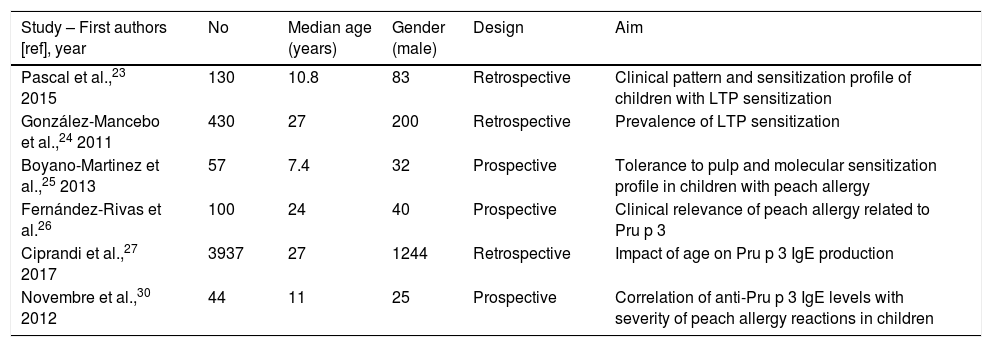
In the literature, the clinical and epidemiological aspects are poorly studied in the pediatric population (Table 2 summarizes the aims of studies regarding LTP sensitization in populations including pediatric patients).
Aims of studies regarding LTP sensitization in populations including pediatric patients.
| Study – First authors [ref], year | No | Median age (years) | Gender (male) | Design | Aim |
|---|---|---|---|---|---|
| Pascal et al.,23 2015 | 130 | 10.8 | 83 | Retrospective | Clinical pattern and sensitization profile of children with LTP sensitization |
| González-Mancebo et al.,24 2011 | 430 | 27 | 200 | Retrospective | Prevalence of LTP sensitization |
| Boyano-Martinez et al.,25 2013 | 57 | 7.4 | 32 | Prospective | Tolerance to pulp and molecular sensitization profile in children with peach allergy |
| Fernández-Rivas et al.26 | 100 | 24 | 40 | Prospective | Clinical relevance of peach allergy related to Pru p 3 |
| Ciprandi et al.,27 2017 | 3937 | 27 | 1244 | Retrospective | Impact of age on Pru p 3 IgE production |
| Novembre et al.,30 2012 | 44 | 11 | 25 | Prospective | Correlation of anti-Pru p 3 IgE levels with severity of peach allergy reactions in children |
The main difficulty to study it is that in most of the articles the adverse reactions to fruits and vegetables could be attributed to LTP or other panallergens (storage proteins, profilins, PR 10 proteins, etc.) because the patients may not be monosensitized.
In the most cited article36 about LTP sensitization in children with a clinical history of plant-food reactions, the prevalence of LTP-proteins sensitization (only LTPs and not any other plant-food panallergens) was 26.2% (34/130 pts). In this subgroup, they demonstrated a prevalence of 83.1% for peach (rPru p 3), 77.7% for walnut (nJug r 3), 56.2% for peanut (rAra h 9), 55.4% for hazelnut (rCor a 8) and 26.2% for wheat (rTri a 14) detected by microarray, while 96.9% for rPru p 3 by ImmunoCAP.
In these 130 young patients, sensitization to a particular plant-food LTP did not always cause clinical symptoms and indeed allergy. In fact, 69% and 63% of peach and walnut tolerant subjects had positive rPru p 3 and nJug r 3 IgE, respectively. Similarly, 39.1% of hazelnut tolerant individuals had positive rCor a 8, whereas for peanut 36.8% Ara h 9 and for wheat 26.2% rTri a 14. These findings concur with a previous observation of frequent asymptomatic Pru p 3 sensitization in an older Spanish cohort, in which only 9% of patients had food allergy (median age: 27 years, range: 15–41).37
With regard to the prevalence of LTP allergy in pediatric age, Boyano-Martinez et al.38 studied 57 children with a previous history suggestive of peach immediate hypersensitivity reactions who underwent oral food challenge (OFC) with peach pulp. They observed that almost all children had serum sIgE to rPru p 3, 6 to rPru p 1 and 4 to rPru p 4. These data confirm that in populations from Southern Europe, the major peach allergen is rPru p 3. In this case the proportion seems to be higher than in another study,39 probably because there are few pollinic patients, in whom sensitization to other panallergens is more common.
