Moths are a significant source of indoor and outdoor aeroallergens. High prevalence of IgE-mediated sensitization was demonstrated in a group of patients with allergic respiratory diseases. There are no studies on adult stage of these moth species allergens involved in allergic respiratory reactions - the aim of this study.
Material and Methods36 participants were included in an experimental study, submitted to skin prick test with Bombyx mori wing extract and six other common allergens, as well as Western blot analysis with incubated nitrocellulose membrane impregnated with silkworm moth extract and human IgE-antibody. The participants were divided into 3 groups: 1) 21 allergic patients whose skin prick test was positive to Bombyx mori wing extract, 2) eight allergic patients whose skin prick test was positive to mite and negative to Bombyx mori extract 3) seven negative non-allergic subjects.
ResultsAmong the 21 participants from group 1, 19 serum samples reacted to Bombyx mori extract by Western blot. All of them reacted to a protein at 80 kDa and five other proteins (66, 50, 45, 37 and 30 kDa) were identified in more than 50% of the individuals tested, considered as major allergenic proteins. Sera from seven out of eight patients sensitized to house dust mite demonstrated IgE-reactivity to Bombyx mori extract by Western blot analysis. Serum samples from healthy participants did not react at all.
ConclusionSix major reactive proteins by immunoblot analysis from moth’s wings sensitized patients can be potential allergens. The one at 80 kDa is the major protein, seen in all IgE-reactive patients from group 1 and in none from group 2, yet to be identified. Future studies should be conducted to better characterize these proteins.
There are around 150,000 species of Lepidoptera, including moths and butterflies, distributed worldwide.1 Some of these are involved in the development of allergic diseases.2,3 Inhalant insect allergens are present in airborne particles and have been implicated as triggers for respiratory allergic symptoms.4,5 The high frequency of moth as aeroallergen has been verified by skin prick test and specific serum IgE in a group of patients with asthma and allergic rhinitis.6
Silkworm moths are significant sources of inhalant allergens, both indoors and outdoors. Their wings are covered with scales, which detach and remain suspended in the air, therefore potentially able to sensitize and cause symptoms of respiratory allergy when inhaled by predisposed individuals.7 Their aeroallergens can cross-react with other species of moths and butterflies. Patients with allergic respiratory diseases may develop symptoms with environmental or occupational exposure to their allergens.8,9
To date, few proteins related to Bombyx mori (Bm) respiratory allergies have been identified and characterized, none of them from the adult stage: silkworm moth. Most of the allergens described are related to food allergies after pupa ingestion, particularly in Asian countries where it is a traditional food10 and after exposure to larvae and pupa through skin or respiratory mucosa in silk industry workers.11
For a better understanding of respiratory allergic diseases related to Bm sensitization, studies on characterization and the identification of new allergens are required. The main objective of the present study was to identify silkworm moth Bm major proteins that might be involved in allergic respiratory symptoms within patients with asthma and/or allergic rhinitis.
Materials and methodsPreparation of Bombyx mori wing extract (BmWE)2.8 g of Bm moths’ wings were mechanically macerated and homogenized in lysis buffer (PBS, pH 7.2, 5% Glycerol, 300 mM NaCl, 0.1% Triton X-100, 1 mM EDTA, 0.5 mM PMSF) 1:10, weight/volume at 4 °C overnight. The resulting extract was clarified through double centrifugation (15 min, 22,000 xg, 4 °C), filtered (0.22 μm, Millipore, Bedford, MS, USA) and stored at −20 °C. For skin prick test (SPT), the extract was diluted in sterile glycerin 50% (1:10) and stored in a refrigerator at 4 °C.
