Allergic diseases have been a global problem over the past few decades. The effect of allergic diseases on healthcare systems and society is generally remarkable and is considered as one of the most common causes of chronic and hospitalized disease. The functional ability of probiotics to modulate the innate/acquired immune system leads to the initiation of mucosal/systemic immune responses. Gut microbiota plays a beneficial role in food digestion, development of the immune system, control/growth of the intestinal epithelial cells and their differentiation. Prescribing probiotics causes a significant change in the intestinal microflora and modulates cytokine secretion, including networks of genes, TLRs, signaling molecules and increased intestinal IgA responses. The modulation of the Th1/Th2 balance is done by probiotics, which suppress Th2 responses with shifts to Th1 and thereby prevent allergies. In general, probiotics are associated with a decrease in inflammation by increasing butyrate production and induction of tolerance with an increase in the ratio of cytokines such as IL-4, IL-10/IFN-γ, Treg/TGF-β, reducing serum eosinophil levels and the expression of metalloproteinase-9 which contribute to the improvement of the allergic disease’s symptoms. Finally, it can be said that the therapeutic approach to immunotherapy and the reduction of the risk of side effects in the treatment of allergic diseases is the first priority of treatment and the final approach that completes the first priority in maintaining the condition and sustainability of the tolerance along with the recovery of the individual
Allergic diseases have been a global problem over the past few decades.1 The prevalence of eczema (atopic dermatitis), food allergies and asthma have increased dramatically during this period, especially in Western societies. It is believed that between 20–30% of people living in Western countries are suffering from at least one type of allergic disease. The effect of allergic diseases on health care systems and society is generally remarkable and is one of the most common causes of chronic and hospitalized illness.2,3 Allergic diseases are described with an inadequate immune response of T helper (Th2) cell lymphocytes to environmental or food antigens. Activating this response results in the secretion of interleukins (IL)-4, IL-5, IL-13, and the production of IgE specifically for allergens. The induction of Th2 cytokine responses also inhibits Th1 activity primarily via interferon (IFN)-γ, which helps maintain an allergic phenotype. The stability of the Th1/Th2 balance is governed by the expression of the transcription factors GATA-3 (Th2) and T-bet (Th1).4,5
Several studies have shown that in patients with allergies, the number and function of regulatory T cells (Tregs) is associated with a decrease in the immune response, while the mutation in the FoxP3 Treg transcription factor leads to severe immunosuppression. The mechanisms that cause allergic diseases in early life are not yet fully understood.6 One of the well-known and very prominent ideas is the impact of intestinal microbiota, where the composition and structure of commensal bacteria are associated with the developing immune system. Such interactions can affect the maturity of the immune system, which potentially results in Th2-type allergic responses. Similarly, preventive or therapeutic strategies targeting intestinal microbiota are the subject of increasing scientific research.7 In this review, the beneficial effects of probiotics on the prevention and treatment of some allergic diseases are critically discussed.
The prevalence of allergic diseases and asthma are increasing worldwide, particularly in low and middle-income countries. Allergic diseases are common in children and adolescents and result in high costs to the health care system, as well as having an adverse impact on the quality of life.8 Complexity and severity of allergic diseases include life-threatening anaphylaxis, food allergies, certain forms of asthma, rhinitis, conjunctivitis, angioedema, urticaria, eczema, eosinophilic disorders, include eosinophilic esophagitis, and drug and insect allergies continue to escalate particularly in children and young adults, who are bearing the highest burden of these trends.8,9 Globally, 300 million people suffer from asthma and about 200–250 million from food allergies.9 Moreover, one-tenth of the population are affected by drug allergies and 400 million from rhinitis.10 In the United States, the prevalence of asthma is approximately 7%. Allergic rhinitis (AR) is more prevalent than asthma, ranging from 10% to 20% (500 million) worldwide and in the United States ranging from 10% to 40%.11
The prevalence of atopic disease has reportedly been on the rise in the United States and around the world, although rates may have plateaued recently. The International Study of Asthma and Allergies in Childhood reported that the lifetime prevalence rates of atopic disease stabilized at 20%.12 This plateau is a mixture of a substantial increase in the prevalence of the atopic disease in Mexico, Chile, Kenya, Algeria, and Southeast Asia, with decreases in New Zealand and the United Kingdom, which previously had high rates.13
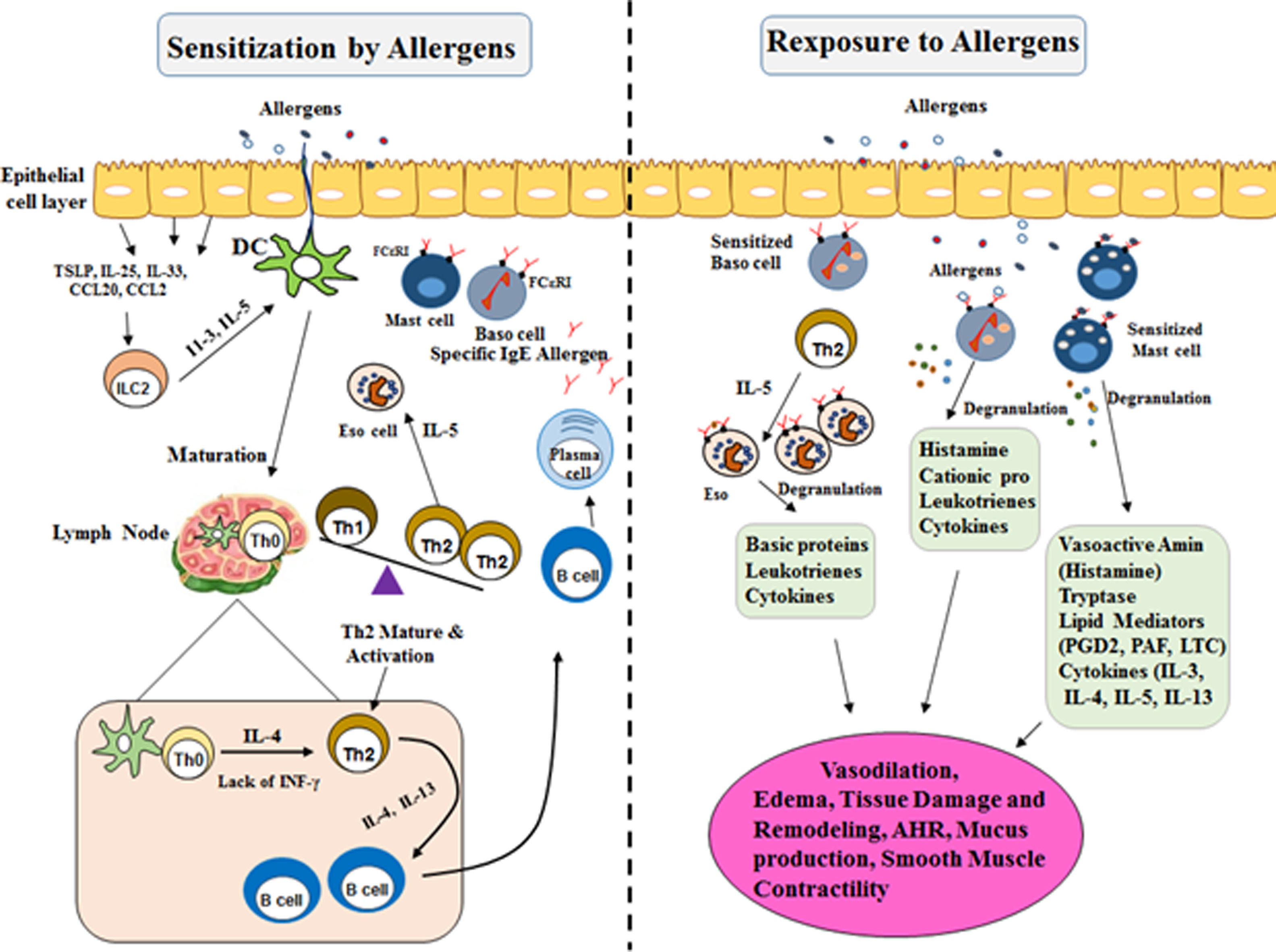
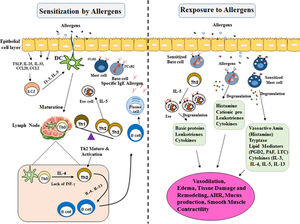
The increase of urbanization in the developing world may contribute to the higher occurrence of aeroallergic disease in an urban area as compared with rural locations. The hygiene hypothesis by industrialization/urbanization led to reduced microbial exposure and infectious agents (biasing the immune system away from a Th1 immune-mediated response toward to Th2, in early life in contrast to rural societies. In addition, another risk factor of the aeroallergic disease includes prolonged and high exposure to dust mite populations, cockroach, or rodent allergens, lifestyle and obesity, elevated concentrations of air pollution14,15 (Fig. 1).
