Allergy to galactose-α-1,3-galactose (alpha-gal) is a peculiar form of food allergy generally manifesting as an anaphylactic reaction hours after mammalian meat consumption, due to the presence of specific IgE against this oligosaccharide. In addition, immediate anaphylaxis may develop after exposure to other sources of alpha-gal, such as monoclonal antibody cetuximab, vaccines, plasma expanders or anti-snake venoms. Sensitization to alpha-gal has also been implicated in the rapid degeneration of biological valve implants, and recognized as a cause of occupational disease in cattle raisers. The implication of tick bites in this type of sensitization has been accepted by all the research groups dedicated to this disease.
Patients and methodThe present study describes the clinical and sensitization characteristics of 39 patients diagnosed with alpha-gal allergy in the hospitals of our province (Lugo, Monforte de Lemos and Burela, Spain).
ResultsMost patients were middle-age males. Of note, is the fact that the series includes the first pediatric patient reported in Spain to date. The predominant clinical manifestations were urticaria or delayed anaphylaxis after consumption of mammalian meat. Seventy-four percent of the patients reported having suffered a previous tick bite, and the clinical presentation of anaphylaxis was significantly more prevalent in those with a persistent local reaction following the bite than in those with no such reaction (p = 0.032).
ConclusionsA review is also made of the disorder which, due to its variable clinical expression, is referred to as alpha-gal syndrome. The study concludes that a diagnosis of alpha-gal allergy should be considered in patients with urticaria-anaphylaxis of uncertain origin or manifesting after the administration of vaccines or products of bovine/porcine origin.
The irruption of alpha-gal allergy has questioned classical principles of IgE-mediated allergic reactions. The latency period of hours; the variability of the appearance of symptoms after exposure to the allergenic source; and the fact that the causal epitope is a carbohydrate are all features that challenge the dogmas of allergy, and define a unique disease condition that proves difficult to deal with.
Galactose-α-1,3-galactose (alpha-gal) is an oligosaccharide that glycosylates different proteins of non-primate mammals. Humans, apes and old-world monkeys do not express the alpha-gal epitope, since the α-1,3-galactosyltransferase gene is inactivated in these species, although they do produce IgM/IgG antibodies targeted to this carbohydrate and which are responsible for graft rejection of organs from pigs.1
The first descriptions of alpha-gal disease correspond to Van Nunen2 in Australia, who published cases of delayed allergy to meat in people with local reactions to tick bites. Previously, Adeyoyin3 reported that 31% of patients with cat allergy recognized cat IgA/IgM (IgA being Fel d5) – this sensitization depended on a carbohydrate. Concomitantly, O’Neil et al.4 reported an unexpected increase in the incidence of immediate reactions with the first infusion of cetuximab in the south-eastern United States, which Chung et al.5 attributed to preformed antibodies against the alpha-gal epitope, since cetuximab was also available without this epitope. Furthermore, Commins et al.6 related this phenomenon to late reactions to beef, and this in turn to tick bites.7 In 2011, the first five cases in Spain were reported in the region of Galicia.8
Specific IgE against alpha-gal is generally produced after tick bites, causing urticaria/delayed anaphylaxis (approximately 4–6 hours) after the consumption of mammalian meat.
The reactions occurring with cetuximab are immediate, severe and manifest with the first dose of the drug.4
Patients allergic to alpha-gal may suffer symptoms with sweets containing gelatin of porcine origin, such as Haribo gummy bears,9 as well as following the administration of intravenous plasma expanders (Gelofusine, Haemaccel,10 Gelafundin9). Another female patient11 suffered immediate anaphylaxis as a result of the application of an antifungal ovule containing alpha-gal in the capsule. Positive skin tests can also be observed with pancreatic extracts containing hydrolyzed bovine amylases, lipases, proteases or pepsin and blood proteins, as in the drugs Creon and Enzynorm f12 (marketed as Kreon and Pankreoflat in Spain).