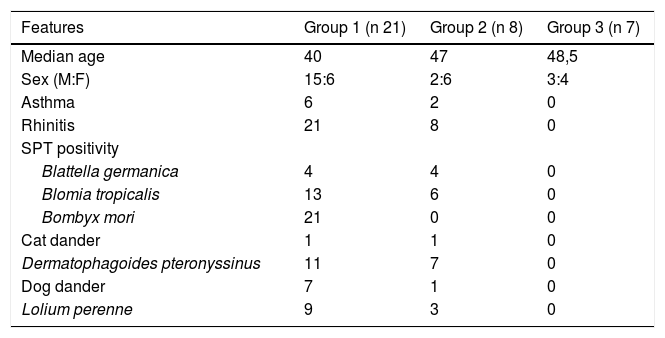
Serum collection and patients’ identificationSerum samples were obtained from 36 participants (Table 1) selected from a tertiary hospital staff outpatient in Curitiba, Brazil. These individuals were submitted to SPT with allergenic glycerinated extracts of in-house Bm extract, house dust mites (Dermatophagoides pteronyssinus) and Blomia tropicalis, cockroach (Blattella germanica), grass pollen (Lolium multiflorum), dog and cat dander (IPI-ASAC Brasil®). A 27 mm needle was introduced through a drop of allergen extract after wiping the forearm’s skin with alcohol. A new disposable needle was used for each allergen. Positive (histamine 10 mg/mL) and negative (glycerinated saline) controls were also used. After 15 min, the mean wheal diameter was measured and considered positive if greater than 3 mm.12
Epidemiological, clinical and SPT sensitivity data from participants (n 36).
| Features | Group 1 (n 21) | Group 2 (n 8) | Group 3 (n 7) |
|---|---|---|---|
| Median age | 40 | 47 | 48,5 |
| Sex (M:F) | 15:6 | 2:6 | 3:4 |
| Asthma | 6 | 2 | 0 |
| Rhinitis | 21 | 8 | 0 |
| SPT positivity | |||
| Blattella germanica | 4 | 4 | 0 |
| Blomia tropicalis | 13 | 6 | 0 |
| Bombyx mori | 21 | 0 | 0 |
| Cat dander | 1 | 1 | 0 |
| Dermatophagoides pteronyssinus | 11 | 7 | 0 |
| Dog dander | 7 | 1 | 0 |
| Lolium perenne | 9 | 3 | 0 |
The subjects were divided into groups based on SPT reactivity and clinical diagnosis: positive to BmWE (group 1) and positive to house dust mite (group 2). Patients from both groups 1 and 2 were clinically diagnosed with asthma and/or allergic rhinitis. Serum samples from seven individuals who were not allergic and exhibited no positive reaction to the allergens tested by SPT were also included as healthy controls (group 3). The study was approved by the Ethics Committee of Complexo Hospital de Clinicas, Federal University of Parana and all participants provided written informed consent.
ImmunodetectionTotal BmWE (7 u L per-well) was applied to 10% Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) and eletrophoretically transferred to nitrocellulose membrane (Amersham Biosciences) in semi-dry system (Trans-Blot SD semi-dry transfer cell, Bio-Rad). Membranes were blotted with 5% (weight/volume) skim powdered milk (MOLICO® - Nestlé) diluted in PBS-T buffer (PBS 1x, pH 7,4 com 0,15% Tween-20) and cut into small strips (about 4 mm wide) for serum incubation (1/30) with 21 BmWE sensitized patients, eight mite sensitized patients and seven healthy controls in PBS-T for 16 h at 4 °C. The strips were then blotted with alkaline phosphatase conjugated human anti-IgE antibody (1/5000 both antibodies), (Sigma-Aldrich) for 1:30 h at room temperature. After each step, membranes were washed five times with PBS-T. Reaction was revealed with 50 mg/mL BCIP (Bromo-Chloro-Indolyl Phosphate), NBT (Nitro Blue Tetrazolium) (Promega, Madison, WI, USA) solution diluted in 10 mL alkaline phosphatase buffer (100 mM Tris-HCl pH 9.5, 100 mM NaCl, 5 mM MgCl2) for one minute and interrupted with 10 mM EDTA in distilled water. A positive control of the assay was used with recombinant arginine kinase (GenScript®).
Allergenic protein characterizationPigment containing membrane areas corresponding to the allergens detected by Western blot from group 1 and 3 patients were sent to mass spectrometry analysis using MALDI-TOF spectrometer (LTQ Orbitrap XL, Thermo Scientific®). Simultaneously, we conducted a search on the NCBI and PDB databanks for Bm proteins with approximate molecular weight to the reactive proteins found in Western blot analysis that were described from the adult form of Bm moth and/or were involved in allergic processes. We evaluated only the major allergens, considered when the protein was detected by binding in more than 50% of the participants serum.
ResultsSera from the 21 Bm SPT positive patients, eight house-dust mite SPT positive patients and seven healthy controls were submitted to Western blot analysis in order to identify patterns of IgE reactivity to BmWE.