Mechanisms involved in allergic reactions. Sensitization to a specific antigen is a prerequisite for the onset of allergic diseases. Differentiation and expansion to Th2 cell subtypes lead to the production of inflammatory cytokines (IL-4, IL-5, and IL-13). They drive immunoglobulin E (IgE) class-switch in B cells and the recruitment and activation of pro-inflammatory cells in mucosal target organs. Allergic reactions are triggered when allergens cross-link preformed IgE bound to the high-affinity receptor FcεRI on Mast cells and Basophil cells. In the early phase of allergic reactions they alert the local immune cells and induce inflammatory reactions by secreting chemical mediators, leukotrienes, and cytokines. Mast cell and Basophil cell degranulation followed by a more constant inflammation, recognized as the late-phase response. This late response includes the employment of other effector cells, particularly Th2 lymphocytes, eosinophils, and basophils, which contribute significantly to the immunopathology of an allergic response. These activations contribute to the development of the inflammation and the symptoms of allergic disease.
TLR: Toll-like Receptor; TNF-α: Tumor Necrosis Factor A; IFN-γ: Interferon γ; TGF-β: Transforming Growth Factor B; IL-10: Interleukin 10; IL-3: Interleukin 3; IL-4: Interleukin 4; IL-5: Interleukin 5; IL-13: Interleukin 13; IL-23: Interleukin 23; IL-25: Interleukin 25; Th0: T Cell Naive; Th2: Type 2 Helper T Cell; Th1: Type 1 Helper T Cell; IgE: Immunoglobulin E; DC: Dendritic Cells.
In the allergic response, the immune system must identify the allergens and induce a dynamic immune response. Sensitization to a specific antigen is an essential part of allergic immune responses mediated by naive T and B lymphocytes. Primary, recognized and processed allergens presented by antigen-presenting cells (APC) to naive T cells (Th0).16 Th2 cell differentiation is linked to the local cytokine environment provided by interactions among the epithelium, resident dendritic cells (DCs), and regional lymph nodes. The crucial cytokines responsible for the allergic response include IL- 4, IL-5, and IL-13, as well as innate lymphoid (ILC-2) cells which may intensify and preserve local Th2-driven allergic inflammation. These interleukins switched immunoglobulin production by heavy chain gene rearrangement to class IgE in B cells17 (Fig. 1).
Allergy treatment and allergen immunotherapyThe primary and best way to prevent allergy symptoms is to avoid allergens as much as possible. This includes removing the source of allergens from your home and other places where you spend time. There are many harmless prescription medicines to relieve allergy symptoms. Non-pharmacological treatment of these patients may cause allergy and manifestation of painful problems such as sinus or ear infections. Allergy medications include nasal corticosteroids, antihistamines, mast cell stabilizers, corticosteroid, epinephrine.18,19
The third treatment is immunotherapy as an appropriate therapeutic strategy chosen for some allergy patients. The most common types of immunotherapy are allergy shots and sublingual immunotherapy (SLIT).20 Allergy shots involve giving injections of allergens in an increasing dose over time. The person becomes progressively less sensitive to that allergen. Allergy shots can work well for some people with allergies to pollen, pets, dust, bees or other stinging insects, as well as for asthma. Allergy shots do not usually work well for allergies to food, medicines, feathers, or for hives or eczema. SLIT is another way to treat certain allergies without injections.21 Allergists give patients small doses of an allergen under the tongue. This exposure improves tolerance to the substance and reduces symptoms. SLIT is fairly safe and effective for the treatment of nasal allergies and asthma. SLIT tablets are currently available for dust mites, grass, and ragweed. While allergy shots are fairly safe, there is a chance of a severe, life-threatening allergic reaction to the injections, so they must always be given in an allergist’s office under observation from a medical professional.22
Merits and demerits of immunotherapy and new strategies for treating allergic diseasesWhen oral drugs and allergies are not able to control allergic reactions, allergy shots known as immunotherapy treatment may be the solution to this problem. There are many benefits to patients who receive an allergy shot, including some people who do not tolerate oral medication. While the cost of allergy shots is higher than oral medications, ultimately, the longer the cost of this treatment is less than the anti-allergic drug and should be spent by these patients who do not choose this treatment.23 Oral allergy medications treat allergic symptoms, but allergy shots treat the cause of allergic reactions. When immunotherapy is successful, patients are recovered, because shots instead of symptoms cure the underlying causes of allergies, but like other types of medical treatments, the desensitization of allergy also has disadvantages. Some of the common disadvantages of this type of allergy include: Immunotherapy does not affect all types of allergy. Shots do not have a good effect on common allergies such as pet, pollen, dust. Shots are not effective in treating allergies to food and hives. In addition, allergy to insect bites or spider bites cannot be prevented by allergic shots. In some patients, the reactions are reddening, swelling and hypersensitivity of the place of the injection. Losing these opportunities can delay or eliminate the treatment of allergies. Allergy shots will make allergic symptoms worse when the infusion regimen starts. Since allergic shots rarely cause anaphylaxis, patients should wait 30 min at the treatment faculty to reduce the risk of anaphylaxis.24
One of the newest therapeutic approaches is allergenic changes that include the use of recombinant proteins, the combination of antigens with infectious carrier proteins, peptide immunotherapy and genetic vaccines containing code for allergic proteins, these methods include are oral and fecal immunotherapy, intralymphatic immunotherapy, and epicutaneous immunotherapy.25,26 The first priority in the treatment of allergic diseases is the therapeutic approach to immunotherapy and the reduction of the risk of side effects, and in the second stage, the maintenance of the tolerance and recovery of the individual.27