Two patients with biological aortic valve prostheses developed alpha-gal allergy,13 with rapid implant degeneration requiring replacement with mechanical prostheses. There have also been descriptions of cases of perioperative allergic reactions in bovine or porcine valve replacement surgery14 in patients with elevated alpha-gal specific IgE titers.
Anti-snake venoms also contain alpha-gal,15 and a reaction to such antidotes has been reported.16 Similar phenomena could also be expected with other antidotes (e.g., Spiders, Jellyfish, Scorpions).
Our group has recently described three cases of urticaria and anaphylaxis secondary to contact with cow amniotic fluid in cattle raisers presenting alpha-gal allergy,17 with confirmation of the presence of alpha-gal in the amniotic fluid. One of these cases was recognized as an occupational disease by the corresponding official organisms.
With regard to the impact of alpha-gal disease in pediatric patients, 47 children with alpha-gal positive IgE titers and symptoms of recurrent urticaria, anaphylaxis or angioedema have been reported in the United States,18 A five-year-old child with alpha-gal allergy suffered immediate anaphylaxis19 on receiving three vaccines: measles-mumps-rubella (MMR), varicella and diphtheria-tetanus-pertussis-polio (DTaP/IPV), with positive testing to MMR, varicella and gelatin. A previous report20 described an immediate systemic reaction in an adult woman with alpha-gal allergy following herpes zoster vaccination. No previous pediatric cases of the disease have been reported in Spain.
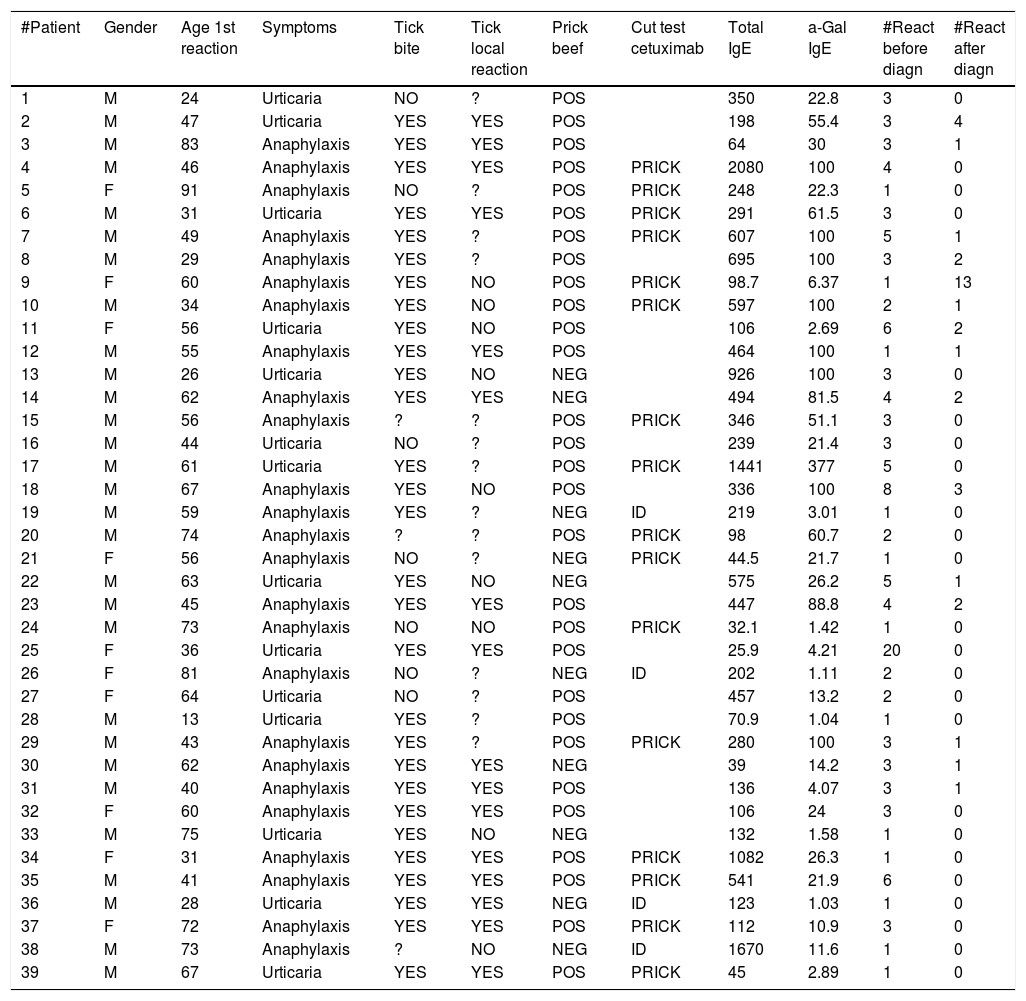
Material and methodsThe study involved 39 patients referred to the Allergy Units of the three hospitals of our province (Lugo, Monforte de Lemos and Burela, Spain) because of episodes of urticaria/delayed anaphylaxis and diagnosed with allergy to mammalian meat due to sensitization to alpha-gal between the years 2010 and 2018. The clinical characteristics of the patients and the allergological tests made, along with other parameters such as a history of tick bites are detailed in Table 1.
