We have observed that some cases of food anaphylaxis were followed by severe thrombosis associated to anticardiolipin antibodies. Food anaphylaxis associated with antiphospholipid syndrome has seldom been published.
ObjectiveThe aims were: 1) to test anticardiolipin antibodies in an important number of patients with anaphylaxis due to vegetal foods and their relationship with possible thrombosis; and 2) to study seed and fruit hypersensitivity in patients with previous thrombotic events associated with antiphospholipid antibodies (aCL).
MethodsWe included 30 patients diagnosed of thrombosis associated with aCL, 52 patients who suffered from anaphylaxis due to seeds or fruits, and 120 control patients. Haematological, cardiopulmonary vascular and rheumatologic studies had been performed as needed. In vivo and in vitro allergy tests with a large battery of vegetal allergens were carried out in all the patients. Measurement of IgG aCL antibodies and specific IgE to vegetal food was done by ELISA and CAP-FEIA (Phadia). Immunodetection and inhibitions with lipoproteins belonging to seeds were performed.
ResultsSeventy-five percent of the patients diagnosed as having antiphospholipid primary syndrome had specific IgE against different proteins from different vegetable allergens, most of them seeds, and clearly against lipoproteins that were also recognised by the patients with food anaphylaxis but not by the control cases. Among the patients with anaphylaxis, 28% had anticardiolipin antibodies and 17.3% thrombosis.
ConclusionOur study suggests that seed lipoproteins which cause severe food anaphylaxis might have a potential role in the antiphospholipid syndrome and related thrombosis.
IgE-mediated reactions to fruits and vegetables are not uncommon in patients with allergic symptoms caused by pollen.1 Recently, different families of plant defence proteins, such as class I Chitinases, lipid transfer proteins (LTPs), cereal inhibitors of α-amylases and thaumatins have been identified as major allergens in allergies produced by plant foods and pollens.2–4
Severe food allergic reactions have been reported to involve the gastrointestinal, cutaneous, ocular, respiratory and cardiovascular systems. Anaphylactic reactions to foods almost always occur immediately and can be diagnosed by skin tests, specific IgE determination and food challenges if necessary, but the delayed clinical complications of this syndrome have not been completely determined yet.
We reported four patients with food-anaphylaxis followed by severe thrombosis.5 In all of them, anaphylaxis was due to the ingestion of vegetable allergens (seeds and fruits). The anaphylactic episode was followed by thrombosis of both iliac veins and lower cava. We found moderate-high levels of anticardiolipin (aCL) IgG antibodies and finally, the patients were diagnosed as having a primary antiphospholipid syndrome (APS) associated to anaphylaxis.
Here, we include 52 patients who suffered from anaphylaxis due to seeds and fruits in order to determinate levels of anticardiolipin (aCL) IgG antibodies and possible related symptoms. On the other hand, we have checked allergic hypersensitivity to seeds and fruits in 30 patients with previous thrombosis associated with aCL.
MethodsPatientsWe reviewed the records of 21,879 patients who had been admitted to our Allergy Department in the last 22 years. We randomly selected 52 patients who suffered from severe anaphylaxis (grade III-IV by Müller) due to seeds or fruits. We also studied 30 patients with thrombosis and aCL antibodies, 20 having primary APS and 10 with APS secondary to systemic lupus erythematosus (SLE). As control group we included 120 subjects: 10 who suffered from anaphylaxis due to egg; 10 with anaphylaxis by Anisakis simplex; and 100 healthy non-atopic people. All the 202 subjects were studied in the same way: a detailed clinical allergy history followed by skin tests with a complete battery of 36 allergens, including prick-by-prick with suspected vegetal allergen, specific IgE measured by CAP method and lung function tests. Double blind placebo controlled oral challenge (DBPCOC) was performed only if necessary and in patients with non-severe anaphylaxis. Informed consent was obtained in all patients. Serum tests were done on individual samples from the 52 patients with anaphylaxis, 30 patients with thrombosis, as well as on pools from 100 non-allergic patients and from the 20 controls with anaphylaxis not due to vegetable foods.
Sensitisation to seeds or fruits was regarded as the presence of: a) one or more positive skin tests (with a wheal of 3mm larger than the negative control to these allergens or an area of 7mm2); b) a positive CAP-FEIA Phadia test >0.35 IU/mL; or c) a positive specific challenge. All these patients were informed of the objectives of the study and tested in order to try to identify an association of sensitisation to a vegetal allergen with thrombosis.
Seed and fruits extractCommercial vegetable extract was supplied by ALK-ABELLO, Madrid, Spain (protein concentration 0.5mg/mL). We performed extracts with fresh cereals seeds (wheat, barley, rye, and rice grains), legume seeds (soy bean, lentil, peanut, green pea), nuts (almond, hazelnut, chestnut, pine nut), garden produce (asparagus, tomato, leak), fruits (peach, apple, melon, banana), and mustard.
For in vitro tests, the different seeds and fruits were defatted, ground into small pieces and extracted by magnetic stirring in agitation in 50mM phosphate-buffered saline (PBS) at pH 7.5 during 16h at 4°C. The samples were centrifuged at 5600g for 30minutes; the supernatant was dialysed against water. The dialysed extract was filtered through a 0.22 (m-pore diameter membrane and freeze-dried.
Skin prick testsSkin prick tests (SPT) with extracts from different vegetables were performed according to standard procedures, over the anterior side of the forearm; one sterile device was used for each test. Histamine phosphate (10mg/ml) and sterile 0.9% saline were used as positive and negative controls, respectively. A mean diameter of 3mm or greater than the negative control and a mean area of 7mm2, 15minutes after puncture, was considered a positive response. SPT with all these extracts were also performed in all patients of the control group.
Bronchial challenge testsIn patients in whom asthma was the predominant symptom of anaphylaxis, specific bronchial challenge tests (BCT) were done, following the procedure proposed by Chai et al., with modifications.4 Aerosolised particles generated by a continuous pressurised De Vilbiss 646 nebuliser were inhaled for 2minutes at normal breathing volume, starting with control PBS solution, and followed - at 10minutes intervals - by progressive concentrations of the extract. Pulmonary function tests were obtained 30 and 60seconds after each dose. A positive response was defined as a greater than 20% fall from basal FEV1. After specific BCT, hourly peak expiratory flow (PEF) measurements were recorded for 9hours. We used an extract of fresh-seed and fruits with a concentration 1/10 w/v.
Specific IgE measurementsTotal IgE to different seed and fruits was determined by the CAP System IgE FEIA (Phadia Diagnostics, Uppsala, Sweden), following the manufacturer's instructions. The results were expressed in kU/L.
Anticardiolipin antibodiesIgG anticardiolipin antibodies were measured by ELISA following the commercial recommendations (INOVA Diagnostics. Inc. Anticardiolipin IgG –HRP). Patients were tested again for anticardiolipin IgG antibodies after two months when positive. The laboratory criteria that we have adopted following the commercial manufacturer indications in the kit were: 10-20GPL/ML low positive, 20-80 medium levels and >80 GPL/ML high positive.
