In the context of a mass vaccination campaign, rare adverse events can be detected through passive surveillance.1 The Brazilian Post-Vaccine Adverse Event Surveillance System received notifications of allergic reactions at a rate of 0.95 cases/100,000 doses of the Measles-Mumps-Rubella vaccine (MMR) produced in Italy (Morupar® Chiron), in the period from 2000 to 2003. However on August 21st 2004 (the first day of the National Measles Follow-up Campaign), an increase in allergic reactions was reported with a rate of 11.56 cases/100,000 doses administered of this vaccine. As a result, and as a preventive measure, the use of this product was discontinued in Brazil. (Ministério da Saúde. Brasília. – Nota Técnica N°97/04).
Studies have shown an association between anaphylaxis following administration of the MMR vaccine and subsequent detection of anti-gelatin IgE antibodies. 2,3 According to the manufacturer’s certification, the gelatin was not present and the Morupar® vaccine had residual traces of egg, hydrolysed casein and dextran 70.
Dextran, a high-molecular-weight polysaccharide used as a stabiliser in some vaccines, is rarely associated with hypersensitivity reactions. These reactions result from circulating immune complexes formed by preexisting anti-dextran IgG antibodies and dextran injected with the vaccine, causing complement activation and mast cell and basophil degranulation by anaphylatoxins.4
We evaluated 19 children (aged 34.9±16.3 months) who reported reactions within two hours after receiving the vaccine, in Curitiba, state of Paraná. Thirty-one age/gender matched children, living in the same area, who had received on the same date and who had no adverse reactions were included as a control group. Blood samples were collected approximately five to six weeks after vaccine administration, while skin tests were performed four to seven months after vaccination.
Serum specific IgE antibodies (casein, egg) were determined by a fluoroenzyme immunoassay method (ImmunoCAP-Pharmacia®) and levels greater than 0.35KU/L were considered positive.
Levels of specific IgE and IgG to dextran 70 were determined by a time-resolved fluorescent lanthanide immunoassay (DELFIA, PerkinElmer, Boston, MA, US). The dissociation-enhanced method can be used to study antibody binding to solid-phase proteins or peptides. Dextran 70 from Leuconostoc ssp (Molecular weight ∼70kDa, Fluka, Sigma Aldrich, Milan, Italy) was used at a concentration of 400μg/ml to coat the Delfia plates. Serum samples were diluted 1:50 in bovine serum albumin 1% and incubated overnight at 4–8°C. Bound antibodies were detected by a europium-labelled anti-human IgG or IgE antiserum (PerkinElmer). Optical density values higher than the mean plus two standard deviations of europium counts in the control group were considered positive.
For allergy skin tests we used undiluted vaccine from the same batch as that employed in the Vaccination Campaign.
The study was approved by the Federal University of Parana’s Institutional Review Board of Hospital de Clinicas, and a voluntary informed-consent was signed by children’s guardians.
No prior allergic reactions to vaccines were reported in either group, and there was no association with history of atopy or allergic reactions to medication and/or food.