The effect of microbiota and development of the immune system on allergic diseasesReducing exposure to microorganisms in early life is one of the main mechanisms for increasing the prevalence of allergic diseases over the past few decades. This is typically referred to as the "Health Hypothesis", which was originally used by Strachan (1989). Today, reducing exposure to microorganisms (increasing allergic conditions) has been pointed out to factors such as diet, antibiotic use, vaccination, households, and health improvements.28 The digestive microbiota provide a significant amount of microbial contamination for infant growth. There are more than 1015 microorganisms which contain 1000 different species that are colonized in the gastrointestinal tract. Under normal conditions, these bacteria play a beneficial role in food digestion, development, and growth of the immune system, control, and growth of the intestinal epithelial cells and their differentiation.29 Commensal bacteria also play an important role in the fermentation of dietary fiber, in addition to essential vitamins, a significant amount of short chain fatty acids (SCFAs) are also released. The colonization of commensal bacteria occurs immediately after birth and during the first years of life. The gastrointestinal microbiota acquisition affects the type of parturition, maternal microbiota, as well as genetic factors, breastfeeding, and other environmental factors.30
One of the important functions of the intestinal microbiota is to contribute to the development of the immune system. The extensive antigenic stimulation after microbiota acquisition helps to organize the immune system through the development of gut-associated lymphoid tissue (GALT) and tolerogenic responses to harmless antigens, including food. These responses are performed by sampling and initial detection of antigens by intestinal epithelial cells and intrinsic immune cells through pattern recognition receptors (PRRs).31 These receptors are bonded to pathogen-associated molecular patterns (PAMPs), expressed by microorganisms. PAMPs include lipoteichoic acid on gram-positive bacteria and lipopolysaccharide on gram-negative bacteria. Identification of PAMPs by PRRs such as toll-like receptor (TLR)-2 and TLR4, triggers signals that determine the type of immune response, leads to the formation of a combination of regulatory and executive functions by DCs, Treg cells, chemokines, and cytokines.32
The study of germ-free (GF) mice confirmed the key role of microbiota and their antigens in the development of GALT and inadequate immune responses. Several studies have shown that GF mice have dramatically reduced the number of intra-epithelial lymphocytes, payer’s patches with incomplete germinal centers, and produce fewer IgA antibodies by plasma cells in the gastrointestinal lamina propria regions (which is actually the GALT executive region). Restoring different types of microbial species can help rebuild the GALT function in these mice, as well as the development and growth of the immune system. Additionally, in GF mice, there is no tolerance to prescriptive antigens due to the deficiency of Treg cells. However, oral administration of microorganisms leads to stabilization of tolerance.33–35
ProbioticsProbiotics are defined as live microorganisms that confer a health benefit on the host when administered in adequate amounts.36–38 The history of the use of probiotics with extensive access to them reflects their high degree of safety39 These findings highlight the important role of microbiota in immune system development and its potential role for immune disorders, such as allergies and autoimmune disease, resulting in intestinal dysbiosis.40,41 The identification and use of probiotics date back to the early 20th century. Generally, probiotic bacteria need to have a set of criteria for inducing their beneficial effects, including: (1) resistance to PH, bile and digestive enzymes; (2) preventing the binding of pathogens and oral antigens to epileptic cells; (3) increasing the probability of biological efficacy in humans; (4) direct effect on toxicity of bacteria, viruses, fungi and parasites; and (5) proper tolerance and their importance in clinical use for their safety.42,43
Safety of probioticsThe human body is colonized (mainly gastrointestinal tract) by 10–100 trillion microbes, including bacteria, archaea, viruses, and eukaryotic microbes. The indigenous intestinal microbiota is known to be a principal contributor in maintaining usual physiology, immune homeostasis, and energy production during life.44 Protein products and metabolites of these microbes play an important role in the development and homeostasis of a number of body functions. The communication between these microorganisms and hosts has grown throughout the entire mammalian evolution, resulting in a distinctive gastrointestinal microbial community in humans.45 The microbial community remains stable over time, moreover, diet and other environmental factors can affect the microbial community. Commensal microorganisms, in addition to inhibiting invasion of other pathogenic microorganisms; donate fermentation of non-digestible fibers; help to absorption of minerals and produce vitamins, SCFAs, and carbohydrates and also regulate inflammatory responses and the immune system.46 A defect to maintain the balance among them (commensal microbiota and the host) interrupting the complex homeostasis (defined as dysbiosis) may cause a variety of syndromes (e.g. intestinal, metabolic, and immune disorders).47
Probiotics are live microorganisms that are regarded as safe because of their long history of use in foods and dairy products for over a hundred years. Common probiotics are Lactobacillus and Bifidobacterium, some strains of Enterococcus and generally Saccharomyces species.48 Because probiotics have been shown to affect both the innate and adaptive immune systems, a theoretical concern about the potential of probiotics to stimulate the immune response in some individuals, possibly leading to autoimmune or inflammation has not been reported in any human subjects.34
The gut acts as an extremely discerning barrier and communication organ between the luminal bacterial environment and the host. Therefore, failure of this communication due to the loss of barrier function or failure of tolerance mechanisms have been associated with diseases, usually as opportunistic infections in some predisposing patients and development of inflammation. Additionally, the presence of transferable antibiotic resistance genes, which comprises a theoretical risk of transfer to a less harmless member of the gut microbial community, must also be considered.49