SDS-PAGE ImmunoblottingWe performed the immunodetection with hazelnut in selected patients sensitised to cereal grains, nuts and fruits. We selected hazelnut because it is the only allergen that can be used successfully in treatment with specific immunotherapy.9
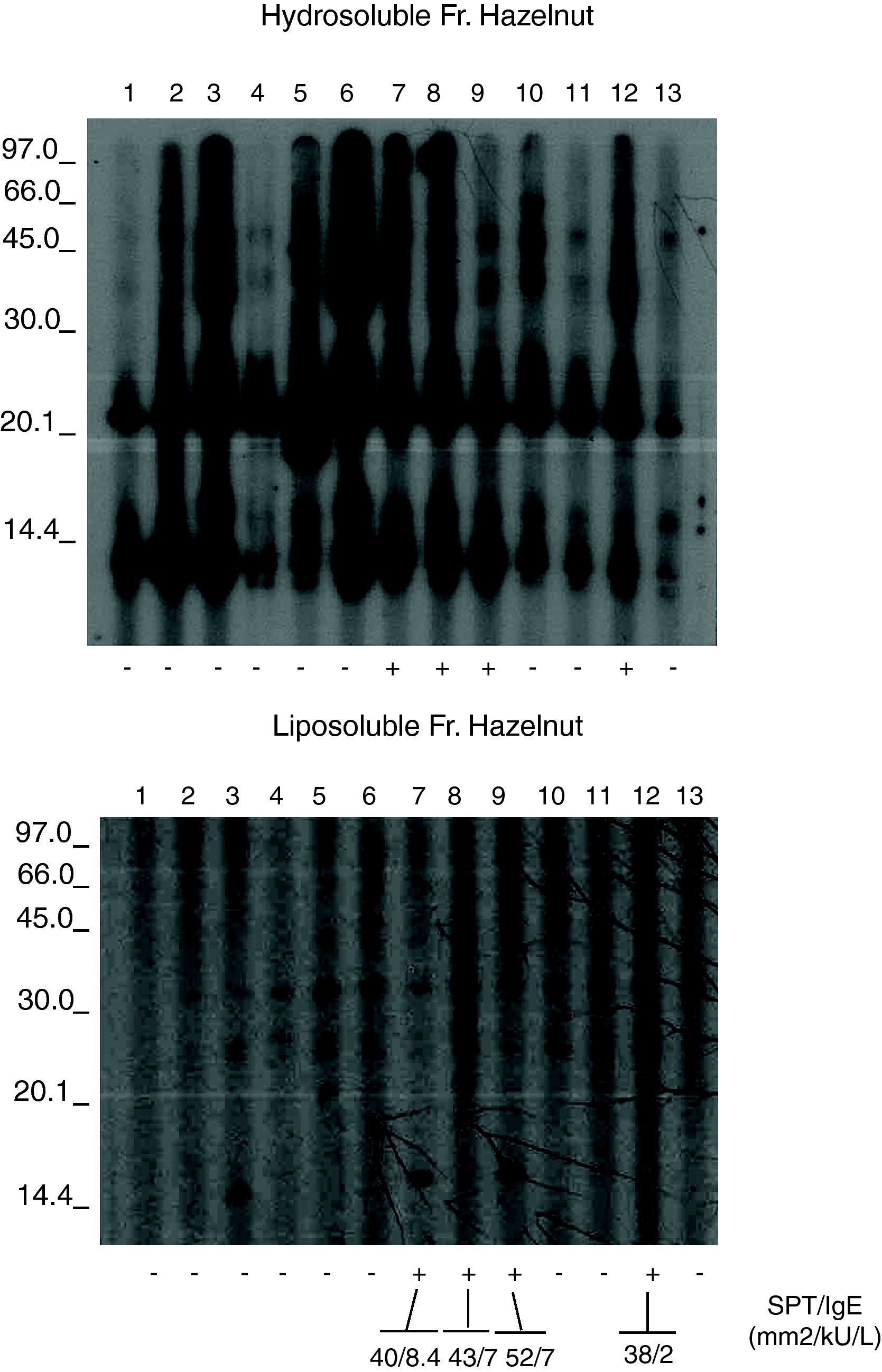
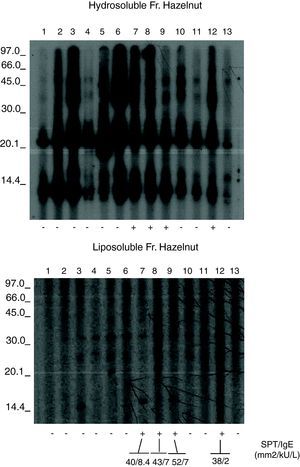
The hazelnut liposoluble and hydrosoluble fractions were separated by 15% SDS-PAGE and transferred to PVDF membranes (Immobilon, MilliporeCo., USA). The transferred fractions were placed in contact with the patient sera,6 and the IgE bound to the proteins was detected using mouse anti-human IgE (Fc)-HRP (SouthernBiotech, Birmingham, USA). Development in turn was based on chemiluminescence (ECL Plus WB Detection System, G.E. Healthcare, USA). (Fig. 1). Immunoblots were analysed in a GS-710 Image Analyser using the Diversity Database program (Bio Rad Laboratories, Hercules, CA, USA) following previously described methods.7–11
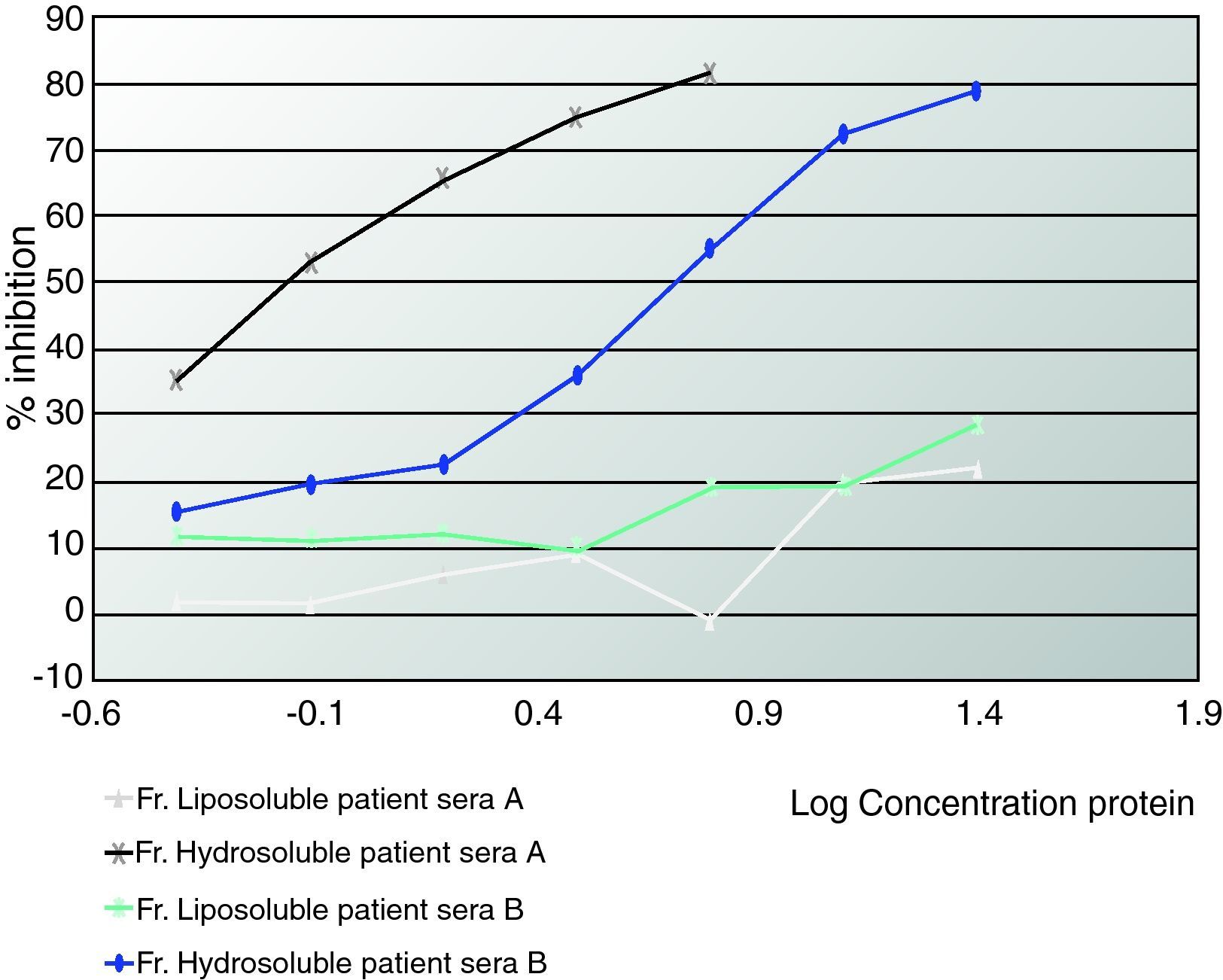
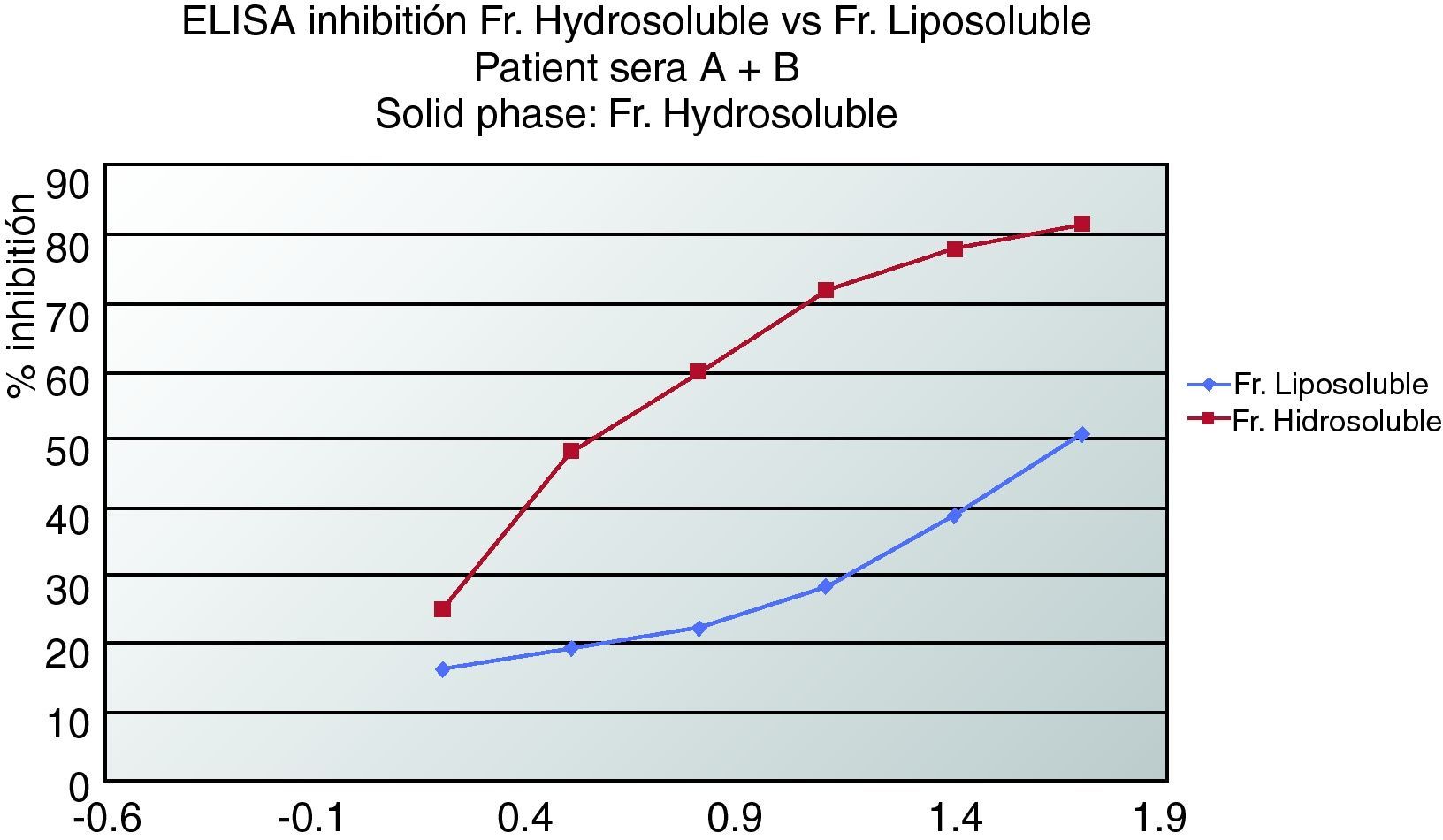
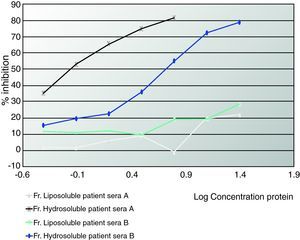
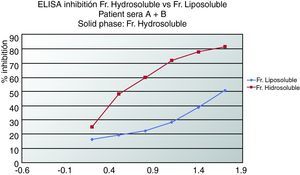
ELISA inhibitionPlates were coated with 0.02mg/mL of hydrosoluble proteins. Sera from patients who suffered from anaphylaxis were classified into two groups. Patients A had negative prick to seeds but positive IgE and immunodetection. Patients from group B presented positive