We know that age may be a critical factor involved in the progression from sensitization to allergy. In this regard, Ciprandi et al.40 recently tested the differences of Pru p 3 (peach LTP) sensitization across Italy mainly concerning the impact of age. They reported that the sensitization percentages significantly diminished from childhood to aging and the serum IgE levels progressively increased from childhood to young adulthood with a peak between 21 and 30.9 years and then decreased until aging. Moreover, there was no difference among centers. Unfortunately, as suggested from the authors, this experience had the limitation of clinical data that was lacking, and the sizes of the single age classes were inconsistent.
Despite these limitations, that study confirmed the data reported by the previous research conducted by Tosca et al. where the peak of sensitization for Pru p 3 was reached at school age.41
Pastorello et al.42 investigated a possible correlation between specific IgE levels to Pru p 3 and the age at onset of peach allergy. They demonstrated a significant inverse correlation between the age at onset of peach allergy and anti-rPru p 3 IgE levels at diagnosis: when peach allergy starts at a younger age, it is likely associated with Pru p 3 sensitization, and the younger the onset, the higher the IgE title.
Therefore, age plays a relevant impact on the sensitization pattern as well as on the serum levels. These findings underline the relevance of considering age when IgE are interpreted and the practical importance of adequately paying attention to this issue in real life.
Regarding the severity of symptoms, in Italian children with peach allergy, the presence of specific IgE to Pru p 3 is not associated with systemic reactions and the levels of specific IgE to Pru p 3 do not seem to correlate with their severity.43 These results do not confirm the previous observations in an adult population,44,45 which reported that peach allergic patients with systemic symptoms had significantly higher levels of anti-rPru p 3 specific IgE than patients with OAS. This discrepancy could be explained as suggested from the authors because few patients were monosensitized in this investigation.
It is unclear why some patients with low levels of specific IgE to fruit develop systemic symptoms, whereas others with high levels do not, despite similar exposure to the allergen. Perhaps other factors should be considered; for instance in pollen-related food allergies, high food-allergen specific IgG4/IgE ratios seem to be associated with food tolerance, potentially because specific IgG4 blocks IgE binding to food allergens.46
In the article by Pascal et al.36 the authors proposed to describe the clinical pattern and sensitization profile of children with plant-food allergy and LTP sensitization from the Northeast of Spain. The results show that symptom severity was highly variable and a wide spectrum of plant-foods were involved in reactions.
Additionally, Pascal et al. observed that subjects sensitized to pollen LTPs had sIgE for a broader spectrum of plant-food LTPs in agreement with the literature data of the adults.2–47 Indeed, the main plant-foods involved in reactions were peach, nut, peanut, apple and walnut in the LTP mono-sensitized patients.
However, in contrast to their previous study in adults from the same geographical area, co-sensitization to other plant-food panallergens, such as storage proteins, was very common in the studied children. In fact, in addition to LTPs, 65% of cases were sensitized to storage proteins, which was associated with experiencing anaphylaxis and nut allergy. On this basis, it may be important to understand whether the LTP sensitization is relevant in these patients or whether this likely silent LTP sensitization might become clinically relevant over time and/or in the presence of co-factors. At present, all these questions require further prospective longitudinal studies.