Therefore, the safety of probiotics is related to the nature of the specific microbe proposed use, dose, and duration.50 Overall, probiotics when administered in adequate amounts constitute a health benefit on the host. Agency for Healthcare Research and Quality (AHRQ) in support with National Institutes of Health and the FDA released a report which analyzed 622 studies and clinical trials that used organisms from six species (Lactobacillus, Bifidobacterium, Saccharomyces, Streptococcus, Enterococcus, and Bacillus) and revealed no evidence of increased risk in those studies.51,52
Overall, the evaluation of probiotics safety includes consideration of a variety of factors: isolation history and taxonomic classification of the applicant probiotic; monitoring of contamination; lack of transferable antibiotic resistance genes; physiological status of the consuming population; dose and method administration (oral or otherwise); lack of allergenic material (for example, dairy proteins) for allergic populations.53
Functional mechanisms of probioticsProbiotics cause beneficial effects in the host via several mechanisms, which are divided into physiological and immunological sections.40
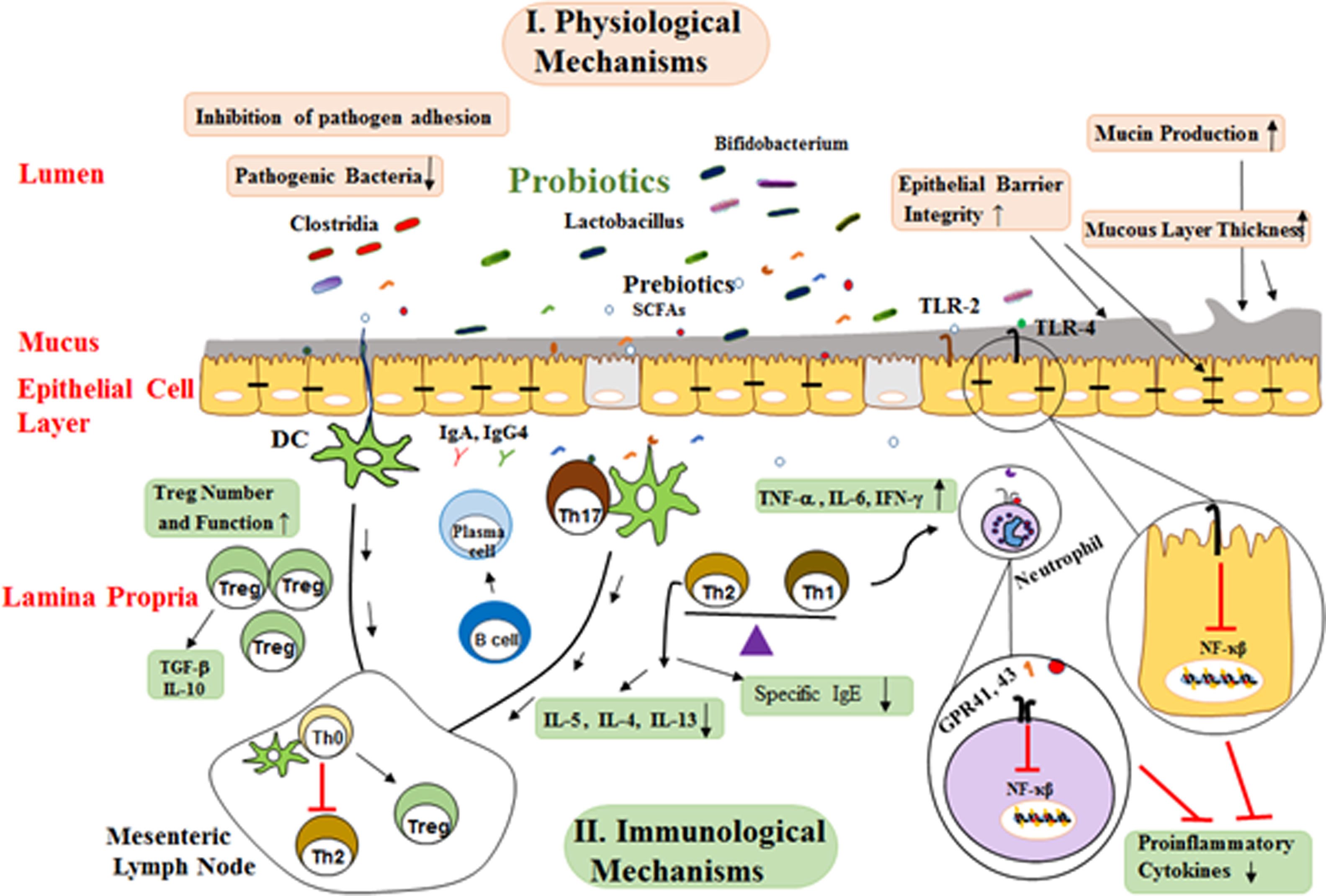
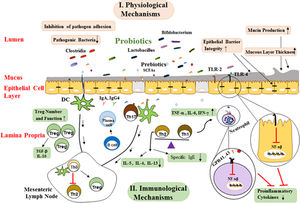
Physiological functionsProbiotics are capable of modulating the intestinal microbiota. In this regard, studies have shown that probiotic supplements, such as Lactobacillus rhamnosus GG (LGG) or Lactobacillus casei, are capable of modulating the intestinal microbiota that involves the reduction of pathogenic bacteria, including cholesterol, and, conversely, an increase in the level of beneficial Bifidobacteria in the neonate with allergies.54 These limiting physiological changes include the appropriate pH for the growth and reproduction of these pathological bacteria. Probiotics can also prevent invasion by competing with other microorganisms to bind to the host epithelial cells.55 Treatment with Bifidobacteria and Lactobacilli in newborns results in modulation of the intestinal microbiota in early years of newborn life. It also stimulates the growth of other beneficial bacteria and their products in humans and other species. Modifying the colonization by probiotic bacteria can affect the prolonged presence of harmful pathogens in the intestine lumen, and thus facilitate their clearing by the immune system.56 In addition, some of these strains prevent the growth of bacterial pathogens by producing bacteriocin. For example, the bacteriocin produced by Lactobacillus acidophilus La-14 inhibits the growth of Listeria monocytogenes bacteria57 (Fig. 2).
Possible mechanisms whereby probiotics affect allergic diseases. These mechanisms are generally divided into two groups of mechanisms of physiological and immunological mechanisms. SCFAs binding to various GPRs and TLR-2/TLR-4 activate several signaling pathways I. physiological mechanisms include: a) Probiotics create competitive conditions and inhibit bacterial adherence to mucosal layer, b) Enhancement epithelial barrier integrity and improved barrier function, c) Mucus production can also be increased by probiotics that stimulate goblet cells leading to activation of mucin gene expression and therefore altering colonization and persistence condition. II. Immunological mechanisms: a) Probiotics, directly and indirectly, affect the epithelial cells and modulate signaling pathways that lead to a reduced expression of inflammatory cytokines by suppressing NF-kB signaling, b) Primary mode of action of probiotics includes restoration of Th1/Th2 cytokine balance and enhancement of Th2 cytokines (IL-4, IL-5, IL-13), c) Probiotics with their products stimulate dendritic cells might be leading to Treg differentiation induction of CD4+ Foxp3+ Treg cells and production of TGF-β and IL-10, d) Probiotics changes the cytokine profiles through effects on dendritic cells and so increase the production of secretory IgA and IgG4 by B cell and reduction in allergen-specific IgE by B cells.
(SCAFs: short chain fatty acids; TLR: Toll-like Receptor; TNF-α: Tumor Necrosis Factor A; IFN-γ: Interferon γ; TGF-β: Transforming Growth Factor B; IL-3: Interleukin 3; IL-4: Interleukin 4; IL-5: Interleukin 5; IL-6. Interleukin 6; IL-10: Interleukin 10; Th0: T Helper Cell Naive; Th2: Type 2 Helper T Cell; Th1: Type 1 Helper T Cell; Treg: Regulatory T Cell; IgE: Immunoglobulin E; DC: Dendritic Cells).
Another of the probiotic functional mechanisms is their effect on epithelial barrier integrity and regulation of the expression of the proteins which are involved in tight junctions and secretion of mucus.58 Treatment with Streptococcus thermophilus and Lactobacillus acidophilus increased transepithelial resistance as well as the phosphorylation of proteins involved in cellular tight junctions, such as actinin and occluding.59 Probiotics also release a significant amount of SCFAs during the fermentation of food fibers and the induction of strong anti-inflammatory activity. Butyrate is one of these SCFAs that is involved in modulating the expression of tight junction proteins, including cingulin, Zo and occludin, which improve the integrity of the epithelial barrier.60 Another SCFA is acetate, which has beneficial effects in animal models by reducing inflammation in asthma and colitis. Neutrophils interfere with their anti-inflammatory effects by acting via G protein-coupled receptors (GPR 41, 43) through which they can attach to SCFAs.61 These effects of SCFAs are accomplished by modulating the NF-κB signaling and the activity of their cytokines network. It also appears that SCFAs have the potential to inhibit histone-deacetylase and alter the structure and function of chromatin, and so the downstream signal affects the gene expression62Fig. 2.
Immunological functionsThe activation of TLRs by microorganisms can lead to immune responses that have mucosal and systemic effects. Lactobacillus reduces pro-inflammatory responses by regulating NF-κB signaling. Probiotic bacteria also modulate the maturation of DCs towards anti-inflammatory cytokines such as IL-10. Although DCs derived from human monocytes treated with probiotics in culture can release IL-10 that cause differentiation and survival of Treg cells.43,63B. animals and B. longum have been shown to induce the release of IFN-γ and TNF-α by DCs, while only B. bifidum can activate Th17 cells through the release of IL-17.64 Sufficient evidence indicates that the Th1/Th2 balance is modulated by probiotics, thereby preventing inflammatory diseases such as allergies. Peripheral blood mononuclear cell (PBMCs) isolated from allergic patients in vitro with several lactic acid bacteria, including L. plantarum, L. lactis, L. casei, and Lactobacillus GG, before induction with house dust mite, reduced the Th2 responses, which is characterized by the reduction of IL-4 and IL-5 production. LGG and B. lactis reduce the allergic symptoms of asthma mouse models by inducing TGF-β secreting65,66 (Fig. 2).