prick, IgE and immunodetection. Patient sera from group A, patient sera from group B and both (A+B) were preincubated with hydrosoluble and liposoluble proteins at different protein concentration for two hours at room temperature. The bound IgE was detected by biotinylated mouse monoclonal antihuman IgE antibody (1:1000, 50 mcL/well; Operon, Zaragoza, Spain) followed by streptavidin-horseradish peroxidase-labelled antimouse IgG antibody (1:5000, 50 mcl/well; Sigma-Aldrich, St Louis, USA); IgE binding was detected by using a solution of 3,3’,5,5’-tetramethylbenzidine (50mcL/well; Sigma-Aldrich, St Louis, USA) and optical densities were read at 450nm. (Fig. 2, 3).
ELISA inhibition with “pools” of patients’ sera with aCL that detected liposoluble fractions from seeds (oleosins).The patients that inhibited with oleosins (group A) were all women with primary APS.
Group A: Patients with Prick (-), IgE (+) and immunodetection (+).
Group B: Patients with Prick (+), IgE (+) and immunodetection (+).
Sera from patients not sensitised to fruit, as well as dilution buffer, were used as negative controls.
Statistical analysisData were analysed using the package SPPS for Windows v. 11.5 (SSPS Inc, 1989-1999 Chicago IL, EEUU).
Informed consentWritten informed consent was obtained from all study participants.
Ethical ApprovalEthical approval was obtained from the Ethical Committee of the Rio Hortega University Hospital.