Description of the patient characteristics.
| #Patient | Gender | Age 1st reaction | Symptoms | Tick bite | Tick local reaction | Prick beef | Cut test cetuximab | Total IgE | a-Gal IgE | #React before diagn | #React after diagn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 24 | Urticaria | NO | ? | POS | 350 | 22.8 | 3 | 0 | |
| 2 | M | 47 | Urticaria | YES | YES | POS | 198 | 55.4 | 3 | 4 | |
| 3 | M | 83 | Anaphylaxis | YES | YES | POS | 64 | 30 | 3 | 1 | |
| 4 | M | 46 | Anaphylaxis | YES | YES | POS | PRICK | 2080 | 100 | 4 | 0 |
| 5 | F | 91 | Anaphylaxis | NO | ? | POS | PRICK | 248 | 22.3 | 1 | 0 |
| 6 | M | 31 | Urticaria | YES | YES | POS | PRICK | 291 | 61.5 | 3 | 0 |
| 7 | M | 49 | Anaphylaxis | YES | ? | POS | PRICK | 607 | 100 | 5 | 1 |
| 8 | M | 29 | Anaphylaxis | YES | ? | POS | 695 | 100 | 3 | 2 | |
| 9 | F | 60 | Anaphylaxis | YES | NO | POS | PRICK | 98.7 | 6.37 | 1 | 13 |
| 10 | M | 34 | Anaphylaxis | YES | NO | POS | PRICK | 597 | 100 | 2 | 1 |
| 11 | F | 56 | Urticaria | YES | NO | POS | 106 | 2.69 | 6 | 2 | |
| 12 | M | 55 | Anaphylaxis | YES | YES | POS | 464 | 100 | 1 | 1 | |
| 13 | M | 26 | Urticaria | YES | NO | NEG | 926 | 100 | 3 | 0 | |
| 14 | M | 62 | Anaphylaxis | YES | YES | NEG | 494 | 81.5 | 4 | 2 | |
| 15 | M | 56 | Anaphylaxis | ? | ? | POS | PRICK | 346 | 51.1 | 3 | 0 |
| 16 | M | 44 | Urticaria | NO | ? | POS | 239 | 21.4 | 3 | 0 | |
| 17 | M | 61 | Urticaria | YES | ? | POS | PRICK | 1441 | 377 | 5 | 0 |
| 18 | M | 67 | Anaphylaxis | YES | NO | POS | 336 | 100 | 8 | 3 | |
| 19 | M | 59 | Anaphylaxis | YES | ? | NEG | ID | 219 | 3.01 | 1 | 0 |
| 20 | M | 74 | Anaphylaxis | ? | ? | POS | PRICK | 98 | 60.7 | 2 | 0 |
| 21 | F | 56 | Anaphylaxis | NO | ? | NEG | PRICK | 44.5 | 21.7 | 1 | 0 |
| 22 | M | 63 | Urticaria | YES | NO | NEG | 575 | 26.2 | 5 | 1 | |
| 23 | M | 45 | Anaphylaxis | YES | YES | POS | 447 | 88.8 | 4 | 2 | |
| 24 | M | 73 | Anaphylaxis | NO | NO | POS | PRICK | 32.1 | 1.42 | 1 | 0 |
| 25 | F | 36 | Urticaria | YES | YES | POS | 25.9 | 4.21 | 20 | 0 | |
| 26 | F | 81 | Anaphylaxis | NO | ? | NEG | ID | 202 | 1.11 | 2 | 0 |
| 27 | F | 64 | Urticaria | NO | ? | POS | 457 | 13.2 | 2 | 0 | |
| 28 | M | 13 | Urticaria | YES | ? | POS | 70.9 | 1.04 | 1 | 0 | |
| 29 | M | 43 | Anaphylaxis | YES | ? | POS | PRICK | 280 | 100 | 3 | 1 |
| 30 | M | 62 | Anaphylaxis | YES | YES | NEG | 39 | 14.2 | 3 | 1 | |
| 31 | M | 40 | Anaphylaxis | YES | YES | POS | 136 | 4.07 | 3 | 1 | |