Probiotics function in allergic disordersOther effects of probiotics that make them suitable for modulation of allergic diseases include stimulating the levels of mucosal IgA, as well as allergen-specific T cells and B cells. These interactions are very complex, and include a network of genes, TLRs, signaling molecules and an increase in IgA intestinal responses.67,68 Many animal and in vitro studies, as well as several human experiments, show the positive effects of probiotics in allergic diseases. Several randomized studies have showed that when Lactobacillus GG or placebo was given to pregnant mothers with a serious family history of eczema, allergic rhinitis, or asthma and after to newborns during the first six months from delivery, the incidence of atopic dermatitis in children decreased by 50%, 44% and 36% at two years, four years and seven years, respectively.69,70
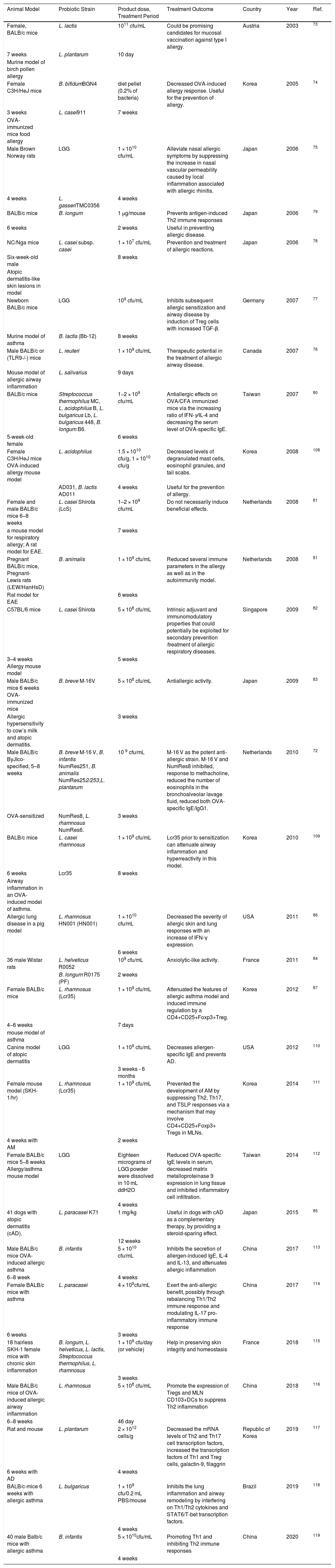
Animal studiesStudies on probiotic effects in allergic diseases in animal models have promising results. In the dermatologic atopic model induced by house dust mite and dinitrochlorobenzene, treatment with probiotic mixture L. acidophilus, L. casei, L. reuteri, B. bifidum and Streptococcus thermophiles, was associated with an increase in the number of CD4+FOXP3+Treg cells parallel with the regression of clinical symptoms, including the specific and total IgE level, and the amount IL-4, IL-5, IL-13.71 In mouse allergic airway disease (AAD), which is a human asthma model, the probiotic efficacy has been studied. Hougee et al. demonstrated that six probiotic strains, when administered to an ovalbumin (OVA)-induced animal model of AAD led to improvement of lung function and the number of eosinophils, specific IgE levels, IL-4, IL-5 and IL-10 in the bronchoalveolar lavage fluid (BALF). Specifically, treating rats with L. casei and L. plantarum before sensitization with OVA prevented the development of airway hypersensitivity (AHR) and increased eosinophil count and total IgE levels. L. reuteri can significantly reduce AAD and eosinophilia of the respiratory tract, AHR, and level of TNF-α, IL-5, and IL-13 in the BALF. It has also been shown that this bacterium can induce Treg CD4+ CD25+ FOXP3+ cells in the spleen and prevent the development of AAD in OVA-sensitive mice.72
In animal models, L. lactis and L. plantarum cause a shift in response to Th1 and reduce the clinical symptoms associated with allergic diseases.73B. bifidum and L. casei reduces the incidence of OVA-induced allergy symptoms.74 LGG can reduce the expression of metalloproteinase 9 (MP-9) and cell infiltration and in combination with L. gasseri, L. reuteri and L. salivarius reduce the symptoms of allergy75,76 A mixture of LGG with B. lactis induces Treg responses along with increased TGF-β and leads to inhibition of allergic responses and decreased IgE levels.77
B. longum and L. casei have a role in preventing Th2 responses and allergic diseases.78,79Streptococcus thermophilus, L. acidophilus, L. bulgaricus and B. Longum cocktail had an anti-allergic effect which is manifested mainly by increasing the IFN-γ/IL-4 ratio and lowering the serum IgE level.80 In the use of bacteria, L. casei Shirota results were variable and it was not found to be beneficial in a BALB-c animal model, while in cases of C57BL6, there was a reduction of allergic disease symptoms.81,82 Simultaneous use of B. animalis, B. brave, L. helveticus and L. paracasei improves allergic responses and alleviates the clinical symptoms of allergic disease.72,83–85B. infantis and L. rhamnosus also reduce the level of eosinophil and specific IgE antibodies leading to an anti-allergic effect.86 Moreover, by increasing the level of IFN-γ, it reduces the symptoms of the disease and, by increasing Treg cells, reduces allergic asthma in the BALB-c model.72,87 (Probiotics used to prevent and treat allergic diseases in animal models are shown in Table 1).
Probiotics used to prevent and treat allergic diseases in animal models.
| Animal Model | Probiotic Strain | Product dose, Treatment Period | Treatment Outcome | Country | Year | Ref. |
|---|---|---|---|---|---|---|
| Female, BALB/c mice | L. lactis | 1011 cfu/mL | Could be promising candidates for mucosal vaccination against type I allergy. | Austria | 2003 | 73 |
| 7 weeks | L. plantarum | 10 day | ||||
| Murine model of birch pollen allergy | ||||||
| Female C3H/HeJ mice | B. bifidumBGN4 | diet pellet (0.2% of bacteria) | Decreased OVA-induced allergy response. Useful for the prevention of allergy. | Korea | 2005 | 74 |
| 3 weeks | L. casei911 | 7 weeks | ||||
| OVA-immunized mice food allergy | ||||||
| Male Brown Norway rats | LGG | 1 × 1010 cfu/mL | Alleviate nasal allergic symptoms by suppressing the increase in nasal vascular permeability caused by local inflammation associated with allergic rhinitis. | Japan | 2006 | 75 |
| 4 weeks | L. gasseriTMC0356 | 4 weeks | ||||
| BALB/c mice | B. longum | 1 μg/mouse | Prevents antigen-induced Th2 immune responses | Japan | 2006 | 79 |
| 6 weeks | 2 weeks | Useful in preventing allergic disease. | ||||
| NC/Nga mice | L. casei subsp. casei | 1 × 107 cfu/mL | Prevention and treatment of allergic reactions. | Japan | 2006 | 78 |
| Six-week-old male | 8 weeks | |||||
| Atopic dermatitis-like skin lesions in model | ||||||
| Newborn BALB/c mice | LGG | 109 cfu/mL | Inhibits subsequent allergic sensitization and airway disease by induction of Treg cells with increased TGF-β. | Germany | 2007 | 77 |
| Murine model of asthma | B. lactis (Bb-12) | 8 weeks | ||||
| Male BALB/c or (TLR9-/-) mice | L. reuteri | 1 × 109 cfu/mL | Therapeutic potential in the treatment of allergic airway disease. | Canada | 2007 | 76 |
| Mouse model of allergic airway inflammation | L. salivarius | 9 days | ||||