ResultsThe mean age of the patients who suffered from anaphylaxis was 20.9±9.7 years. Of these patients, 21 were males and 31 females. Among the patients sensitised to nuts, all in group A (negative prick, positive IgE) were female, age 20±6.2 years, whereas all in B were male, age 18±2.6years.
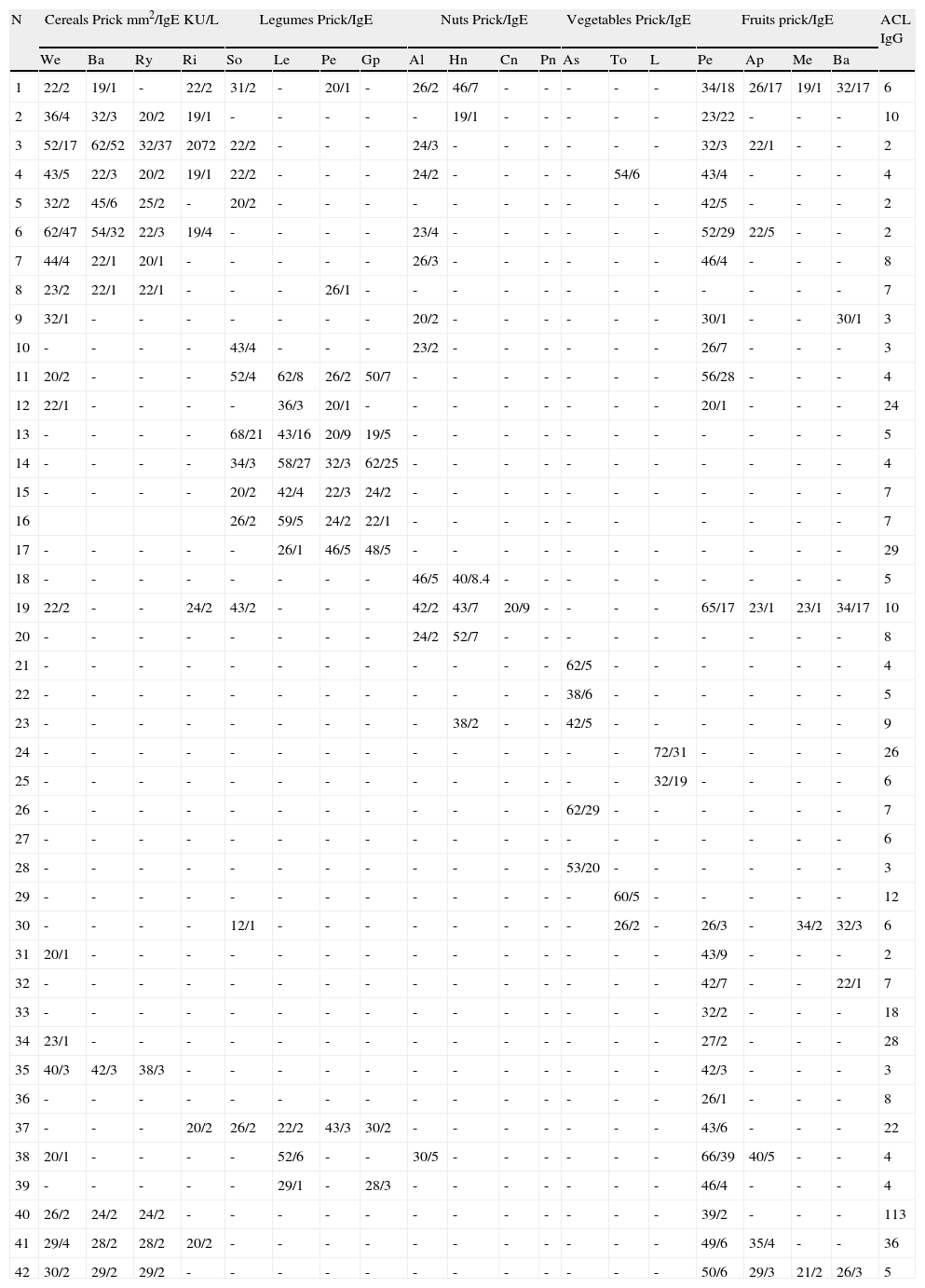
Among the 52 patients affected with anaphylaxis, 15 (28%) presented aCL (levels moderate-high) and related symptoms: 12 patients suffered from thrombosis: two of them of both iliac veins, four of either iliac or cava, one intestinal bleeding due to mesenteric thrombosis and three women presented foetal losses. Their sensitisation to different allergens can be seen in Tables 1.
Patients suffering from anaphylaxis due to different seeds, vegetables and fruits:.
| N | Cereals Prick mm2/IgE KU/L | Legumes Prick/IgE | Nuts Prick/IgE | Vegetables Prick/IgE | Fruits prick/IgE | ACL IgG | ||||||||||||||
| We | Ba | Ry | Ri | So | Le | Pe | Gp | Al | Hn | Cn | Pn | As | To | L | Pe | Ap | Me | Ba | ||
| 1 | 22/2 | 19/1 | - | 22/2 | 31/2 | - | 20/1 | - | 26/2 | 46/7 | - | - | - | - | - | 34/18 | 26/17 | 19/1 | 32/17 | 6 |
| 2 | 36/4 | 32/3 | 20/2 | 19/1 | - | - | - | - | - | 19/1 | - | - | - | - | - | 23/22 | - | - | - | 10 |
| 3 | 52/17 | 62/52 | 32/37 | 2072 | 22/2 | - | - | - | 24/3 | - | - | - | - | - | - | 32/3 | 22/1 | - | - | 2 |
| 4 | 43/5 | 22/3 | 20/2 | 19/1 | 22/2 | - | - | - | 24/2 | - | - | - | - | 54/6 | 43/4 | - | - | - | 4 | |
| 5 | 32/2 | 45/6 | 25/2 | - | 20/2 | - | - | - | - | - | - | - | - | - | - | 42/5 | - | - | - | 2 |
| 6 | 62/47 | 54/32 | 22/3 | 19/4 | - | - | - | - | 23/4 | - | - | - | - | - | - | 52/29 | 22/5 | - | - | 2 |
| 7 | 44/4 | 22/1 | 20/1 | - | - | - | - | - | 26/3 | - | - | - | - | - | - | 46/4 | - | - | - | 8 |
| 8 | 23/2 | 22/1 | 22/1 | - | - | - | 26/1 | - | - | - | - | - | - | - | - | - | - | - | - | 7 |
| 9 | 32/1 | - | - | - | - | - | - | - | 20/2 | - | - | - | - | - | - | 30/1 | - | - | 30/1 | 3 |
| 10 | - | - | - | - | 43/4 | - | - | - | 23/2 | - | - | - | - | - | - | 26/7 | - | - | - | 3 |
| 11 | 20/2 | - | - | - | 52/4 | 62/8 | 26/2 | 50/7 | - | - | - | - | - | - | - | 56/28 | - | - | - | 4 |
| 12 | 22/1 | - | - | - | - | 36/3 | 20/1 | - | - | - | - | - | - | - | - | 20/1 | - | - | - | 24 |
| 13 | - | - | - | - | 68/21 | 43/16 | 20/9 | 19/5 | - | - | - | - | - | - | - | - | - | - | - | 5 |
| 14 | - | - | - | - | 34/3 | 58/27 | 32/3 | 62/25 | - | - | - | - | - | - | - | - | - | - | - | 4 |
| 15 | - | - | - | - | 20/2 | 42/4 | 22/3 | 24/2 | - | - | - | - | - | - | - | - | - | - | - | 7 |
| 16 | 26/2 | 59/5 | 24/2 | 22/1 | - | - | - | - | - | - | - | - | - | - | 7 | |||||
| 17 | - | - | - | - | - | 26/1 | 46/5 | 48/5 | - | - | - | - | - | - | - | - | - | - | - | 29 |
| 18 | - | - | - | - | - | - | - | - | 46/5 | 40/8.4 | - | - | - | - | - | - | - | - | - | 5 |
| 19 | 22/2 | - | - | 24/2 | 43/2 | - | - | - | 42/2 | 43/7 | 20/9 | - | - | - | - | 65/17 | 23/1 | 23/1 | 34/17 | 10 |
| 20 | - | - | - | - | - | - | - | - | 24/2 | 52/7 | - | - | - | - | - | - | - | - | - | 8 |
| 21 | - | - | - | - | - | - | - | - | - | - | - | - | 62/5 | - | - | - | - | - | - | 4 |