| 32 | F | 60 | Anaphylaxis | YES | YES | POS | 106 | 24 | 3 | 0 | |
| 33 | M | 75 | Urticaria | YES | NO | NEG | 132 | 1.58 | 1 | 0 | |
| 34 | F | 31 | Anaphylaxis | YES | YES | POS | PRICK | 1082 | 26.3 | 1 | 0 |
| 35 | M | 41 | Anaphylaxis | YES | YES | POS | PRICK | 541 | 21.9 | 6 | 0 |
| 36 | M | 28 | Urticaria | YES | YES | NEG | ID | 123 | 1.03 | 1 | 0 |
| 37 | F | 72 | Anaphylaxis | YES | YES | POS | PRICK | 112 | 10.9 | 3 | 0 |
| 38 | M | 73 | Anaphylaxis | ? | NO | NEG | ID | 1670 | 11.6 | 1 | 0 |
| 39 | M | 67 | Urticaria | YES | YES | POS | PRICK | 45 | 2.89 | 1 | 0 |
The skin prick tests were made using a standard battery (Laboratorio Alk-Abelló, Madrid, Spain) of aeroallergens (Dermatophagoides pteronyssinus, Lepidoglyphus destructor, Tyrophagus putrescentiae, Chortoglyphus arcuatus, Glycyphagus domesticus, Alternaria, Cladosporium, Aspergillus fumigatus, Betula verrucosa, Cupressus arizonica, Lolium perenne, Phleum pratense, Plantago, Parietaria judaica, profilin, dog, cat, cow, horse and rabbit) and food allergens (milk, egg, walnut, peanut, lentil, soybean, gliadin, white fish, wheat flour, Anisakis, shrimp, apple, peach LTP, kiwi, latex and beef) routinely employed in our Units. As positive control we used histamine 10 mg/ml, with saline solution as negative control. Wheal size was measured 20 min after the test, and the result was considered positive when the size of the wheal induced by the allergen was at least equal to the diameter of the wheal induced by histamine.
In addition, 14 patients underwent skin prick tests with 5 mg/ml of cetuximab (Erbitux, Merck S.L., Madrid, Spain). If the prick test proved negative, intradermal skin testing (0.5 mg/ml) was performed following the instructions of the European Committee21 on the volar surface of the forearm. The result was considered positive when at least one wheal measuring 5 mm in diameter was obtained, with erythema, 20 min after the test.
Total and specific IgE against alpha-gal were determined. Specific IgE against other meats was tested in some patients. The ImmunoCAP technique (Thermo Fischer Scientific, Uppsala, Sweden) was used for both total IgE and specific IgE.
In patients with the implication of non-steroidal anti-inflammatory drugs (NSAIDs) as possible cofactors, controlled exposure testing was carried out with progressively increasing doses until the final therapeutic dose was reached.