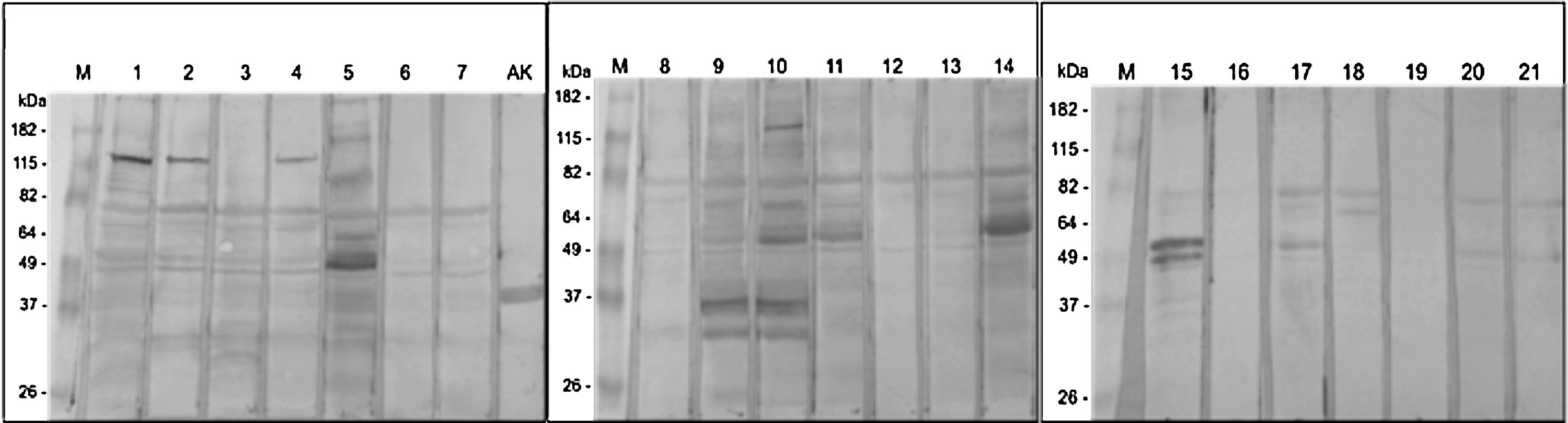
Among the 21 moth SPT positive patients, two sera (numbers 16 and 19) showed no reactivity to BmWE by Western blot. The remaining 19 reactive patients demonstrated different patterns of reactivity. Some reacted strongly to few proteins, some weakly to several ones. All of them reacted towards a protein at 80 kDa (Fig. 1). Five other proteins at approximately 66, 50, 45, 37 and 30 kDa were identified in more than 50% of the individuals tested which were considered major allergens.13
Western blot of BmWE with sera from 21 allergic SPT positive to moth extract patients. AK) Recombinant arginine kinase as reaction positive control M) Pre-stained molecular mass marker (Bench marker). A, B, C) Membranes incubated with positive patients’ sera. 1-21) Lanes with respective sera from group 1 patients.