| 22 | - | - | - | - | - | - | - | - | - | - | - | - | 38/6 | - | - | - | - | - | - | 5 |
| 23 | - | - | - | - | - | - | - | - | - | 38/2 | - | - | 42/5 | - | - | - | - | - | - | 9 |
| 24 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 72/31 | - | - | - | - | 26 |
| 25 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 32/19 | - | - | - | - | 6 |
| 26 | - | - | - | - | - | - | - | - | - | - | - | - | 62/29 | - | - | - | - | - | - | 7 |
| 27 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 6 |
| 28 | - | - | - | - | - | - | - | - | - | - | - | - | 53/20 | - | - | - | - | - | - | 3 |
| 29 | - | - | - | - | - | - | - | - | - | - | - | - | - | 60/5 | - | - | - | - | - | 12 |
| 30 | - | - | - | - | 12/1 | - | - | - | - | - | - | - | - | 26/2 | - | 26/3 | - | 34/2 | 32/3 | 6 |
| 31 | 20/1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 43/9 | - | - | - | 2 |
| 32 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 42/7 | - | - | 22/1 | 7 |
| 33 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 32/2 | - | - | - | 18 |
| 34 | 23/1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 27/2 | - | - | - | 28 |
| 35 | 40/3 | 42/3 | 38/3 | - | - | - | - | - | - | - | - | - | - | - | - | 42/3 | - | - | - | 3 |
| 36 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 26/1 | - | - | - | 8 |
| 37 | - | - | - | 20/2 | 26/2 | 22/2 | 43/3 | 30/2 | - | - | - | - | - | - | - | 43/6 | - | - | - | 22 |
| 38 | 20/1 | - | - | - | - | 52/6 | - | - | 30/5 | - | - | - | - | - | - | 66/39 | 40/5 | - | - | 4 |
| 39 | - | - | - | - | - | 29/1 | - | 28/3 | - | - | - | - | - | - | - | 46/4 | - | - | - | 4 |
| 40 | 26/2 | 24/2 | 24/2 | - | - | - | - | - | - | - | - | - | - | - | - | 39/2 | - | - | - | 113 |
| 41 | 29/4 | 28/2 | 28/2 | 20/2 | - | - | - | - | - | - | - | - | - | - | - | 49/6 | 35/4 | - | - | 36 |
| 42 | 30/2 | 29/2 | 29/2 | - | - | - | - | - | - | - | - | - | - | - | - | 50/6 | 29/3 | 21/2 | 26/3 | 5 |
Anaphylaxis due to cereals: Patients 1 to 9: We: wheat, Ba: barley, Ry: Rye, Ri: Rice.
Anaphylaxis due to legumes: Patients 10 to 17: So: Soy bean, Le: lentil, Pe: peanut, Gp: Green pea.
Anaphylaxis due to nuts: Patients 12 to 23: Al: Almond, Hn: Hazel nut, Cn: chestnut, Pn: Pine nut.
Anaphylaxis due to vegetables: Patients 24 to 29: As: Asparagus, To. Tomato, Le: leak.
Anaphylaxis due to fruits: Patients 30 to 42. Pe: peach, Ap: apple, Me: melon, Ba: banana.
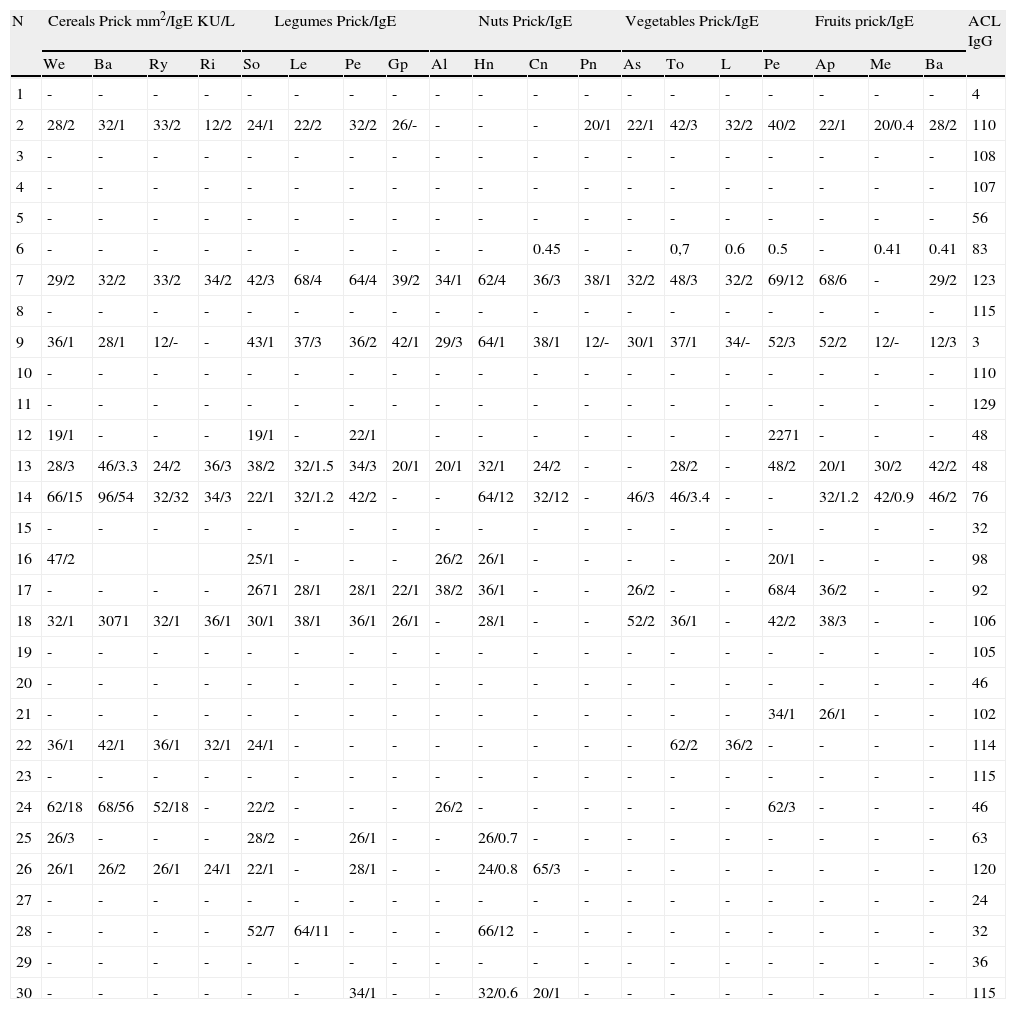
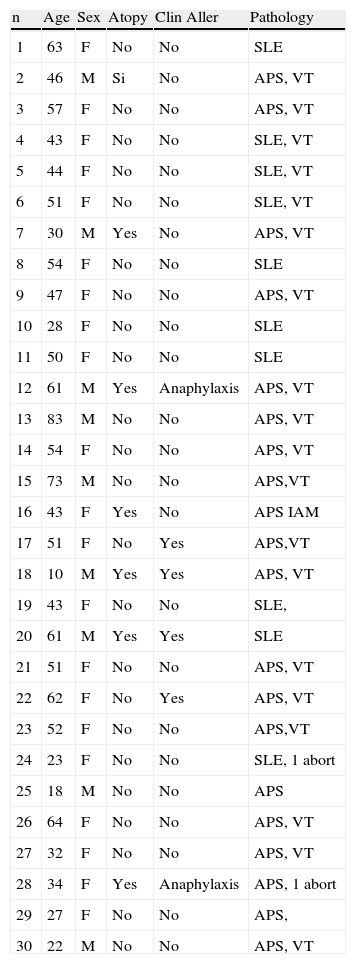
Among the 30 patients diagnosed previously of APS and thrombosis (Table 3), nine were male and 21 female, mean age 45.9±19.9 years. Their mean age was significantly higher than the mean age of the persons with anaphylaxis (p<0.0001). Both in the primary APS and in SLE, women were more common (60% and 90% respectively). As a whole - 15 patients- they all suffered from APS, and presented hypersensitivity to seeds and fruits, confirmed by allergy study. Two of these patients suffered anaphylaxis but they did not know the reason. None of the patients with SLE presented sensitisation to vegetables. None of the control patients had positive aCL levels. Symptoms and allergic response can be seen in Table 2.