Statistical analysisThe chi-squared test, Student t-test, Kruskal-Wallis test, Mann-Whitney U-test and Wilcoxon test were used to analyze the results obtained. The SPSS version 25.0 statistical package was used throughout.
ResultsAll the patients were Caucasians. Twenty-nine of the 39 patients were males (74%). There were no statistically significant differences in the presence of urticaria or anaphylaxis between males and females (p = 0.652). The mean patient age was 53 years (range 13–91), and a full 69% of the patients were between 40–75 years of age. The mean age of the patients with urticaria was 45.3 years, versus 57.68 years among those with anaphylaxis – the difference between groups being statistically significant (p = 0.0043). The present study reports a delayed reaction to pork in a 13-year-old boy, which constitutes the first pediatric case published in Spain to date.
All the patients were referred by their doctors due to symptoms hours after exposure to meat, except two: one male diagnosed with an immediate reaction to cetuximab, and a female diagnosed with an intraoperative immediate reaction related to the use of Gelaspan (plasma expander).
Twenty-five of the patients (64%) presented anaphylaxis with the rest presenting urticaria. The mean latency between meat consumption and the appearance of symptoms was five hours (range 2–10). The types of meat implicated in the reactions were beef, pork and lamb. One patient also developed symptoms in relation to wild boar meat. Two patients with symptoms in response to beef experienced faster reactions (two hours) on consuming kidney.
Among the patients with anaphylaxis, 13 suffered at least one further reaction after the diagnosis (52%). Before the diagnosis the patients experienced an average of 2.76 episodes, versus 1.16 episodes after the diagnosis – the difference was statistically significant (p = 0.000).
Of the patients with urticaria, three suffered at least one further reaction after the diagnosis (25%). Before the diagnosis the patients experienced an average of 4.07 episodes, versus 0.5 episodes after the diagnosis – the difference was statistically significant (p = 0.002).
With regard to tick bites, 29 patients recalled having been bitten (74%), three did not remember, and seven denied having been bitten by ticks (18%). There was no statistically significant association between tick bites and the severity of the symptoms, i.e., the presence of urticaria or anaphylaxis (p = 0.390). Of the patients who had been bitten by ticks, 58% developed a local reaction as a result, and this reaction could last for months. In the patients with urticaria, the bites caused a local reaction in 45.45% of the cases, versus in 61.1% of the patients with anaphylaxis. The Kruskal-Wallis test showed older age and the presence of a local reaction to tick bites to be associated to an increased risk of anaphylaxis (p = 0.032) – a situation not seen in the patients with urticaria (p = 0.815). The reaction to meat manifested weeks or months after the bite.
The total IgE titers ranged between 25–2080 IU/ml, with an arithmetic mean of 403 IU/ml (after excluding the patients that yielded values of 2080, 1670 and 1441 IU/ml, the mean total IgE titer was 293 IU/ml) and a median of 248 UI/ml. There were no statistically significant differences between the patients with urticaria and those who suffered anaphylaxis (p = 0.621).
The specific IgE titers against alpha-gal ranged between 1.03 and >100 kU/l, with a mean of 47.97 kU/l. In seven patients the titers exceeded 100 kU/l, which is the upper limit of detection in our laboratory. There were no statistically significant differences between the patients with urticaria and those who suffered anaphylaxis (p = 0.157).
The 14 patients subjected to skin testing with cetuximab all yielded positive results (12 with prick testing and two with the intradermal test). The only patient in our series who had been referred due to anaphylaxis with the first cetuximab dose had never experienced problems with meat, and the intradermal test proved positive.
In five of the patients in our series (three of them have been described in a recent article published by our group17), a diagnosis of contact urticaria or anaphylaxis due to cow amniotic fluid allergy was established, demonstrating the presence of alpha-gal in the amniotic fluid.
The 26 patients whose blood group was recorded all had groups other than group B.
Of the 39 patients, none reported symptoms caused by milk or dairy products, or gelatins. Cured ham was tolerated by all the patients.
Skin testing with commercial beef extract proved positive in 29 patients (74%) and negative in 10 (26%). A positive test was obtained in 71.43% of the patients with urticaria and in 76% of those with anaphylaxis – the difference was not significant (p = 0.753).
