INTRODUCTION
Food protein induced enterocolitis syndrome (FPIES), typically appears from the first month of life, usually induced by cow's milk and or soy protein. It was first described by Gryboski in 1967 1, and its main symptoms consist on vomiting ensues, diarrhea, failure to thrive in infant and may progress to acidemia and shock. Symptoms resolve after the responsible protein is removed from the diet, and recur after the reintroduction of the protein, generally within 2 hours, with symptoms that may lead to lethargy, dehydration, hypotension and metabolic acidosis 2,3.
There are an increase number of reports pointing of additional causatives solid food such as rice 2,4,5, poultry, egg, grains, vegetables and peanut 2,6,7, that could trigger this syndrome. There is only one case report of FPIES induced by fish 8, even though this food is often clinically suspected in pediatric patients by allergist. The disorder is a form of cell-mediated, non-immunoglobulin E (IgE) antibody- associated food hypersensitivity. Recent studies suggest that tumor necrosis factor α (TNF-α) from T cells upon milk stimulation is involved in the pathophysiology of FPIES 9. A relative lack of expression of transforming growth factor-β (TGF-β) may also be implicated 10.
Our group has studied 14 infants with FPIES due to dietary fish protein. In this study we report the clinical characteristics of these patients and their clinical course.
MATERIAL AND METHODS
We reviewed the medical history of those patients sended to our Allergy Department referring gastrointestinal symptoms after ingestion of fish in which hypersensitivity IgE mediated was to ruled out.
The patients underwent a detailed clinical and nutritional history, physical examination, Skin Prick Test (SPT) for food allergens, Atopy Patch Test (APT), serum specific IgE antibodies against fish, and an open oral food challenge.
SPT were performed with commercial extracts to fish (Leti Laboratories), and Anisakis simplex (IPI Laboratories), and prick by prick with the suspicious fish (hake, sole or cork float), positive (histamine) and negative (Glycerol saline) were included. SPT were considered positive if the mean wheal diameter was at least 3 mm greater than the saline control.
Serum fish-specific IgE antibodies were measured by the CAP System TM (Pharmacia-Uppsala, Sweden), being the lower limit of detection 0, 35 KU/L.
