Wheat and cereal grains have a broad range of cross-reactivity, but the clinical relevance of this cross-reactivity is uncertain. This study aimed to evaluate clinical and in vitro cross-reactivity with barley, oat, and Job’s tears among wheat-allergic patients.
Materials and MethodsPatients aged 5 to 15 years with IgE-mediated wheat allergy were enrolled. Skin prick test (SPT) and specific IgE (sIgE) to wheat, barley, and oat, and SPT to Job’s tears were performed. Oral food challenge (OFC) was conducted if the SPT was ≤5 mm in size and there was no history of anaphylaxis to each grain. Profiles of sIgE bound allergens of wheat, barley, and oat, and inhibition ELISA of IgE binding to barley and oat with wheat were performed.
ResultsTen patients with a median age of 8 years were enrolled. Nine of those patients had a history of wheat anaphylaxis. The median SPT size and sIgE level to wheat was 7.3 mm and 146.5 kUA/l, respectively. The cross-reactivity rate for barley, oat, and Job’s tears was 60.0%, 33.3%, and 20.0%, respectively. Significantly larger SPT size and higher sIgE level were observed in patients with positive cross-reactivity to barley and oat when compared to patients without cross-reactivity. Barley and oat extracts inhibited 59% and 16% of sIgE bound to wheat gliadins and glutenins, respectively.
ConclusionThe cross-reactivity rate was quite low for oat and Job’s tears compared to that of barley; therefore, avoidance of all cereal grains may be unnecessary in patients with severe wheat allergy.
The prevalence of wheat allergy is currently increasing, with a reported prevalence of 0.4-4% in the general population.1,2 The prevalence of wheat allergy is also rising among the Thai population, and wheat is now another leading cause of food allergy, in addition to cow’s milk and egg.3,4 It is clear that wheat has a broad range of in vitro cross-reactivity among other cereal grains in the same family (Poaceae), and prolamines are responsible for the observed cross-reactivity between gliadins in wheat and hordeins in barley, avenins in oat, secalins in rye, and coixins in Job’s tears.5–12 However, only a few studies have described the clinical relevance of this cross-reactivity, and the results of those studies were inconclusive,13–15 especially for Job’s tears (Coix lachryma-jobi L.). Job’s tears is another type of cereal grain that has gained in popularity among Asians,11,16 and it can be consumed in a variety of forms (Supplementary Fig. 1). In addition to the necessary adoption of the wheat elimination diet, patient concerns about cross-reactivity between wheat and other grains has led to unnecessary avoidance of related grains.17 The aim of this prospective study was to evaluate the clinical cross-reactivity of cereal grains (barley, oat, and Job’s tears) in children with IgE-mediated wheat allergy as the primary outcome. Profiles of IgE bound allergens of wheat, barley, oat, and inhibition ELISA of IgE binding to barley and oat with wheat were also performed to determine cross-reactivity.
MethodsPatients aged 5 to 15 years old with a history of hypersensitivity reactions within four hours after wheat ingestion during 2000–2015 were identified from the Allergy Clinic, Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand. Diagnosis of IgE-mediated wheat allergy was made based on either convincing clinical history of reactions during the past one year before enrollment combined with positive skin prick test (SPT) to wheat extracts (1:10 w/v) in Coca’s solution and 10% alcohol ≥3 mm18, and/or a level of specific IgE (sIgE) to wheat >26 kUA/l19, or a positive physician-supervised oral food challenge (OFC) test result to wheat within one year.
We excluded patients with wheat-allergic reactions greater than four hours or wheat-dependent exercise-induced anaphylaxis. This study was approved by the Siriraj Institutional Review Board (SIRB) (COA no. 299/2016). Written informed consent from parents or guardians and assent from children older than 7 years of age were obtained.
Demographic data and clinical manifestations were recorded. The diagnosis of other allergic diseases and other types of food allergy were performed by allergists. Anaphylaxis was defined according to the clinical criteria from the World Allergy Organization anaphylaxis guidelines.20
SPT and sIgE to cereal grainsSPT with wheat, barley, oat, and Job’s tears extracts (1:10 w/v) in Coca’s solution and 10% alcohol was performed on the patient’s forearm using a lancet. The extraction method was previously reported for wheat18 and was modified for other grains in this study. Histamine phosphate (10 mg/mL) and glycerinated saline were used as positive and negative controls, respectively. The result of the SPT was considered to be positive if the size of the mean wheal diameter (MWD) was at least 3 mm compared to the negative control.