Patients with APS and thrombosis.
| N | Cereals Prick mm2/IgE KU/L | Legumes Prick/IgE | Nuts Prick/IgE | Vegetables Prick/IgE | Fruits prick/IgE | ACL IgG | ||||||||||||||
| We | Ba | Ry | Ri | So | Le | Pe | Gp | Al | Hn | Cn | Pn | As | To | L | Pe | Ap | Me | Ba | ||
| 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 4 |
| 2 | 28/2 | 32/1 | 33/2 | 12/2 | 24/1 | 22/2 | 32/2 | 26/- | - | - | - | 20/1 | 22/1 | 42/3 | 32/2 | 40/2 | 22/1 | 20/0.4 | 28/2 | 110 |
| 3 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 108 |
| 4 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 107 |
| 5 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 56 |
| 6 | - | - | - | - | - | - | - | - | - | - | 0.45 | - | - | 0,7 | 0.6 | 0.5 | - | 0.41 | 0.41 | 83 |
| 7 | 29/2 | 32/2 | 33/2 | 34/2 | 42/3 | 68/4 | 64/4 | 39/2 | 34/1 | 62/4 | 36/3 | 38/1 | 32/2 | 48/3 | 32/2 | 69/12 | 68/6 | - | 29/2 | 123 |
| 8 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 115 |
| 9 | 36/1 | 28/1 | 12/- | - | 43/1 | 37/3 | 36/2 | 42/1 | 29/3 | 64/1 | 38/1 | 12/- | 30/1 | 37/1 | 34/- | 52/3 | 52/2 | 12/- | 12/3 | 3 |
| 10 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 110 |
| 11 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 129 |
| 12 | 19/1 | - | - | - | 19/1 | - | 22/1 | - | - | - | - | - | - | - | 2271 | - | - | - | 48 | |
| 13 | 28/3 | 46/3.3 | 24/2 | 36/3 | 38/2 | 32/1.5 | 34/3 | 20/1 | 20/1 | 32/1 | 24/2 | - | - | 28/2 | - | 48/2 | 20/1 | 30/2 | 42/2 | 48 |
| 14 | 66/15 | 96/54 | 32/32 | 34/3 | 22/1 | 32/1.2 | 42/2 | - | - | 64/12 | 32/12 | - | 46/3 | 46/3.4 | - | - | 32/1.2 | 42/0.9 | 46/2 | 76 |
| 15 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 32 |
| 16 | 47/2 | 25/1 | - | - | - | 26/2 | 26/1 | - | - | - | - | - | 20/1 | - | - | - | 98 | |||
| 17 | - | - | - | - | 2671 | 28/1 | 28/1 | 22/1 | 38/2 | 36/1 | - | - | 26/2 | - | - | 68/4 | 36/2 | - | - | 92 |
| 18 | 32/1 | 3071 | 32/1 | 36/1 | 30/1 | 38/1 | 36/1 | 26/1 | - | 28/1 | - | - | 52/2 | 36/1 | - | 42/2 | 38/3 | - | - | 106 |
| 19 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 105 |
| 20 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 46 |
| 21 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 34/1 | 26/1 | - | - | 102 |
| 22 | 36/1 | 42/1 | 36/1 | 32/1 | 24/1 | - | - | - | - | - | - | - | - | 62/2 | 36/2 | - | - | - | - | 114 |
| 23 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 115 |
| 24 | 62/18 | 68/56 | 52/18 | - | 22/2 | - | - | - | 26/2 | - | - | - | - | - | - | 62/3 | - | - | - | 46 |
| 25 | 26/3 | - | - | - | 28/2 | - | 26/1 | - | - | 26/0.7 | - | - | - | - | - | - | - | - | - | 63 |
| 26 | 26/1 | 26/2 | 26/1 | 24/1 | 22/1 | - | 28/1 | - | - | 24/0.8 | 65/3 | - | - | - | - | - | - | - | - | 120 |
| 27 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 24 |
| 28 | - | - | - | - | 52/7 | 64/11 | - | - | - | 66/12 | - | - | - | - | - | - | - | - | - | 32 |
| 29 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | 36 |
| 30 | - | - | - | - | - | - | 34/1 | - | - | 32/0.6 | 20/1 | - | - | - | - | - | - | - | - | 115 |
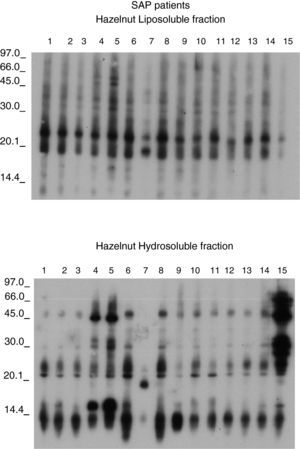
Figures 1 and 2 shows the results of the immunoblotting and ELISA-inhibition experiments carried out. Several IgE- binding bands were detected both in hydrosoluble (ranging from around 25 to 100 KDa) and in liposoluble fractions (membrane lipoproteins) among the oleosins involved (with molecular masses around 16.7 and 14.7 kDa).