The APT was performed in 8 children using the same extracts used for SPT and cooked fish (boiled). We used Curatest adhesive strips on the back, and kept the occlusion for 48 hours. The APT was read 30 minutes after the removal of the cups, and after 96 hours. The APT reading were based on the assessment criteria recommended by ICDRG 11.
The diagnosis of FPIES was based on clinical criteria and in 9 patients on physician supervised oral food challenges after written informed consent was obtained. The open oral challenge was performed initially with the eighth portion of a normal serving size amount per age, and was gradually increased by 2 every 30 minutes to a full serve. The patients remained under physician supervision for three hours, regardless of the outcome of the challenge.
RESULTS
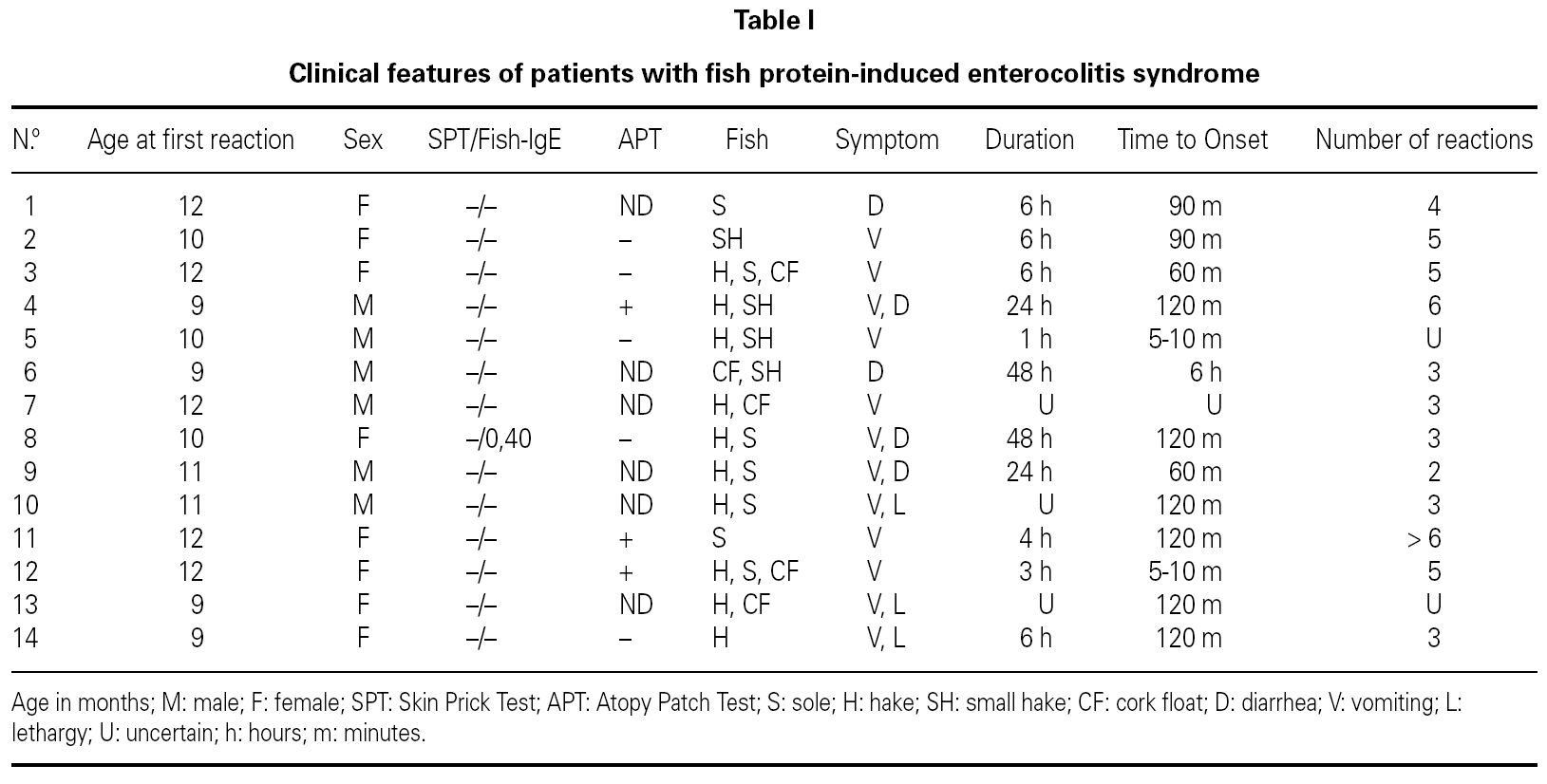
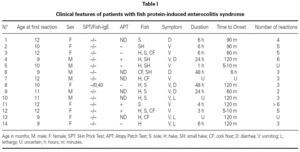
The patient's clinical data are shown in table I.
We report 14 patients (6 males and 8 females), aged between 9-12 months at the time of diagnosis.
Four patients had previous history of atopy (asthma in 4 cases, atopic dermatitis in 2 and one patient presented recurrent urticaria not related to food). Three patients referred family history of atopy. One patient's father had a clinical anamnesis consistent with FPIES induced by fish in his early childhood. The involved fish were hake, sole and cork float; 10 of them (71 %) had symptoms with more than one fish. Symptoms begun a few minutes after ingestion of fish in 2 cases, and between 60 minutes to 6 hours in 12 remaining patients. Symptoms persisted 3 to 48 hours.
Six patients had 2 to 3 reactions to the offending fish's proteins before the diagnosis was established, 5 patients 4-6 episodes and 1 had 6 incidents.
The presenting symptoms included diarrhea in 2 cases, profuse vomiting in 6, 3 infants had recurrent vomiting and subsequently diarrhea. The remaining 3 patients associated septic appearance, apathy and lethargy, to the previous symptoms described.
SPT with commercial extracts to fish and prick-prick with boiled fish, were negative in all cases. APT was positive in 3 patients. Serum food-specific IgE antibodies to the responsible fishes were negative in all cases, except patient n.º 8, who had positive serum-specific IgE antibodies to hake (0,40 KU/L).