Specific IgE against rabbit, lamb, pork and beef was positive in 19 of the 23 patients in whom such testing was requested (83%). The IgE titers corresponding to bovine serum albumin (BSA) were generally low. Specific IgE against chicken was negative in all cases.
Before the diagnosis, the patients had experienced between 1–20 episodes, with an average of 3.2 episodes per patient (after excluding the patients with 20 episodes of recurrent urticaria, the mean was 2.7 episodes per patient). Following the diagnosis, the patients experienced between 0–13 episodes, with an average of 0.92 episodes per patient (after excluding the single patient with 13 episodes, the mean was 0.6 episodes per patient).
With regard to atopy (defined as a positive skin test for some aeroallergen), 12 of the total patients (30%) yielded positive skin tests: 10 to dust mites, one to pollen and one to dust mites/pollen. An additional patient had positive skin tests for LTP, and another one for bee venom. The tryptase values were within normal ranges.
Controlled exposure to NSAIDs was well tolerated by four (ibuprofen in three and naproxen in one) of the six patients in whom those drugs possibly intervene as a cofactor; two patients tolerated alternatives and rejected testing.
Lastly, physical exercise and alcohol consumption were evaluated as possible cofactors in the respective reactions. Posterior exposure to these two cofactors caused no adverse reaction in any of the patients.
DiscussionGalactose-α-1,3-galactose (alpha-gal) syndrome is a disorder that has become known during the last decade. In 2011, our group reported the first five cases in Spain, and the present study describes the data corresponding to 39 diagnosed patients.
Carbohydrates as such were not regarded as allergens, and although the confounding effect they produced in the context of in vitro allergy testing was known, it was not considered to be of clinical relevance.22–24
Alpha-gal was known to be responsible for hyperacute porcine transplant rejection in humans, although in that context the reaction is mediated by IgM/IgG antibodies. It is present in the tissues of mammals (except humans, apes and old-world monkeys). The existing evidence indicates that the development of specific IgE against alpha-gal occurs as a result of tick bites, and does not seem to be caused by food.7 The disorder is characterized by urticaria/delayed anaphylaxis (3–6 hours) following the consumption of non-primate mammalian meat. The symptoms develop faster with the consumption of viscera, particularly kidney.25 It is not clear why these reactions occur, although the way in which the meat is prepared for consumption appears to play a role. Nevertheless, this epitope is known to be resistant to both heat26 and pepsin-mediated lysis27; as a result, the symptoms do not manifest every time the patient consumes meat – a fact that complicates clinical suspicion and diagnosis. Some authors18 have offered hypotheses attempting to explain why the consumption of mammalian meat containing alpha-gal does not always produce an allergic reaction, including variations in the metabolic process (digestion and absorption) of carbohydrates, in the amount of alpha-gal that reaches the bloodstream, the amount of alpha-gal found in different foods, the food dose or portion consumed, preparation of the food, and the latency period from the last mammalian meat intake.
Once this particular antigen started to be taken into account, the diagnosis of idiopathic anaphylaxis in regions of high alpha-gal intake such as Tennessee (USA) decreased from 59% to 35%. In effect, while alpha-gal allergy was once practically unknown, it now represents the most commonly identified cause of anaphylaxis in patients of this kind.28 A link has even been established between specific IgE against alpha-gal in people from Virginia (USA) and an increased atheroma and unstable plaque burden - thus representing a possible new risk factor for coronary artery disease.29
Tick bites can produce a local reaction or cause anaphylaxis secondary to sensitization to the tick salivary proteins.30 Alternatively, some time after the bite, sensitization to alpha-gal may develop, producing the syndrome addressed in our study.
The tick Amblyomma sculptum contains alpha-gal in its saliva,31 and Ixodes ricinus contains alpha-gal in its intestine,32 although the literature does not specify whether alpha-gal was endogenous to the tick itself or exogenous (i.e., originating from the ingested mammalian blood). In one study,7 the bites of Amblyomma americanum (lone star tick) induced both IgE against tick proteins and IgE against alpha-gal, yet this was not seen in the case of Dermacentor variabilis (brown dog tick).