General data of 30 patients diagnosed of thrombosis associated with aCL.
| n | Age | Sex | Atopy | Clin Aller | Pathology |
| 1 | 63 | F | No | No | SLE |
| 2 | 46 | M | Si | No | APS, VT |
| 3 | 57 | F | No | No | APS, VT |
| 4 | 43 | F | No | No | SLE, VT |
| 5 | 44 | F | No | No | SLE, VT |
| 6 | 51 | F | No | No | SLE, VT |
| 7 | 30 | M | Yes | No | APS, VT |
| 8 | 54 | F | No | No | SLE |
| 9 | 47 | F | No | No | APS, VT |
| 10 | 28 | F | No | No | SLE |
| 11 | 50 | F | No | No | SLE |
| 12 | 61 | M | Yes | Anaphylaxis | APS, VT |
| 13 | 83 | M | No | No | APS, VT |
| 14 | 54 | F | No | No | APS, VT |
| 15 | 73 | M | No | No | APS,VT |
| 16 | 43 | F | Yes | No | APS IAM |
| 17 | 51 | F | No | Yes | APS,VT |
| 18 | 10 | M | Yes | Yes | APS, VT |
| 19 | 43 | F | No | No | SLE, |
| 20 | 61 | M | Yes | Yes | SLE |
| 21 | 51 | F | No | No | APS, VT |
| 22 | 62 | F | No | Yes | APS, VT |
| 23 | 52 | F | No | No | APS,VT |
| 24 | 23 | F | No | No | SLE, 1 abort |
| 25 | 18 | M | No | No | APS |
| 26 | 64 | F | No | No | APS, VT |
| 27 | 32 | F | No | No | APS, VT |
| 28 | 34 | F | Yes | Anaphylaxis | APS, 1 abort |
| 29 | 27 | F | No | No | APS, |
| 30 | 22 | M | No | No | APS, VT |
Pathology: SLE: Systemic Lupus Erythematosus, APS: Antiphospholipid primary Syndrome, VT: Venous thrombosis.
The recognition patterns obtained from the patients’ sera presented a common result not found in any controls. The nine patients with negative prick showed negative specific IgE but a positive recognition of proteins from hydrosoluble and liposoluble fractions of hazelnut. All these patients were young women (20±6 years). These responses were both soluble protein (up in the figure) and to lipoproteins (below). The response in patients with negative prick could be explained because the commercial available extracts are defatted, without lipoproteins.
In Figure 3 we can see an ELISA-inhibition test with sera pools from patients with aCL and which detected lipoproteins from seeds. The patient's sera that inhibited with lipoproteins were all from women suffering from APS.
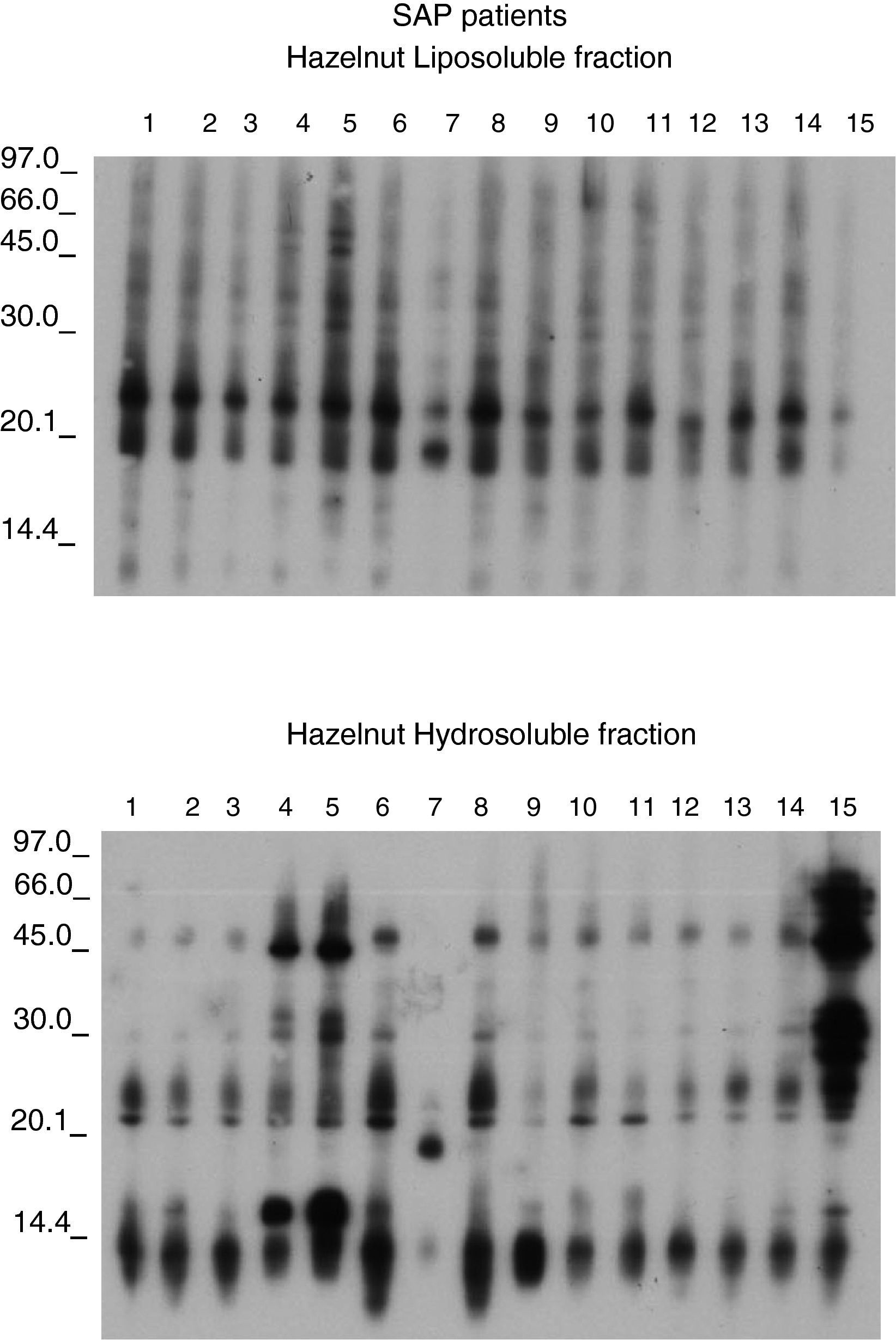
The sera from the 15 patients with primary APS showed IgE detection to lipoproteins belong to the membrane oleosins (16.7 and 14.7 KDa). (Figure 4). The membrane lipoproteins from the oleosin fraction were able to produce allergic sensitisation, a fact not demonstrated to date.
In summary, 75% of the patients diagnosed as having antiphospholipid primary syndrome had specific IgE against different proteins from different vegetable allergens, most of them seeds, and clearly against lipoproteins that were also recognised by the patients with food anaphylaxis but not by the control cases. Among the patients with anaphylaxis, 28% had anticardiolipin antibodies and 17.3% thrombosis.
DiscussionAnaphylaxis is a potential life-threatening condition. Prevention of anaphylaxis and anaphylactoid reactions concerns all physicians. To maximise the chances of preventing recurrences, the aetiology should be determined.12 Nevertheless, it can be difficult to identify the causes, as well as to predict unexpected complications.