Moreover, also regarding the reaction's severity in this study, specific IgE levels to LTPs did not correlate with the reaction's severity on history, as previously described. Additionally, sensitization to storage proteins was associated with anaphylaxis, suggesting that these allergens might involve higher potential for severe reactions than LTPs, in agreement with other articles.48
In disagreement with the literature data of LTP allergic adults, in this study the authors identified the involvement of a co-factor (exercise) in the genesis of the reactions only in three young patients (with a total of five reactions). This result could be explained on the basis that the LTP patients amount to only 26.2% of the studied sample, and maybe the parents’ difficulties to identify them. Moreover, regarding the co-factor involvement, Mota et al.49 reported three young cases of food-dependent exercise-induced anaphylaxis (FDEIA) (respectively 11, 16, and 18 years old) in a study on 43 cases of anaphylaxis induced by LTP.
In LTP allergy, according to the study by Pascal et al.,23 a heterogeneous model of symptoms was observed: OAS (34/45, 75.6%), urticaria (30/45, 66.7%), contact urticaria (5/45, 11.1%), gastrointestinal disorders (25/45, 55.6%) and anaphylaxis (34/45, 75.6%). Thirty-four (75.6%) subjects were diagnosed with pollinosis, while all had rhinitis, in 14 cases (31.1%) associated with asthma IgEs determination by ImmunoCAP ISAC 103 allergens. Since patients with food allergies do not often spontaneously report abdominal disorders with food intake, this observation highlights the need to carefully control the digestive symptoms in these patients also because they may precede the appearance of angioedema or urticaria that usually occur in older children and adolescents, especially if cofactors are involved.
Interestingly, a high prevalence of concomitant atopic dermatitis is found in pediatric patients with asymptomatic sensitization to LTP,36 this could suggest an easier and earlier sensitization to LTP through an impaired skin barrier, as reported for other food allergens.50
Finally, we cite again the previously described work of Boyano-Martinez et al.,38 where the authors proposed to ascertain the frequency of tolerance to peach pulp in children with a previous history of allergic reactions after ingestion or contact with the fruit. In more than 90% of the patients, tolerance to the pulp was confirmed in children (mean 7.4 years, range 2–7 years).
In another article51 the authors performed an OFC with peach peel and pulp separately and found that 68% of the studied population exclusively presented symptoms upon ingestion of the pulp. This discrepancy may be linked to the fact that the latter study included both adults and children. Therefore, the higher age of the latter population (mean age 20±8 years) may have determined a higher sensitization to pollen and thus, a higher prevalence of sensitization to panallergens such as profilins or PR-10 present in the pulp.
ConclusionsIn conclusion, this review of the literature shows that allergy to lipid transfer protein probably represents the most difficult type of food allergy, in terms of preventive strategies. In fact, the widespread diffusion of the protein, along with its variable degree of cross-reactivity from one patient to another, makes it virtually impossible to predict which foods the patients will react to, and what the clinical expression of adverse events will be.
All these aspects affect the quality of life of patients and, in the case of children, of parents and caretakers because they are prompted to avoid foods usually consumed in their environment. They must also be aware of possible accidental-allergic reactions, which in some cases may be life threatening. For these reasons, it is important that dietary avoidance is well grounded on the basis of clinical allergy and not only on sensitization, which can be asymptomatic.
Finally, based on this, we believe that LTP sensitization and allergy should be correctly diagnosed and managed on an individual basis both in adult and overall in pediatric patients to improve their wellness and quality of life.
Current evidence in the field of molecular-based diagnosis in plant-food allergy could help the clinician in routine decision-making in terms of individual risk assessment or discrimination between allergy and tolerance.
Moreover, some variables should be carefully addressed, including age, area of residence, co-sensitization, co-morbidity, and sports practice after eating.
An early diagnosis of this allergy in childhood or adolescence is useful for the clinician in providing adequate dietary advice and provides the basis for future therapeutic approaches so as to avoid severe reactions from occurring.
Ethics approval and consent to participateNot applicable. This article does not contain any studies with human participants or animals performed by any of the authors.
Availability of data and materialData sharing not applicable to this article as no datasets were generated or analyzed during the current study.
FundingThere is no funding source.
Conflict of interestThe authors declare that they have no conflict of interest.
The authors are responsible for the content and the writing of this paper.