| BALB/c mice | Streptococcus thermophilus MC, L. acidophilus B, L. bulgaricus Lb, L. bulgaricus 448, B. longum B6. | 1−2 × 109 cfu/mL | Antiallergic effects on OVA/CFA immunized mice via the increasing ratio of IFN-γ/IL-4 and decreasing the serum level of OVA-specific IgE. | Taiwan | 2007 | 80 |
| 5-week-old female | 6 weeks | |||||
| Female C3H/HeJ mice OVA-induced allergy mouse model | L. acidophilus | 1.5 × 1010 cfu/g, 1 × 1010 cfu/g | Decreased levels of degranulated mast cells, eosinophil granules, and tail scabs. | Korea | 2008 | 108 |
| AD031, B. lactis AD011 | 4 weeks | Useful for the prevention of allergy. | ||||
| Female and male BALB/c mice 6–8 weeks | L. casei Shirota (LcS) | 1–2 × 109 cfu/mL | Do not necessarily induce beneficial effects. | Netherlands | 2008 | 81 |
| a mouse model for respiratory allergy; A rat model for EAE. | 7 weeks | |||||
| Pregnant BALB/c mice, Pregnant- Lewis rats (LEW/HanHsD) | B. animalis | 1 × 109 cfu/mL | Reduced several immune parameters in the allergy as well as in the autoimmunity model. | Netherlands | 2008 | 81 |
| Rat model for EAE | 6 weeks | |||||
| C57BL/6 mice | L. casei Shirota | 5 × 108 cfu/mL | Intrinsic adjuvant and immunomodulatory properties that could potentially be exploited for secondary prevention /treatment of allergic respiratory diseases. | Singapore | 2009 | 82 |
| 3–4 weeks Allergy mouse model | 5 weeks | |||||
| Male BALB/c mice 6 weeks OVA-immunized mice | B. breve M-16V | 5 × 108 cfu/mL | Antiallergic activity. | Japan | 2009 | 83 |
| Allergic hypersensitivity to cow’s milk and atopic dermatitis. | 3 weeks | |||||
| Male BALB/c ByJIco-specified, 5–8 weeks | B. breve M-16 V, B. infantis NumRes251, B. animalis NumRes252/253,L. plantarum | 10 9 cfu/mL | M-16 V as the potent anti-allergic strain. M-16 V and NumRes8 inhibited, response to methacholine, reduced the number of eosinophils in the bronchoalveolar lavage fluid, reduced both OVA-specific IgE/IgG1. | Netherlands | 2010 | 72 |
| OVA-sensitized | NumRes8, L. rhamnosus NumRes6. | 3 weeks | ||||
| BALB/c mice | L. casei rhamnosus | 1 × 109 cfu/mL | Lcr35 prior to sensitization can attenuate airway inflammation and hyperreactivity in this model. | Korea | 2010 | 109 |
| 6 weeks | Lcr35 | 8 weeks | ||||
| Airway inflammation in an OVA-induced model of asthma. | ||||||
| Allergic lung disease in a pig model | L. rhamnosus HN001 (HN001) | 1 × 1010 cfu/mL | Decreased the severity of allergic skin and lung responses with an increase of IFN-γ expression. | USA | 2011 | 86 |
| 6 weeks | ||||||
| 36 male Wistar rats | L. helveticus R0052 | 109 cfu/mL | Anxiolytic-like activity. | France | 2011 | 84 |
| B. longum R0175 (PF) | 2 weeks | |||||
| Female BALB/c mice | L. rhamnosus (Lcr35) | 1 × 109 cfu/mL | Attenuated the features of allergic asthma model and induced immune regulation by a CD4+CD25+Foxp3+Treg. | Korea | 2012 | 87 |
| 4−6 weeks | 7 days | |||||
| mouse model of asthma | ||||||
| Canine model of atopic dermatitis | LGG | 1 × 109 cfu/mL | Decreases allergen-specific IgE and prevents AD. | USA | 2012 | 110 |
| 3 weeks - 6 months | ||||||
| Female mouse model (SKH-1/hr) | L. rhamnosus (Lcr35) | 1 × 109 cfu/mL | Prevented the development of AM by suppressing Th2, Th17, and TSLP responses via a mechanism that may involve CD4+CD25+Foxp3+ Tregs in MLNs. | Korea | 2014 | 111 |
| 4 weeks with AM | 2 weeks | |||||
| Female BALB/c mice 5−8 weeks Allergy/asthma mouse model | LGG | Eighteen micrograms of LGG powder were dissolved in 10 mL ddH2O | Reduced OVA-specific IgE levels in serum, decreased matrix metalloproteinase 9 expression in lung tissue and inhibited inflammatory cell infiltration. | Taiwan | 2014 | 112 |
| 4 weeks | ||||||
| 41 dogs with atopic dermatitis (cAD). | L. paracasei K71 | 1 mg/kg | Useful in dogs with cAD as a complementary therapy, by providing a steroid-sparing effect. | Japan | 2015 | 85 |
| 12 weeks | ||||||
| Male BALB/c mice OVA-induced allergic asthma | B. infantis | 5 × 1010 cfu/mL | Inhibits the secretion of allergen-induced IgE, IL-4 and IL-13, and attenuates allergic inflammation | China | 2017 | 113 |
| 6−8 week | 4 weeks | |||||
| Female BALB/c mice with asthma | L. paracasei | 4 × 109cfu/mL | Exert the anti-allergic benefit, possibly through rebalancing Th1/Th2 immune response and modulating IL-17 pro-inflammatory immune response | China | 2017 | 114 |
| 6 weeks | 3 weeks | |||||
| 18 hairless SKH-1 female mice with chronic skin inflammation | B. longum, L. helveticus, L. lactis, Streptococcus thermophilus, L. rhamnosus | 1 × 109 cfu/day (or vehicle) | Help in preserving skin integrity and homeostasis | France | 2018 | 115 |
| 3 weeks | ||||||
| Male BALB/c mice of OVA-induced allergic airway inflammation | L. rhamnosus | 5 × 108 cfu/mL | Promote the expression of Tregs and MLN CD103+DCs to suppress Th2 inflammation | China | 2018 | 116 |
| 6−8 weeks | 46 day | |||||
| Rat and mouse | L. plantarum | 2 × 1012 cells/g | Decreased the mRNA levels of Th2 and Th17 cell transcription factors, increased the transcription factors of Th1 and Treg cells, galactin-9, filaggrin | Republic of Korea | 2019 | 117 |
| 6 weeks with AD | 4 weeks | |||||
| BALB/c-mice 6 weeks with allergic asthma | L. bulgaricus | 1 × 109 cfu/0.2 mL PBS/mouse | Inhibits the lung inflammation and airway remodeling by interfering on Th1/Th2 cytokines and STAT6/T-bet transcription factors. | Brazil | 2019 | 118 |
| 4 weeks | ||||||
| 40 male Balb/c mice with allergic asthma | B. infantis | 5 × 1010cfu/mL | Promoting Th1 and inhibiting Th2 immune responses | China | 2020 | 119 |
| 4 weeks |
Ova: Ovalbumin; LAB: Lactic Acid Bacteria; L: Lactobacillus; B: Bifidobacterium; EAE: Experimental Autoimmune Encephalomyelitis; LGG: L. rhamnosus GG; AM: Allergic March.