This is the second known report of food anaphylaxis associated to primary antiphospholipid syndrome (APS). APS is diagnosed when arterial or venous thrombosis or recurrent miscarriages occur in a person in whom laboratory tests for antiphospholipid antibodies (aPL), namely lupus anticoagulant (LA), anticardiolipin antibodies (aCL) or antibodies directed to various proteins, mainly beta-2-glycoprotein (β2GPI), are positive.13
Evidence shows that some patients with antibodies to phospholipids are exposed to episodes of venous or arterial thrombosis, repeated foetal loss and thrombocytopenia.14–17 It is also likely that the lupus anticoagulant and anticardiolipin antibody tests detect antibodies with overlapping specificities.18 The term primary APS has been introduced as a mean of categorising and studying those patients with APS who do not meet criteria for SLE or any other well-defined diseases like infections or neoplasia.17 Some clinicians have proposed that livedo reticularis, migraine headaches, chorea, peripheral artery occlusions, endocardial lesions, adult respiratory distress syndrome, avascular necrosis of the bone and transverse mielopathy might be features of APS.17,18 Because of this overlap, the true clinical and serological boundaries of APS are vague and will probably remain so until these patients are studied over time.
Given the heterogeneity of clinical manifestation in APS it is likely that more than one pathophysiological process may play a role.
In the case of our patients, aPL antibodies were persistent and could be related with the previous episodes of anaphylaxis. Deep vein thrombosis was the most frequent manifestation. In 3 cases, foetal losses were suffered recurrently.
The levels of aPL antibodies in our allergic patients were lower than those in the patients previously diagnosed of APS, but the age and evolution time in the last group was higher. In this respect aPL have been found in approximately 12% of elderly populations and in 2% of younger populations.13
Different genetic, environmental,19–21 and immunological responses to infectious agents,22 drugs or neoplasm23 have been related with the production of aPL, but never with food allergy.
Food allergy hypersensitivity reactions have increased in the last decade, and are currently estimated to affect between 2–8% of the population, with a special incidence of food of plant origin in adult patients.24 However, methods of diagnosis and treatment (potential immunotherapy), as well as knowledge of the mechanisms of awareness, development of clinical symptoms and reactions to different allergenic sources (various foods and/or pollens), in general, are inadequate and incomplete.
Our patients’ sera showed an IgE binding to several proteins included in hydrosoluble and liposoluble fractions (membrane lipoproteins) among which oleosins are involved. This response was not diagnosed under conventional protein extraction protocols. Then, membrane lipoproteins (oil body fraction contained) could have the capacity to sensitise.
The allergenic components of foods previously described include primarily glycoproteins. Despite the fact that closely related foods (legumes, cereal grains and other seeds) frequently contain allergens that cross-react immunologically (prick, RAST), they rarely cross-react clinically.25 Allergy to Rosaceae fruits (peach, apple, pear) is frequently associated with birch pollinosis in The United States, Canada, and Central and Northern Europe. This cross-sensitisation has been explained mainly by the presence of homologous allergens in both the pollen from trees of the Fagales order and in the fruit/vegetables mentioned. Proteins of 16 to 18 Kd that share common IgE epitopes with Bet v 1, the major birch pollen allergen, have been characterised in alder, hazel and hornbeam pollens, and in celery, cherry and carrot. Nevertheless, profilin and Bet v 1-related structures are not involved in Rosaceae fruit allergy without pollinosis. In these patients the symptoms are usually more severe, as in our patients.26 The patients allergic to Rosaceae fruits and seeds whose sera were assayed in this study lived in cereal-producing areas free of birch and other Fagales trees and therefore could not be directly sensitised to their pollens. In this type of allergic population, which is frequent in the Mediterranean countries, a major 13 Kd allergen presented in various Rosaceae fruits has been detected.1,2 In our study, the patients presented sensitisation to different vegetable foods, but Rosaceae fruits and seeds appear to have an important clinical role. Recently, we have purified and identified lipid transfer proteins (LTPs) from peach and wheat grain27 and we have previously assayed in immunoblotting experiences a hazelnut extract that behaves as a very stable and useful product to investigate sensitisation to seeds and specific immunotherapy.9 We decided on the use of this extract to unify our results and to find something in common among the patients. Our results fully confirmed the relevance of glycoproteins and membrane lipoproteins allergens because they were recognised clearly by all the sera tested.
It was originally proposed that LTPs play a role in the cellular trafficking of lipids, but this hypothesis has now been largely abandoned because it is not consistent with several properties of this group of polypeptides, mainly with its extracellular location. By contrast, LTPs are truly involved in the defence mechanism of plants against pathogens.28 In this context, it is possible that the lipoproteins detected in this experience might be classified as defence proteins considering the uniformity and ubiquity of the antibodies in these patients.
Is it possible that there is a relationship between the sensitisation to these antigens and the APS suffered from our patients?
In this regard, it is tempting to suggest that these glyco and lipopoproteins, to which our patients had immunological responses, might have a similar behaviour to β2-GP-I, the necessary cofactor for the interaction between aCL and the antigen.29 This is supported by the similar structure of human and vegetable membrane. Lipoproteins have a phospolipid bilayer shared by all eukaryotic cells8 including vascular endothelial and vegetal cells. Studies in APS have shown a remodelling of the membrane phospholipids bilayer in response to activation or apoptosis, that was defined as membrane-coated small vesicles that are membrane-coated and released from the plasma membrane by exocitic budding. These vesicles express negatively charged phospholipids and cell surface antigens characteristics of the cells of origin.8 Surface exposure of phosphatidylserine or tissue factor activity provides a catalytic surface that supports the assembly of clotting enzymes complexes, leading to thrombin generation.
The thrombosis may be due to an alteration of the kinetics of the normal procoagulant and anticoagulant reactions by cross-linking membrane-bound proteins, by blocking protein-protein interactions or by blocking the access of other proteins to the phospholipid membrane.30
There is a platelet role in anaphylaxis, and some data suggest that platelet activation in APS patients is related to anti β2-GP-I antibodies.31,32 In this way, endothelial activation in APS has been described to be mediated through Toll-like receptors (TLR4), resulting in a prothrombotic and pro-inflammatory phenotype with synthesis and secretion of adhesion molecules and pro-inflammatory cytokines very similar to those released during anaphylactic response to vegetal foods.31
The prevalence of food allergies has increased over the past fifteen years. Recent studies have found that 17% of young adults reported that particular food or foods nearly always cause illness or trouble when eaten, presumably due to either food intolerance or food allergy.32 The reasons suggested are changes in dietary behaviour and the evolution of food technologies. New food-processing techniques can increase the allergenicity or create neo-allergens; storage can also induce the synthesis of allergenic stress or PR proteins.33 Genetically-modified plants have risks of allergenicity, and methodological steps of investigations as well as the studies with sera from allergic patients are required. On the other hand, the combination of enzymatic hydrolysis, heating, or the development of genetically-modified plants may offer new alternatives towards hypoallergenic foods.34
With respect to the treatment of APS with thrombosis, there is consensus in treating patients with oral anticoagulation to a target International Normalised Ratio of 2.0 to 3.0.35 The approach for women with obstetric manifestations is based on the use of aspirin plus heparin.36 In addition to this we recommended these measures and a diet without the putative vegetal allergen to our patients. None of our patients has suffered from new episodes of thrombosis in five years and one woman had her first child successfully.
In summary, despite the strong association between aPL and thrombosis, the pathogenic role of these antibodies in the development of thrombosis has not been fully elucidated. A novel mechanism involving hypersensitivity to lipoproteins allergens is described here. Nevertheless, our preliminary observations must be confirmed in further studies. The knowledge of these new pathogenic approaches might identify novel therapeutic targets and therefore may improve the management of these patients.
Conflict of interestThe authors report no conflict of interest.
Declaration of all sources of fundingThis work was evaluated by the General Direction of Public Health, Research, Innovation and Development, Sanidad Castilla y León. (SACYL) and registered in its data base.