Of the children evaluated, all developed skin manifestations (erythema, urticaria or angioedema), associated or not with other systems. All of them received oral antihistamines, two received oral corticosteroids, and subcutaneous adrenaline complemented the therapy.
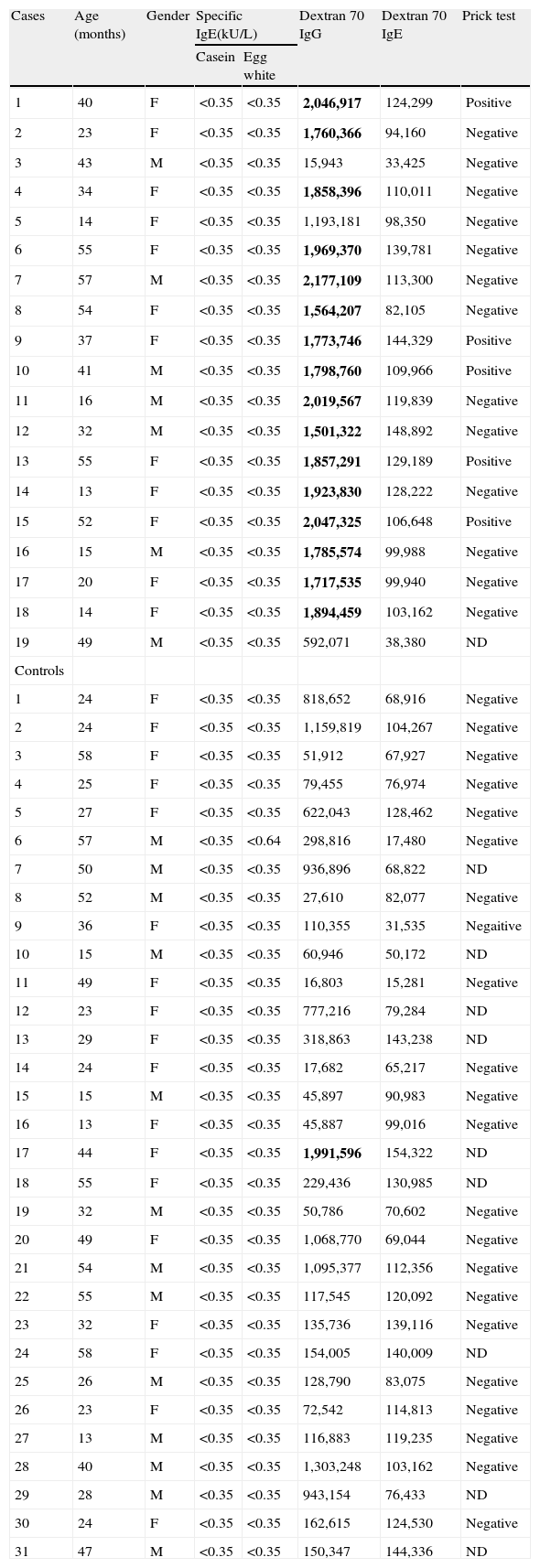
Demographic and laboratory data of subjects and controls are shown in Table 1.
Demographic data, serologic and prick test results of cases and control subjects
| Cases | Age (months) | Gender | Specific IgE(kU/L) | Dextran 70 IgG | Dextran 70 IgE | Prick test | |
| Casein | Egg white | ||||||
| 1 | 40 | F | <0.35 | <0.35 | 2,046,917 | 124,299 | Positive |
| 2 | 23 | F | <0.35 | <0.35 | 1,760,366 | 94,160 | Negative |
| 3 | 43 | M | <0.35 | <0.35 | 15,943 | 33,425 | Negative |
| 4 | 34 | F | <0.35 | <0.35 | 1,858,396 | 110,011 | Negative |
| 5 | 14 | F | <0.35 | <0.35 | 1,193,181 | 98,350 | Negative |
| 6 | 55 | F | <0.35 | <0.35 | 1,969,370 | 139,781 | Negative |
| 7 | 57 | M | <0.35 | <0.35 | 2,177,109 | 113,300 | Negative |
| 8 | 54 | F | <0.35 | <0.35 | 1,564,207 | 82,105 | Negative |
| 9 | 37 | F | <0.35 | <0.35 | 1,773,746 | 144,329 | Positive |
| 10 | 41 | M | <0.35 | <0.35 | 1,798,760 | 109,966 | Positive |
| 11 | 16 | M | <0.35 | <0.35 | 2,019,567 | 119,839 | Negative |
| 12 | 32 | M | <0.35 | <0.35 | 1,501,322 | 148,892 | Negative |
| 13 | 55 | F | <0.35 | <0.35 | 1,857,291 | 129,189 | Positive |
| 14 | 13 | F | <0.35 | <0.35 | 1,923,830 | 128,222 | Negative |
| 15 | 52 | F | <0.35 | <0.35 | 2,047,325 | 106,648 | Positive |
| 16 | 15 | M | <0.35 | <0.35 | 1,785,574 | 99,988 | Negative |
| 17 | 20 | F | <0.35 | <0.35 | 1,717,535 | 99,940 | Negative |
| 18 | 14 | F | <0.35 | <0.35 | 1,894,459 | 103,162 | Negative |
| 19 | 49 | M | <0.35 | <0.35 | 592,071 | 38,380 | ND |
| Controls | |||||||
| 1 | 24 | F | <0.35 | <0.35 | 818,652 | 68,916 | Negative |
| 2 | 24 | F | <0.35 | <0.35 | 1,159,819 | 104,267 | Negative |
| 3 | 58 | F | <0.35 | <0.35 | 51,912 | 67,927 | Negative |
| 4 | 25 | F | <0.35 | <0.35 | 79,455 | 76,974 | Negative |
| 5 | 27 | F | <0.35 | <0.35 | 622,043 | 128,462 | Negative |
| 6 | 57 | M | <0.35 | <0.64 | 298,816 | 17,480 | Negative |
| 7 | 50 | M | <0.35 | <0.35 | 936,896 | 68,822 | ND |
| 8 | 52 | M | <0.35 | <0.35 | 27,610 | 82,077 | Negative |
| 9 | 36 | F | <0.35 | <0.35 | 110,355 | 31,535 | Negaitive |
| 10 | 15 | M | <0.35 | <0.35 | 60,946 | 50,172 | ND |
| 11 | 49 | F | <0.35 | <0.35 | 16,803 | 15,281 | Negative |
| 12 | 23 | F | <0.35 | <0.35 | 777,216 | 79,284 | ND |
| 13 | 29 | F | <0.35 | <0.35 | 318,863 | 143,238 | ND |
| 14 | 24 | F | <0.35 | <0.35 | 17,682 | 65,217 | Negative |
| 15 | 15 | M | <0.35 | <0.35 | 45,897 | 90,983 | Negative |
| 16 | 13 | F | <0.35 | <0.35 | 45,887 | 99,016 | Negative |
| 17 | 44 | F | <0.35 | <0.35 | 1,991,596 | 154,322 | ND |
| 18 | 55 | F | <0.35 | <0.35 | 229,436 | 130,985 | ND |
| 19 | 32 | M | <0.35 | <0.35 | 50,786 | 70,602 | Negative |
| 20 | 49 | F | <0.35 | <0.35 | 1,068,770 | 69,044 | Negative |
| 21 | 54 | M | <0.35 | <0.35 | 1,095,377 | 112,356 | Negative |
| 22 | 55 | M | <0.35 | <0.35 | 117,545 | 120,092 | Negative |