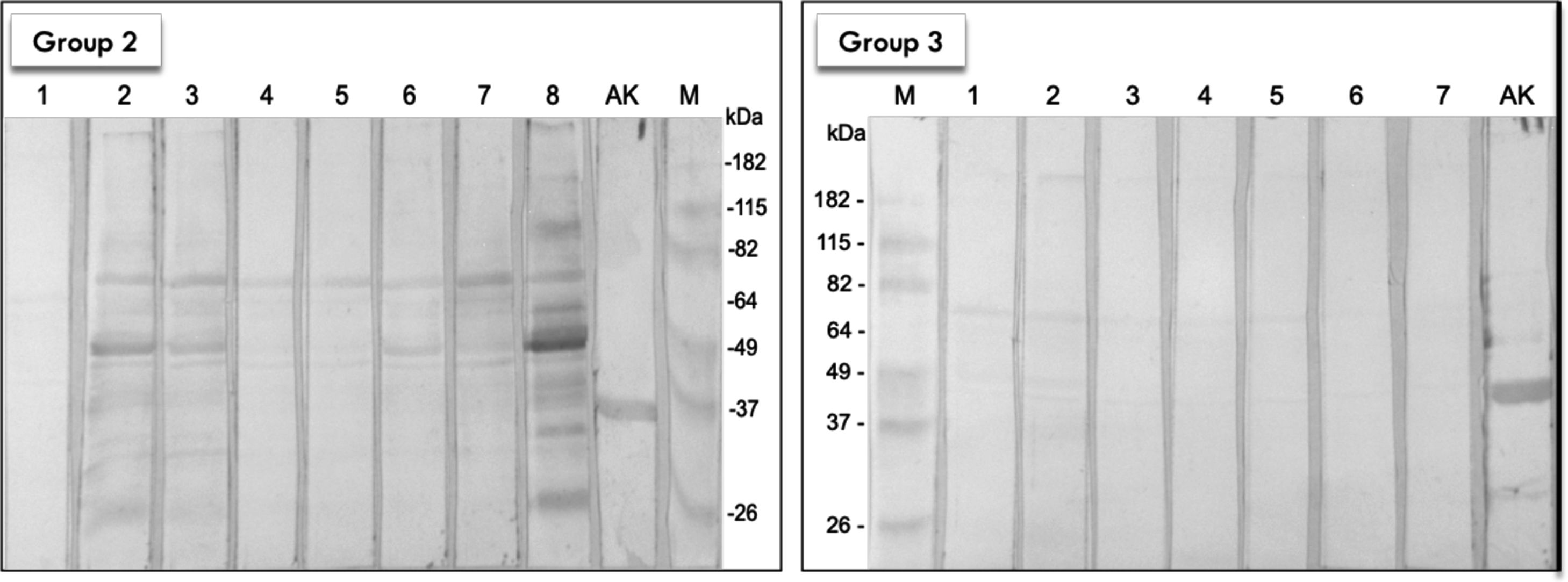
Seven out of eight house-dust mite SPT positive patients demonstrated reactivity to BmWE by Western blot analysis. Their sera showed less and weaker reactions when compared to the 19 moth SPT positive patients. Sera from all subjects of the IgE-reactive group 2 reacted towards a protein at 66 kDa. Two other proteins at approximately 50 and 40 kDa were identified in more than 50% of the individuals tested. On the other hand, serum samples from the healthy individuals did not exhibit reactivity to the BmWE (Fig. 2).
Western blot of BmWE with sera from control groups. AK) Recombinant arginine kinase expressed in E.coli as reaction positive control. M) Pre-stained molecular mass marker (Bench marker). Group 2) Membrane incubated with eight allergic SPT positive to mite-extract patients. Group 3) Membrane incubated with nine non-allergic SPT negative patients. 1-8) Lanes with respective sera from group 2 patients. 1-7). Lanes with respective sera from group 3 patients.
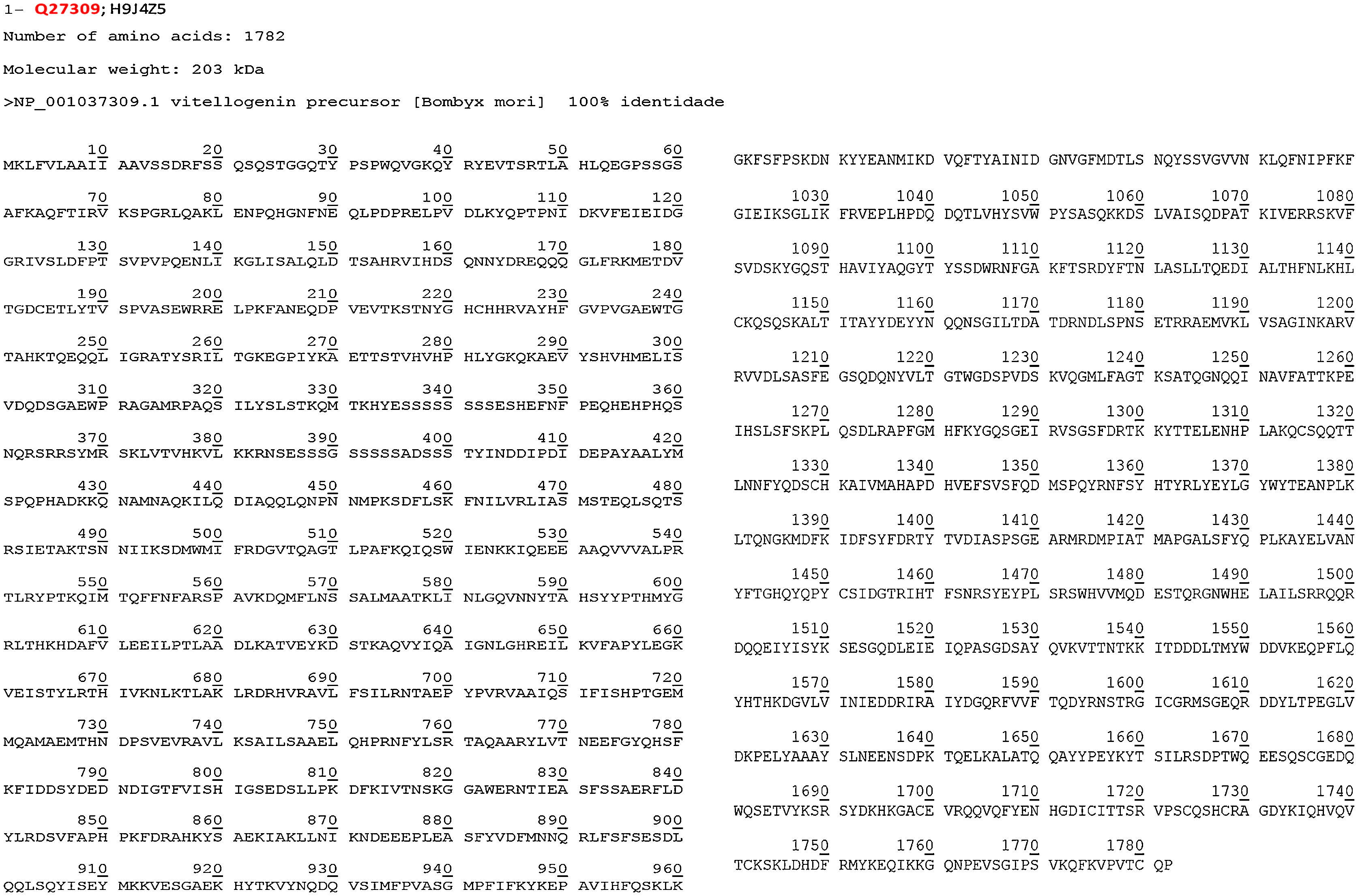
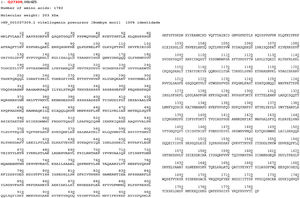
The mass spectrometry analysis revealed only the following two proteins: vitellogenin at 45 kDa (whose amino acid sequence is shown in Fig. 3) and actin at 200 kDa. The last one presented quite a small amount of peptides, and therefore cannot be considered a relevant protein. These results suggest that further investigation should be made to confirm and identify other allergenic proteins from Bm wing extract, perhaps using a gel after Bm extract purification. More than 3000 items describing Bm-related proteins were found in the databank (NCBI and PBD) search. Based on criteria defined in methods, possible allergenic proteins were selected by similar molecular weight of each reactive protein in Western blot analysis as discussed above.