Since Ixodes ricinus is the most common tick in our geographical setting,33 we presume that it is the species that bites and sensitizes our patients.
A recent study has shown that the tick Ixodes scapularis activates the genes that produce alpha-gal in response to infection by Anaplasma phagocytophilum; as a result, the ticks most infected with that type of Rickettsia generate more alpha-gal. These findings could help explain the geographical differences in the prevalence of alpha-gal allergy.34
It has been shown that tick bites induce an increase in the titers of specific IgE against alpha-gal35 and that the levels are higher in patients that have received multiple bites.7 It has been ruled out that persistent local reactions at the bite site are secondary to infection with Borrelia burgdorferi.36
Tick bites were implicated in most of our patients (70%), but not in all of them. This raises the question as to whether there may be other sensitization routes. Over half of our patients developed local reactions to the bite (52%), and these could persist for weeks or months – being considered as a risk factor for the development of symptoms in relation to alpha-gal. A total of 45.45% of the cases of urticaria were associated to local reactions to tick bites, versus 61.1% of the cases of anaphylaxis. We found that the most severe cases (anaphylaxis) corresponded to males of older age and with local reactions to tick bites.
In Spain, in the region of La Rioja,37 15% of the exposed population (forestry workers) had high titers of IgE against alpha-gal, compared with 4% of the controls (although none of them presented symptoms with the consumption of meat). These data can be compared with those obtained in Germany,38 where the prevalence of positive IgE against alpha-gal in hunters and forestry workers was 35% (using CAP > 0.1 kU/l as cut-off point) or 19.3% (using CAP > 0.35 kU/l as cut-off point), but only 8.6% of the latter showed delayed anaphylaxis in response to beef.
The sources of exposure in alpha-gal syndrome are highly varied, with cases having been related to cetuximab, gelatins, plasma expanders, meat/innards, vaccines, sweets, drug capsules, anti-snake venom sera and heart valves. In our setting, the presentation is typically characterized by anaphylaxis (in up to 65% of the patients) occurring in the early morning hours, between 2 and 10 hours after consuming beef or pork. The condition is more often seen in males of about 50 years of age. This is probably because such individuals are more exposed to tick bites (cattle raisers, forestry workers, outdoor sports, hunters, hikers, or subjects involved in walking or bicycling activities). Of note is the fact that our series includes the first case in Spain of a 13-year-old boy with delayed anaphylaxis due to pork, and with positive beef skin test results and specific IgE against alpha-gal.
On the other hand, the condition may also constitute an occupational disease, as in reactions caused by exposure to cow amniotic fluid at calving. We have already documented five such cases to date. Some patients also develop skin symptoms on the arms on kneading raw meat for the preparation of sausages. None of our patients reported symptoms associated with milk or dairy products.
Six patients had taken NSAIDs on the day of the reaction. Controlled exposure testing was thus performed to four and was well tolerated in all of them. The drug possibly may have acted as a cofactor, though in our patients this does not seem likely, since the medication failed to shorten the reaction latency period.
In our setting, the meats most often implicated in alpha-gal syndrome are beef, pork and lamb, although other types of mammal meats can be involved – including wild boar, as in one of our cases. The consumption of viscera such as kidney – which contains abundant alpha-gal26,29 – is associated with shorter latency reactions.
It should be noted that in the subgroup of patients with anaphylaxis, 60% suffered another reaction after the diagnosis of the syndrome. In this regard, we believe that patients find it hard to accept complete avoidance of meat, because they do not trust the diagnosis (e.g., they sometimes eat meat without suffering symptoms); or due to cultural/diet reasons. In this context it is essential to have an epinephrine self-injector available in the event of an emergency. On the other hand, before being diagnosed, the patients had suffered an average of 3.5 episodes each – although one individual had experienced 20 previous reactions. Following the diagnosis, the average dropped to 1.6 episodes per patient, with statistically significant differences in both the patients with urticaria and in those with anaphylaxis – this demonstrates improvement in patient quality of life and prognosis. Furthermore, all the patients were seen to tolerate cured ham, which thus allowed them to eat at least one pork product without problems.