| 23 | 32 | F | <0.35 | <0.35 | 135,736 | 139,116 | Negative |
| 24 | 58 | F | <0.35 | <0.35 | 154,005 | 140,009 | ND |
| 25 | 26 | M | <0.35 | <0.35 | 128,790 | 83,075 | Negative |
| 26 | 23 | F | <0.35 | <0.35 | 72,542 | 114,813 | Negative |
| 27 | 13 | M | <0.35 | <0.35 | 116,883 | 119,235 | Negative |
| 28 | 40 | M | <0.35 | <0.35 | 1,303,248 | 103,162 | Negative |
| 29 | 28 | M | <0.35 | <0.35 | 943,154 | 76,433 | ND |
| 30 | 24 | F | <0.35 | <0.35 | 162,615 | 124,530 | Negative |
| 31 | 47 | M | <0.35 | <0.35 | 150,347 | 144,336 | ND |
Dextran 70IgG: fixed cutoff value, 1,433,119 Europium counts, Dextran 70IgE: fixed cutoff value, 167,336 Europium counts (positive results are written in bold). Gender: male (M), female (F); prick test: not determined (ND).
Casein-specific IgE was not detected in all the subjects. Two controls showed levels > 0.35 KU/L of specific IgE to egg.
Sera from the case and control groups were tested for specific IgE and IgG to dextran 70, which was the most suspected causal component in the Italian case series 5. All the children had specific IgE to dextran 70 below the cut-off value (167,336; mean 93,092, SD 37,122). The cut-off for specific IgG was fixed at 1,433,119, which is higher than the mean plus 2 SDs of europium counts in the control group (mean, 422,899; SD, 505,109×2). Sixteen out of 19 cases (84%) and one out of 31 controls (3%) presented specific IgG levels above the cut-off value (Table 1).
Positive skin prick test with the Morupar vaccine was observed in five out of 18 cases and in none of the 22 controls tested. These five patients also showed high levels of specific IgG to dextran 70, with negativity of specific IgE.
The determination of specific IgE to casein and egg allowed us to exclude that these proteins were part of the cause of reactions to the Morupar vaccine. According to the results of dextran-specific antibodies, immediate allergic reactions reported in Brazil could be possibly induced by dextran 70 like those reported in Italy.5
The positive allergy skin tests to MMR vaccine observed in five cases could be due to a double pathogenetic mechanism in these children, such as in certain drug allergies6, involving specific IgG to dextran and IgE to a different component of the vaccine, although a non-specific reaction due to a direct degranulation of mast cells cannot be ruled out.
We conclude that residual dextran 70 in this particular brand of MMR vaccine, which induced high levels of specific IgG antibodies, could be the culprit of most of the hypersensitivity reactions reported in Brazil.
Research supported byPrograma Nacional de Imunizações, Ministério da Saúde, Brasília/Distrito Federal, Brazil
Secretaria de Estado da Saúde do Paraná, Curitiba/Paraná, Brazil
The Green Channel Reference Centre for Pre-vaccination Consultancy, University Hospital, Verona, Italy.
Conflict of interestThe authors contributed equally to this work and they have no conflict of interest.
We wish to thank the helpful discussion of Drs. Robert T. Chen, Karin R. Luhn and Fides Sbardelotto. We acknowledge Elizabeth Ferraz, Daniel R. C. de Freitas, Gisele C. B. Araújo, Kátia Terêncio and Gisele Madalosso for recruitment and serum collection.