Levels of sIgE to wheat, omega-5 gliadin (ω5G), barley, and oat were measured using the ImmunoCAP System (Phadia, Uppsala, Sweden). This assay has a lower limit of detection of less than 0.35 kUA/l.
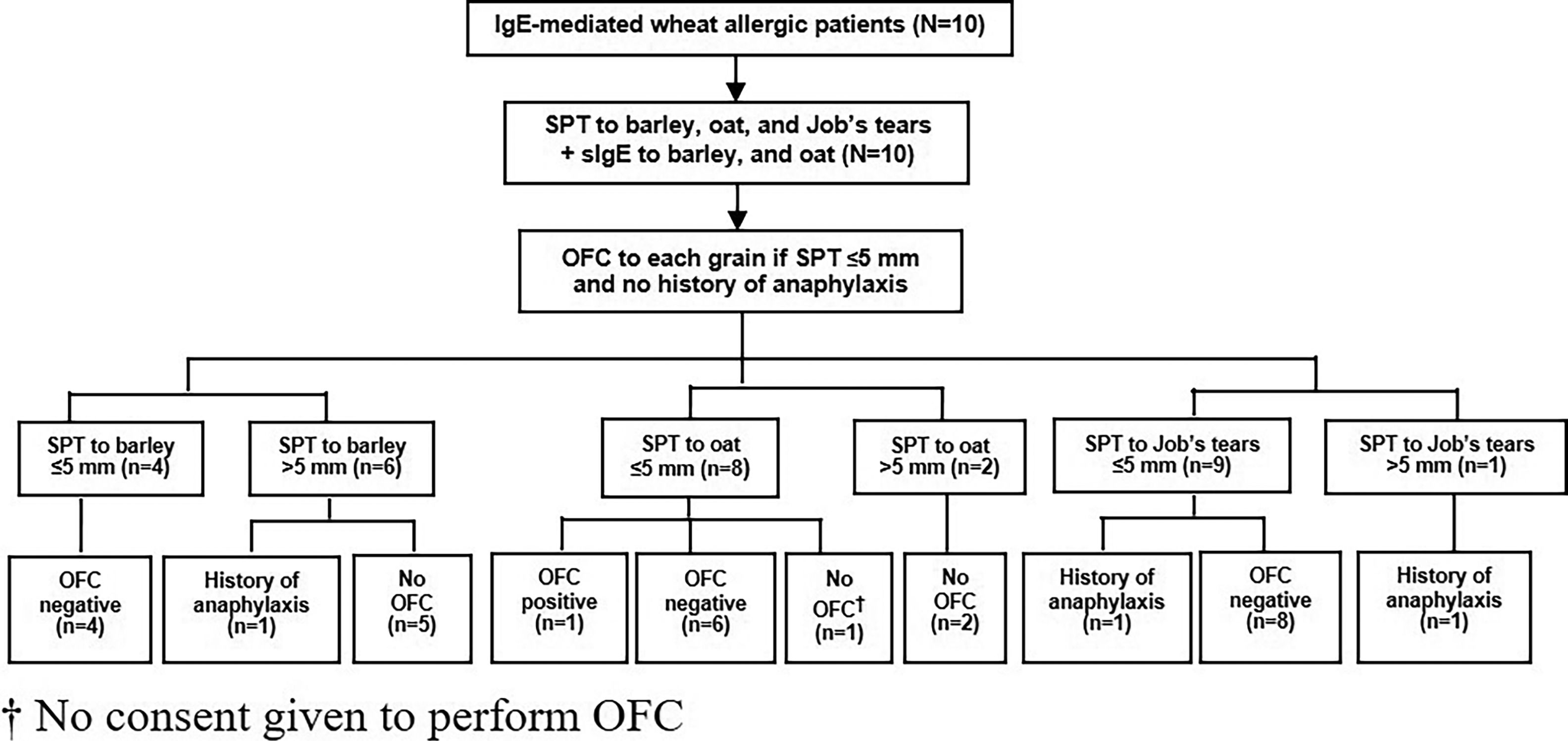
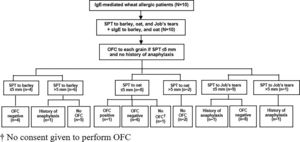
Oral food challenge (OFC)Since severe reaction of IgE-mediated allergy can occur, we performed OFC only in patients with no history of anaphylaxis and in patients with a SPT size for each grain that did not exceed 5 mm. The study flow chart is shown in Fig. 1.