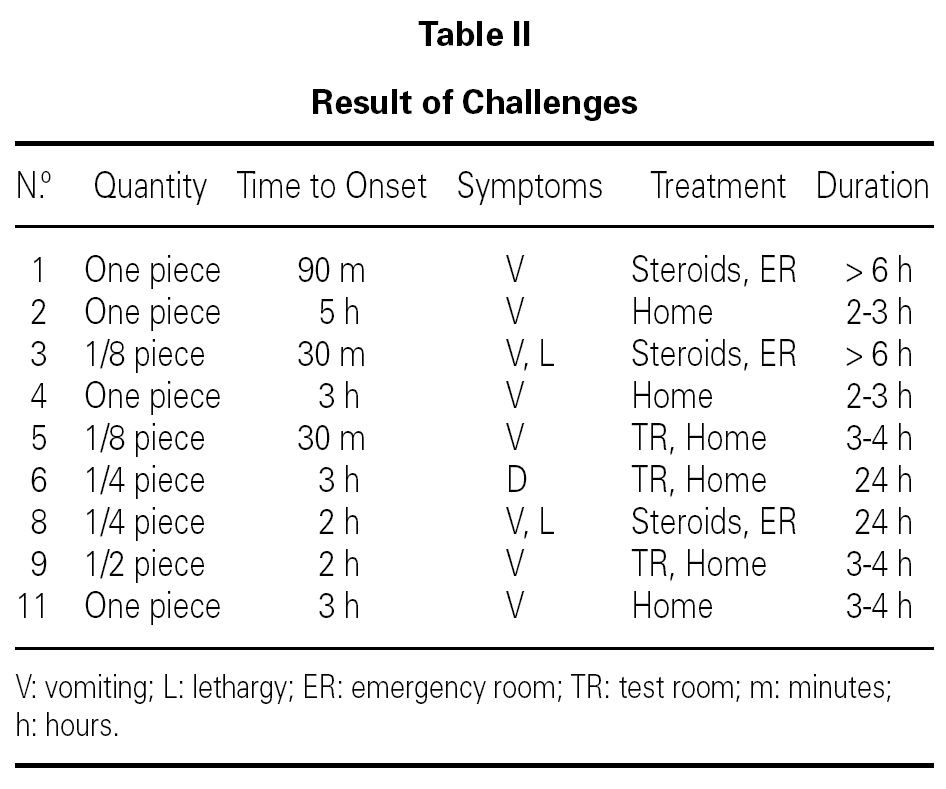
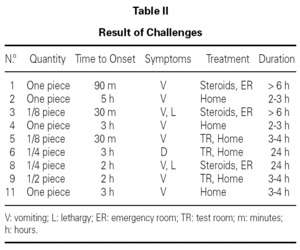
Oral food diagnostic challenge were performed in 9 infants, being positive in all of them (table II). Two patients developed symptoms after the first dose, and in 4, symptoms took place after the hole serving was given. Three patients required emergency treatment, with intravenous fluids and corticosteroids, for a few hours. One of them remained hospitalized for 24 hours. The rest 6 patients overcame symptoms and improved clinically just with oral hydration. The remaining 5 infants did not undergo the oral challenge, because they referred various evocative episodes of FPIES.
Blood test and stool culture was performed in 3 patients after positive oral challenge. Neutrophilia was not found and the stool culture were negative.
One patient suffered celiac disease, and 2 infants had previous history of egg allergy IgE mediated. Once FPIES induced by fish diagnosis was achieved, one patient developed twice, vomiting and diarrhea, 2 hours after melon ingestion. SPT as well as serum specific IgE antibodies to melon were negative.
Follow-up
We monitored patient's clinical evolution 1-7 years after diagnosis. After an elimination diet of 3-4 year, we undertook follow-up oral challenge. Four infants became clinically tolerant to the causal food. Three patients currently tolerate one single fish (swordfish).
Two patients continued fish elimination diet over 5 years, and their parents do not wish to have them re-challenged at the present time.
About the 5 remaining patients, 2 had a positive re-challenge with hake in last visit, so they will continue diet; 2 infants had a recent diagnosis, and finally, patient 8, who removed fish's protein of diet 3 years ago, has developed recently a sensitization to melon that suggest FPIES by melon, so we would prefer to wait for the re-challenge.
DISCUSSION
FPIES is a severe syndrome of vomiting and diarrhea typically caused by cow's milk or soy protein in infants. The disorder is probably cell-mediated and occurs with negative allergy skin prick test and the absence of serum allergen-specific IgE antibodies 2.
The prevalence of FPIES in the general pediatric population is unknown but it is considered to be a rare form of gastrointestinal food hypersensitivity.
We report the first series of infants with FPIES caused by fish proteins. The involved fishes were: hake, sole and cork float, usually consumed by children in our country. Ten patients (71 %) had previous history of reaction to more than one fish. None children reacted to cow's milk, 2 infants presented egg allergy IgE mediated, and one patient had vomiting and diarrhea after melon ingestion.
Pathology related to foods is contributed by its consumption pattern; Spain holds the second place amongst European Union's fish consumers. 12 In Spain, fish is usually introduced in children feeding before the first year of life.