In our patients, the mean total IgE titer was 369 IU/ml (median 248 UI/ml), and the mean titer corresponding to specific IgE against alpha-gal was 47.97 kU/l. The true figure was probably higher, because in seven patients the titer was >100 KU/l, which exceeds the upper detection limit of our laboratory; the real titer is therefore not known. The specific IgE titers ranged between 1.03 and 100 kU/l. Skin testing with beef only proved positive in 76% of the patients. Specific IgE was positive in all cases. When IgE against alpha-gal proves negative but clinical suspicion is high, skin testing with cetuximab can be carried out, with positive results in 100% of the cases, according to Martinez-Arcediano et al.40 In patients with doubtful symptoms and positive specific IgE against alpha-gal, basophil activation testing can be performed, and a positive result would support the clinical suspicion.
Consistent clinical findings and positive IgE against alpha-gal suffice to establish the diagnosis of the disease. Provocation testing is normally not needed, in view of the hypothetical appearance of potentially serious symptoms, and in our opinion it makes no sense to increase the dose as in controlled exposure tests in protein allergies, since the reaction is delayed and does not always appear. If controlled exposure testing is considered essential (a practice we do not advise), it could be performed with viscera (kidney), gelatin, Gelaspan or even cetuximab.
In agreement with Kennedy,18 we do not consider it necessary to conduct testing of IgE against other meats. Likewise, it is of no practical interest to test specific IgE against cat antigens, since it will prove positive in these patients, as shown by Gonzalez-Quintela et al.,39 due to the Fel d5 allergen, which is cat IgA. In the same way, testing of cow’s milk is of little use, since it usually proves positive (with negative results referred to the milk fractions), due to the presence of alpha-gal.18 If such patients develop respiratory symptoms as a consequence of exposure to cat dander, specific IgE against cat antigen could constitute a false-positive result; we thus recommend testing for specific IgE against Fel d1.
Due to the structural similarity between alpha-gal and the blood group B antigen, blood group B status was believed to afford protection against alpha-gal syndrome. However, the latest studies41 have concluded that there is no evidence that blood group B results in lessened susceptibility to sensitization to type I alpha-gal, lowered specific IgE against alpha-gal, or a lesser risk of developing alpha-gal syndrome. The absence of patients with blood group B is explained by the low prevalence of this blood group. In this regard, in our setting (Galicia, Spain), the prevalence of blood group B is 6.8%, and that of blood group AB 3%.
Alpha-gal syndrome does not appear to be more frequent in atopic patients than in non-atopic individuals. In our series, 36% of the patients were atopic – this being consistent with the data published by other authors.13
We believe that thanks to the diagnosis of alpha-gal allergy, panallergens and the study of cofactors, the diagnosis of idiopathic anaphylaxis has decreased drastically in recent years.
In relation to pediatric patients, the literature only reports one boy with a reaction to measles-mumps-rubella (MMR) – varicella vaccine and gelatin positivity,19 and the cases described by Kennedy et al.,18 comprising 51 pediatric patients between 4–17 years of age in the state of Virginia (USA) with a history of recurrent acute urticaria (not chronic urticaria), idiopathic anaphylaxis or angioedema – with positive IgE against alpha-gal in 47 cases. Most of the children had suffered persistent local reactions after tick bites (90% of the patients had suffered bites in the last year).
Lastly, in children suffering recurrent urticaria with no clear causal relationship, anaphylactic reactions (particularly delayed anaphylaxis) or urticaria-like reactions to vaccines and products of bovine or porcine origin, the possibility of alpha-gal allergy as the underlying cause should be evaluated.
Conflict of interestThe authors have no conflict of interest to declare.
Funding sourcesThis research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
The authors wish to thank Professor Constantino Arosa Gómez, Informatic Engineering PhD and Economics PhD for his valuable help in the statistical analysis.