Patients underwent a medically supervised open OFC consisting of a cumulative dose of 30 g each of cooked barley, Job’s tears, and instant oats (0.1, 0.5, 1.0, 2.0, 4.0, 8.0, and 14.4 g at 30 min intervals). Barley and Job’s tears were prepared by soaking in water for 2 h, followed by 15 min of boiling. Instant oats (McGarrett®, Australia) were mixed in hot water. OFC was considered positive if any type of allergic reaction consistent with IgE-mediated symptoms developed within 24 h after the last dose of food taken during the OFC. The first 2 h of observation was performed at the hospital challenge unit, after which parents were instructed to observe their child’s symptoms at home during the remaining 22 h of the 24 h evaluation period. Parents were advised to contact an investigator immediately if any adverse reaction occurred. OFC was considered negative if no reaction occurred after this period. Each challenge was performed at least one week apart, and all challenges were completed within six months after enrollment.
Profiles of IgE bound allergens and inhibition of IgE ELISAExtraction of proteins from wheat, barley, and oat was performed based on previously described serial extraction protocols.18 Protein concentration of extracts was determined by bicinchoninic acid (BCA) protein assay.
For profiles of IgE bound allergens or immunoblot, 20 μg per well of wheat, barley, and oat extracts (alcohol soluble) were resolved in 16% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel before proteins were electro-transferred onto a nitrocellulose membrane. The membrane was incubated in phosphate-buffered saline (PBS) containing 0.2% v/v Tween-20 and 3% w/v skim milk (PBS-A) for 1h at room temperature (RT). The membrane was washed with PBS containing 0.2% v/v Tween-20 (PBS-B) before incubation with diluted sera of patients and with sera of non-sensitized volunteers in PBS-A overnight at 4 °C. The membrane was washed with PBS-B before incubation with 1:10,000 diluted horseradish peroxidase (HRP) conjugated goat IgG anti-human IgE antibody (KPL, Gaithersburg, MD, USA) in PBS-A for 1h. After washing, the membrane was incubated with chemiluminescent HRP substrate (Millipore, Burlington, MA, USA) and the emitted signal was exposed to X-ray film.
For inhibition of IgE ELISA, 500 ng of gliadin and glutenin extracts in PBS was added per well in 96-well Maxisorb plates (Nunc A/S, Roskilde, Denmark) and incubated at 4 °C overnight. The coated 96-well plate was washed with PBS-A. Sera of patients (based on sIgE level from results of ImmunoCap) and sera of controls were diluted 1/5-1/25 in PBS-A. Diluted sera were incubated with serially diluted barley and oat extracts (alcohol soluble) in PBS-A for 2 h at RT. The extract-coated 96-well plate was washed with PBS-A. The absorbed sera were centrifuged at 17,210 g for 10 min before the supernatant was added to the extract-coated 96-well plate and incubated for 2 h at RT. The plate was washed before diluted HRP-labelled goat IgG anti-human IgE antibody was added. Substrate was added to the plate and the color signal of each well was measured by absorbance at OD650 nm. All experiments were performed in duplicate.
Statistical analysisAll analyses were performed using SPSS version 18.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as median, median and interquartile range (IQR), or percentage. Boxplots of SPT size and sIgE level to each grain were generated and compared among patients with positive cross-reactivity to each grain compared to the negative group. Inhibition of IgE ELISA was calculated as mean and standard error of the percent inhibition obtained for each of the different sera, and p-values were calculated using one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons test. A p-value less than 0.05 indicates statistical significance.