Our patients had a median of 4 reactions (2 to 6), before the diagnosis was achieved. This coincides with Nowak-Wegrzyn 7 series of patients, with a median of 2 reactions (range 2 to 5) before diagnosis. Delayed diagnosis is particularly common in these patients, probably because of the uncommon nature of the disorder, the lack of a confirmatory specific diagnostic test, and the unspecificity of the symptoms that similar to episodes of infectious gastroenteritis. The time course of reactions can also confound the diagnosis. Young infants with FPIES to cow's milk or soy which are exposed to these proteins on a daily basis, typically manifest chronic symptoms. In FPIES caused by solids, the presentations tended to be more severe, because the context of intermittent exposures to the dietary food proteins in slightly older infants.
Our report confirmed previous observations that measurements of food allergen-specific IgE antibodies (SPT or serum levels) are typically negative 2,11. Some authors have point out that the development of specific IgE antibodies in FPIES induced by milk or soy, seems to be a sign of poor prognosis for tolerance 2. Only one of our patients had detectable IgE for hake (0, 40 KU/L).
There are increasing reports of FPIES induced by food other than cow's milk and soy. Nowak-Wegrzyn et al 7 reported 14 patients with FPIES induced by grains, vegetables and poultry; 5 of them had only sensitization to solid food and the 9 remaining patients also had food hypersensitivity to milk and/or soy. Levy and Danon 14 reported 6 patients with FPIES induced by poultry, legumes and soy, associating FPIES by milk in 3 of them. We want to point out that in our 14 patient's series, no one had previous history of FPIES provoked by cow's milk. We think this fact could have a hand in monosensitization to fish reported in 11 of our 14 patients, quite the opposite to most previous reports which described sensitization to more than one solid food, such as in Nowak-Wegrzyn et al 7 report, who described that 78 % of patients reacted to more than one food protein.
At diagnosis, all patients aged between 9 to 12 months, coinciding with the median age of diet fish introduction, such as we have commented previously, and clearly distinguished of FPIES due to milk or soy that triggers at an early age. To achieve a correct diagnosis, is necessary to consider the age distribution pattern related with the consumption of diverse food.
Three of our patients, had positive fish APT, without previous history of atopic dermatitis. Hypothetically, the APT, used with variable clinical utility for atopic dermatitis 15,16 or eosinophilic gastroenteritis 17, may be of use in the diagnosis of gastrointestinal allergy without evidence of IgE 18, but it has not been sufficiently evaluated in this disorder.
We performed blood testing to 3 of the patients. The polymorphonuclear leukocyte count of the 3 evaluated patients was normal, even though, we consider these results warily, because of the lack of previous cell counts and the limited number of samples.
Powell 19 suggested specific criteria for the diagnosis of milk or soy enterocolitis based on oral challenge. However, this challenge may be dangerous, so that, a confirmatory challenge would not be needed when the typical symptoms occur after ingestion of the food (particularly more than once) and there are no alternative explanations for the symptoms. Therefore the need for an oral food challenge to confirm the diagnosis must be determined on clinical grounds. Otherwise, this modality is used to monitor development of tolerance. Now a days, the better management for this disease is the dietary avoidance of the implicated food.
In patients follow up, 4 infants (28.5 %) became clinically tolerant after a 3-4 year-period elimination diet. Three patients currently tolerate one single fish (swordfish). Five patients continued fish elimination diet (for having a recent diagnosis in 2 infants, because their parents do not desire re-challenge yet in 2 patients, and for having a recent sensitization to another food in 1 infant). The remaining 2 patients had a positive re-challenge with hake in last visit, so they will continue diet.
In Nowak-Wegrzyn 7 series of 14 patients, 4 became tolerant to the causal food and 5 had new sensitizations. In our study, only one of 14 patients had a new sensitization (to melon), although 2 of them had previous egg IgE-mediated allergy. Only one patient of 6 achieved tolerance in Levy et al 14 report. Vitoria et al 8 described 3 children with cow's milk protein intolerance and associated enteropathy related to fish, rice and chicken respectively. In patient with FPIES induced by fish, after a period of fish dietary elimination, a normal intestinal biopsy was obtained. Afterwards, an oral food fish challenge was performed, being positive, and a second intestinal biopsy was carry out, demonstrating atrophy of jejunal mucosa.
More research is needed to determine the best course of dietary management, develop laboratory test to avoid the need for oral food challenges, address prevention, and to determine specific treatment modalities.