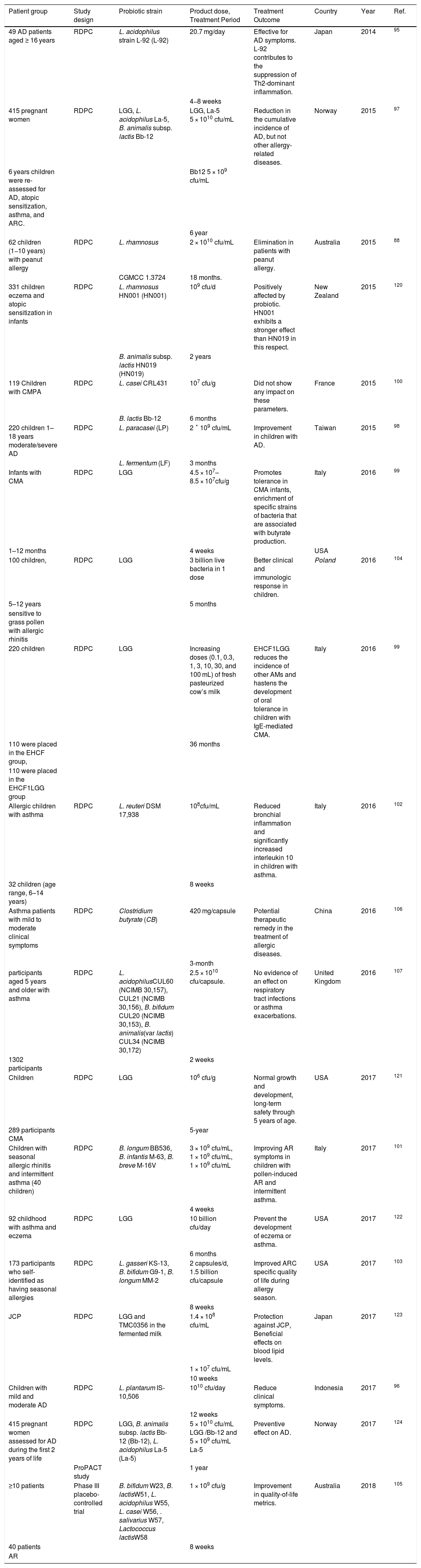
Early study demonstrates that administration for eight weeks of LGG, Bifidobacterium lactis Bb12, or B. breve M-16V strains led to the improvement of eczema symptoms in infants and children compared to a control group.88 In spite of the studies that were later carried out in this field, the results were poor in patients with eczema. Viljanen in 2005 and Sistek in 2006 studies showed that LGG, L. rhamnosus and B. lactis had a better Scorad in children with atopic dermatitis. Viljanen et al. observed that LGG decreased the IL-6 and C-reactive protein and thereby inflammation. On the other hand, it stimulated IL-10 and IgA production which indicated the beginning of repressive responses.89,90 The complementary formulation containing L. acidophilus, B. lactis and fructooligosaccharides (FOS) after eight weeks of treatment significantly reduced Scorad as compared to the control.91 Wu et al. demonstrated that treatment of children with L. casei for 12 weeks significant decreased Scorad scar as compared to placebo. In 2012, Yesilova studied the effect of B. bifidum, L. acidophilus, L. casei, and L. salivarius combination for eight weeks in 1–3 years aged children with eczema. A decrease in Scorad and IL-5, IL-6, IFN-γ, and IgE total compared to the control group was observed. Although the use of a combination of probiotics, prebiotics, and synbiotics, including B. breve M-16 and a mixture of galacto-fructooligosaccharides, was not able to improve the severity of eczema and reduce Scorad.92 In Toh et al., a study in children with moderate to severe eczema, the use of the combination of L. salivarius and FOS for eight weeks resulted in a significant reduction in Scorad severity compared to FOS alone.68 In another similar adult study, treatment with L. salivarius for 16 months significantly decreased with Scorad in comparison with the control group, as well as decreasing the level of IFN-γ, IL-2, and Th1/Th2 cytokines.93
Preventing allergies is one of the major clinical challenges. While there is a lack of evidence, there is a great gap in the use of probiotics in the treatment of allergic diseases.94 Several randomized clinical trials (RCTs) have been conducted to evaluate the effects of various probiotic strains in children and adults with a history of allergic diseases. The duration of treatment with probiotics seems to be one of the key factors determining their beneficial effects.
Among the human studies conducted between 2014 and 2018, the strains that can affect AD (atopic dermatitis) could be L. acidophilus, LGG, B. animalis, L. paracasei, L. fermentum, and L. plantarum. The combination was generally accompanied by suppressing Th2 responses and improving AD clinical symptoms.95–98 In the Cow’s Milk Allergy (CMA) studies, the use of LGG, which caused butyrate production, increased the induction of tolerance in CMA children, but no significant effect was observed in the presence of B. lactis Bb-12 and L. casei on CMA.99,100 The improvement of AR symptoms was reported for several strains such as LGG, L. reuteri, L. casei, L. salivarius, B. Longum, B. infantis, B. breve, B. gasseri, B. bifidum G9-1, and Lactococcus lactis, all of which were effective in reducing clinical symptoms.101–105 In patients with asthma, the effect of L. acidophilus, L. reuteri, and Clostridium butyrate were evaluated. The L. reuteri increased IL-10 and decreased inflammation, leading to symptom improvement in these patients.102,106,107 The probiotics used to prevent and treat allergic diseases in human studies shown in Table 2.
Probiotics used to prevent and treat allergic diseases in human studies.
| Patient group | Study design | Probiotic strain | Product dose, Treatment Period | Treatment Outcome | Country | Year | Ref. |
|---|---|---|---|---|---|---|---|
| 49 AD patients aged ≥ 16 years | RDPC | L. acidophilus strain L-92 (L-92) | 20.7 mg/day | Effective for AD symptoms. L-92 contributes to the suppression of Th2-dominant inflammation. | Japan | 2014 | 95 |
| 4−8 weeks | |||||||
| 415 pregnant women | RDPC | LGG, L. acidophilus La-5, B. animalis subsp. lactis Bb-12 | LGG, La-5 5 × 1010 cfu/mL | Reduction in the cumulative incidence of AD, but not other allergy-related diseases. | Norway | 2015 | 97 |
| 6 years children were re-assessed for AD, atopic sensitization, asthma, and ARC. | Bb12 5 × 109 cfu/mL | ||||||
| 6 year | |||||||
| 62 children (1−10 years) with peanut allergy | RDPC | L. rhamnosus | 2 × 1010 cfu/mL | Elimination in patients with peanut allergy. | Australia | 2015 | 88 |
| CGMCC 1.3724 | 18 months. | ||||||
| 331 children eczema and atopic sensitization in infants | RDPC | L. rhamnosus HN001 (HN001) | 109 cfu/d | Positively affected by probiotic. HN001 exhibits a stronger effect than HN019 in this respect. | New Zealand | 2015 | 120 |
| B. animalis subsp. lactis HN019 (HN019) | 2 years | ||||||
| 119 Children with CMPA | RDPC | L. casei CRL431 | 107 cfu/g | Did not show any impact on these parameters. | France | 2015 | 100 |
| B. lactis Bb-12 | 6 months | ||||||
| 220 children 1–18 years moderate/severe AD | RDPC | L. paracasei (LP) | 2 ˟ 109 cfu/mL | Improvement in children with AD. | Taiwan | 2015 | 98 |
| L. fermentum (LF) | 3 months | ||||||
| Infants with CMA | RDPC | LGG | 4.5 × 107–8.5 × 107cfu/g | Promotes tolerance in CMA infants, enrichment of specific strains of bacteria that are associated with butyrate production. | Italy | 2016 | 99 |
| 1–12 months | 4 weeks | USA | |||||
| 100 children, | RDPC | LGG | 3 billion live bacteria in 1 dose | Better clinical and immunologic response in children. | Poland | 2016 | 104 |
| 5–12 years | 5 months | ||||||
| sensitive to grass pollen with allergic rhinitis | |||||||
| 220 children | RDPC | LGG | Increasing doses (0.1, 0.3, 1, 3, 10, 30, and 100 mL) of fresh pasteurized cow’s milk | EHCF1LGG reduces the incidence of other AMs and hastens the development of oral tolerance in children with IgE-mediated CMA. | Italy | 2016 | 99 |
| 110 were placed in the EHCF group, | 36 months | ||||||
| 110 were placed in the EHCF1LGG group | |||||||
| Allergic children with asthma | RDPC | L. reuteri DSM 17,938 | 108cfu/mL | Reduced bronchial inflammation and significantly increased interleukin 10 in children with asthma. | Italy | 2016 | 102 |
| 32 children (age range, 6–14 years) | 8 weeks | ||||||
| Asthma patients with mild to moderate clinical symptoms | RDPC | Clostridium butyrate (CB) | 420 mg/capsule | Potential therapeutic remedy in the treatment of allergic diseases. | China | 2016 | 106 |
| 3-month | |||||||
| participants aged 5 years and older with asthma | RDPC | L. acidophilusCUL60 (NCIMB 30,157), CUL21 (NCIMB 30,156), B. bifidum CUL20 (NCIMB 30,153), B. animalis(var lactis) CUL34 (NCIMB 30,172) | 2.5 × 1010 cfu/capsule. | No evidence of an effect on respiratory tract infections or asthma exacerbations. | United Kingdom | 2016 | 107 |
| 1302 participants | 2 weeks | ||||||
| Children | RDPC | LGG | 106 cfu/g | Normal growth and development, long-term safety through 5 years of age. | USA | 2017 | 121 |