DiscussionGiven the high prevalence of asthma and rhinitis and its impact on public health worldwide,14,15 identification of the sensitizing allergens is essential. Inhalant allergens from moths, butterflies, mosquitos and cockroaches have been described as triggers of respiratory allergic symptoms, although their importance as an underlying cause of allergic diseases is underestimated.16
Studies on respiratory allergy to silkworm moth, causing symptoms of asthma and allergic rhinitis have been conducted, both in a domestic and an occupational environment.17,18 Nevertheless, the main allergenic proteins involved in these immunological reactions have not been thoroughly described.
More than 300 proteins were identified in emanating fluids during silkworm moth metamorphosis.19 However, only few proteins have been identified as specific allergens of Bm, yet none of them in its adult phase.
Development of a recombinant form, performance of immunoassays and mass spectrometry analysis are required to determine whether a protein is a true allergen.20 In the present study, immunoassays were performed in sera from allergic and non-allergic patients using Bm-wing crude extract. Six major allergenic proteins have been revealed, although only one (vitellogenin) was confirmed by mass spectrometry.
Vitelline is the most abundant protein in insect eggs and its precursor, vitellogenin is important during the reproductive cycle of various insect species. Yano et al. described that protein as an antibacterial agent from Bm pupa.21 Whilst in our study, the 45 kDa protein identified in moth-sensitized participants has an allergenic potential.
Antico et al. described a case of a patient with symptoms of respiratory allergy to the species of Lepidoptera Galleria mellonella who had serum IgE-reactive proteins detected at 27, 40 and 45 kDa22 by immunoblot. As identified in the present study, light chain vitellogenin (45 kDa protein) was seen in about 85% of the patients. There is a 90% protein homology between lepidoptera (moths and butterflies),23 possibly indicating a similar pattern of allergenic proteins.
Plodia interpunctella, a moth species considered a food plague in some developed countries, both in domestic environments and storage locations, was defined as a sensitizing agent in 51% of patients with respiratory allergy. This study identified arginine kinase protein Plo i 1, which cross-reacted with mite, cockroaches and shrimp allergens. Reaction to a component at 66 kDa has also been described.24 It is a potential allergen, still to be identified as a Bm major allergenic component.
In our study, the majority of sensitized Bm patients (approximately 90%) and almost all of those sensitized to mites presented reactivity by Western blot to a 66 kDa protein. This suggests a cross-reactive component between them.
The case of a farmer diagnosed with asthma and allergic rhinitis, whose property was infested by crickets, was investigated. She reacted to three cricket species by SPT as well as bronchial provocation test to a crude-cricket extract. Immunoblot revealed presence of the proteins 107, 80, 58 and 52 kDa.25
Protein at 50 kDa found in our study might correspond to that at 52 kDa, as it is not possible to precisely identify molecular mass in Western blot assays. Some proteins are highly conserved in insects,23 so they might be common among different species of moths and crickets, in addition to a potential cross-reaction. We did not find a correspondent in the data-base research of the 80 kDa protein, present in all reactive moth-sensitized participants and in none of the house-dust mite sensitized ones. It is therefore an important and specific allergenic protein from Bm.
Bomb m 1 was the first allergenic component identified from Bm larvae.26 In our study, nine of 21 patients from group 1 reacted to a 40 kDa protein (arginine kinase), not considered a major protein. That could be explained by population differences, as well as diverse sources and preparation of crude BmWE. In the Chinese study, occupational allergy patients were selected, whilst in our study, sensitization probably occurred in the domestic environment and the extracts were made respectively from larvae and adult moth wings. On the other hand, Liu et al. also described a 50 kDa protein in all sera tested. That was observed in most of our patients, nevertheless, that protein was not the aim of the Chinese study, thus not identified until present time.
Among mite-sensitized patients from group 2, all reactive proteins were common to both groups (1 and 2). Some of these proteins might be pan-allergens like tropomyosin, which is a conserved protein among insects and arthropods.27,28 As described in an Italian study where immunoassays were performed in moths, cockroaches and dust mites detecting a component at 37 kDa, potentially tropomyosin.29
In the present study, most patients from both groups 1 and 2 reacted to a protein at 37 kDa, possibly tropomyosin, which can have a cross-reactive potential. A retrospective analysis of serum IgE to silkworm moth and nine common inhalant allergens among patients with respiratory allergy in China, showed a positive correlation between allergies to silkworm moths and cockroaches or house-dust mites.30
A lipoprotein (present on insect’s hemolymph) was described as another allergic component from Bm pupa in individuals with occupational asthma that corresponded to a 30 kDa protein by immunoblot.11 The same component reacted in approximately 70% of our moth sensitized patients. Some proteins might be conserved during the Bm metamorphosis process from larvae to adult moth, so they may be the same protein.29
In conclusion, this study revealed proteins that could potentially cause respiratory allergic reactions from silkworm moth Bm-sensitized patients. The one at 80 kDa, present in all reactive sera from moth sensitive extract patients and in none of the mite-sensitive extract participants, is an important and specific protein from Bm wings. Some of the other proteins might be pan-allergens, possibly cross-reacting with other insects and arthropods like house-dust mites. Further studies are necessary to completely identify and characterize these proteins.
Ethical disclosuresProtection of human and animal subjects. The authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance whit those of the World Medical Association and the Helsinki Declaration.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consent. The authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author of correspondence is in possession of these documents.
Financial supportCorresponding author was supported by scholarship from CAPES, Brazil.
Conflict of interestThe authors have no conflict of interest to declare.