| 289 participants CMA | 5-year | ||||||
| Children with seasonal allergic rhinitis and intermittent asthma (40 children) | RDPC | B. longum BB536, B. infantis M-63, B. breve M-16V | 3 × 109 cfu/mL, 1 × 109 cfu/mL, 1 × 109 cfu/mL | Improving AR symptoms in children with pollen-induced AR and intermittent asthma. | Italy | 2017 | 101 |
| 4 weeks | |||||||
| 92 childhood with asthma and eczema | RDPC | LGG | 10 billion cfu/day | Prevent the development of eczema or asthma. | USA | 2017 | 122 |
| 6 months | |||||||
| 173 participants who self-identified as having seasonal allergies | RDPC | L. gasseri KS-13, B. bifidum G9-1, B. longum MM-2 | 2 capsules/d, 1.5 billion cfu/capsule | Improved ARC specific quality of life during allergy season. | USA | 2017 | 103 |
| 8 weeks | |||||||
| JCP | RDPC | LGG and TMC0356 in the fermented milk | 1.4 × 108 cfu/mL | Protection against JCP, Beneficial effects on blood lipid levels. | Japan | 2017 | 123 |
| 1 × 107 cfu/mL | |||||||
| 10 weeks | |||||||
| Children with mild and moderate AD | RDPC | L. plantarum IS-10,506 | 1010 cfu/day | Reduce clinical symptoms. | Indonesia | 2017 | 96 |
| 12 weeks | |||||||
| 415 pregnant women assessed for AD during the first 2 years of life | RDPC | LGG, B. animalis subsp. lactis Bb-12 (Bb-12), L. acidophilus La-5 (La-5) | 5 × 1010 cfu/mL LGG /Bb-12 and 5 × 109 cfu/mL La-5 | Preventive effect on AD. | Norway | 2017 | 124 |
| ProPACT study | 1 year | ||||||
| ≥10 patients | Phase III placebo-controlled trial | B. bifidum W23, B. lactisW51, L. acidophilus W55, L. casei W56, . salivarius W57, Lactococcus lactisW58 | 1 × 109 cfu/g | Improvement in quality-of-life metrics. | Australia | 2018 | 105 |
| 40 patients | 8 weeks | ||||||
| AR |
ARC: Allergic Rhinoconjunctivitis; AD: Atopic Dermatitis; AR: Allergic Rhinitis; RDPC: Randomized, Double-blind, Placebo-Controlled trial; CMPA: Cow’s Milk Protein Allergy; JCP: Japanese Cedar Pollinosis; LGG: L. rhamnosus GG.
Currently, the prevention and treatment of allergic diseases is one of the major clinical challenges. The rapid rise in immune system disorders, such as allergic disease, is strongly associated with reduced exposure to early microorganisms. Intestinal microbiota partially stimulates the immune system, and the particular composition of the microbiota of the intestine may affect the risk of allergic disease. Therefore, these findings suggest a therapeutic approach for probiotics and prebiotics used in allergic diseases. Probiotics are selectively stimulating a number of beneficial bacteria that are evaluated in allergy treatment studies. In general, probiotics use major mechanisms to improve clinical symptoms in patients with allergic diseases and prevent them, including: (1) the suppression of Th2 responses and shift response to Th1; (2) butyrate production and increased induction of tolerance; (3) increase of IL-10 and decreased inflammation; (4) decreased eosinophil level and serum specific IgE levels; (5) increasing the IFN-γ/IL-4 ratio; (6) increasing Treg cells and inducing their responses; (7) increasing TGF-β responses and inhibiting allergic responses; and (8) reducing the expression of metalloproteinase 9 and cell infiltration. Finally, it can be said that the therapeutic approach to immunotherapy and the reduction of the risk of side effects in the treatment of allergic diseases is the first priority of treatment. The final approach that completes the first priority is maintaining the condition and sustainability of the tolerance along with the recovery of the individual.
There are several advantages and health profits associated with probiotics or probiotic food products. The primary results of evaluated studies in our review included improvement in the clinical score, influence on the immune system and allergy modulation, also effects on the fecal and plasma biomarkers of inflammation. But these studies also have limitations and disadvantages that will be discussed in detail. The major limitations of some of the studies is the reduced sample size, the randomization process and the small number of bacterial groups used. In addition, some studies do not clearly describe the conditions and goals of the selected probiotic species, inclusion and exclusion criteria and methods of working. However, there are several other risks associated with probiotics therapy in the clinical field, especially in the treatment of allergic diseases.
These risks are mainly concerned with safety in vulnerable target groups such as immune compromised subjects (high risk populations such as pregnant women, babies and the elderly individuals) and some immunodeficiency patients. Probiotics can affect the commensal bacteria and can also have a direct influence on the host in the long term and for these reasons the evolution of these interactions is one of the key concerns for future research. Therefore, immediate clinical and molecular examination is required to improve our knowledge about interaction among candidate probiotics and host microbiome, cells, mucus and immune defenses, in order to create efficacious interventions.
One theoretical concern with the safety of probiotics is that some have been designed or chosen to have good adherence to the intestinal mucosa, and this is considered important for their mechanism of action. Adherence to the intestinal mucosa may also increase bacterial translocation and virulence. The most potent probiotics, therefore, may have increased pathogenicity. The relation between mucosal adhesion and pathogenicity in Lactobacillus spp. is supported by the finding that blood culture isolates of Lactobacillus spp. adhere to intestinal mucus in greater numbers than isolates from human feces or dairy products do. Emerging insights from microbiome research allow an assessment of gut colonization by probiotics, species and strain, interactions with the indigenous microbiome, safety and impacts on the host, physiological effects and potentially beneficial medical indications such as allergic diseases.
Author contributionMajid Eslami: Investigated and supervised the findings of this work, wrote the article. Processed the experimental data. Supervised the project.
Bahman Yousefi: Designed the study, helped supervise the project, conceived the original idea. Processed the experimental data. Developed the theoretical framework.
Mohsen Karbalaei: Provided critical feedback and helped the analysis of the manuscript. Contributed to the interpretation of the results.
Masoud Keikha: Contributed to the final version of the manuscript.
Aisa Bahar: Contributed to the final version of the manuscript.
Nazarii M. Kobyliak: Contributed to the final version of the manuscript.
All authors: Designed the model and the computational framework and analyzed the data.
Conflict of interestThe authors have no conflict of interest to declare.
FundingThis research is not supported by a specific project grant.
Department of Immunology, Semnan University of Medical Sciences, Semnan, Iran, and Cancer Research Center, Semnan University of Medical Sciences, Semnan, Iran.