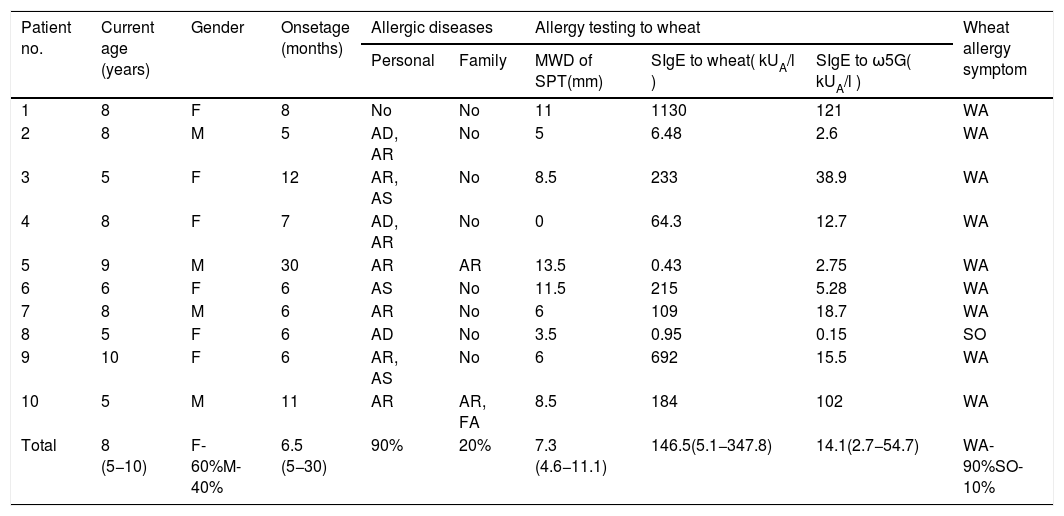
ResultsTen patients were included in this study (six girls and four boys). The median age at enrollment and onset of symptoms was 8 years (range: 5–10) and 6.5 months (range: 5–30), respectively. Nine patients (90%) had other allergic diseases, and four of them (40%) reacted to more than one food. Family history of allergic diseases was found in two patients (20%) (Table 1).
Clinical characteristics of patients, and the results of allergy testing to wheat.
| Patient no. | Current age (years) | Gender | Onsetage (months) | Allergic diseases | Allergy testing to wheat | Wheat allergy symptom | |||
|---|---|---|---|---|---|---|---|---|---|
| Personal | Family | MWD of SPT(mm) | SIgE to wheat( kUA/l ) | SIgE to ω5G( kUA/l ) | |||||
| 1 | 8 | F | 8 | No | No | 11 | 1130 | 121 | WA |
| 2 | 8 | M | 5 | AD, AR | No | 5 | 6.48 | 2.6 | WA |
| 3 | 5 | F | 12 | AR, AS | No | 8.5 | 233 | 38.9 | WA |
| 4 | 8 | F | 7 | AD, AR | No | 0 | 64.3 | 12.7 | WA |
| 5 | 9 | M | 30 | AR | AR | 13.5 | 0.43 | 2.75 | WA |
| 6 | 6 | F | 6 | AS | No | 11.5 | 215 | 5.28 | WA |
| 7 | 8 | M | 6 | AR | No | 6 | 109 | 18.7 | WA |
| 8 | 5 | F | 6 | AD | No | 3.5 | 0.95 | 0.15 | SO |
| 9 | 10 | F | 6 | AR, AS | No | 6 | 692 | 15.5 | WA |
| 10 | 5 | M | 11 | AR | AR, FA | 8.5 | 184 | 102 | WA |
| Total | 8 (5−10) | F-60%M-40% | 6.5 (5−30) | 90% | 20% | 7.3 (4.6−11.1) | 146.5(5.1−347.8) | 14.1(2.7−54.7) | WA-90%SO-10% |
Data presented as median, median and interquartile range, or percentage.
Abbreviations: AC, allergic conjunctivitis; AD, atopic dermatitis; AR, allergic rhinitis; AS, asthma; FA, food allergy; F, female; M, male; MWD, mean wheal diameter; sIgE, specific IgE; SPT, skin prick test; SO, skin only symptom; WA, wheat anaphylaxis.
Nine patients (90%s) had a history of wheat anaphylaxis, and the one remaining patient had only cutaneous symptoms. The median SPT size to wheat extracts was 7.3 mm (IQR: 4.6−11.1). The median sIgE level to wheat and ω5G was 146.5 kUA/l (IQR: 5.1−347.8) and 14.1 kUA/l (IQR: 2.7−54.7), respectively (Table 1).
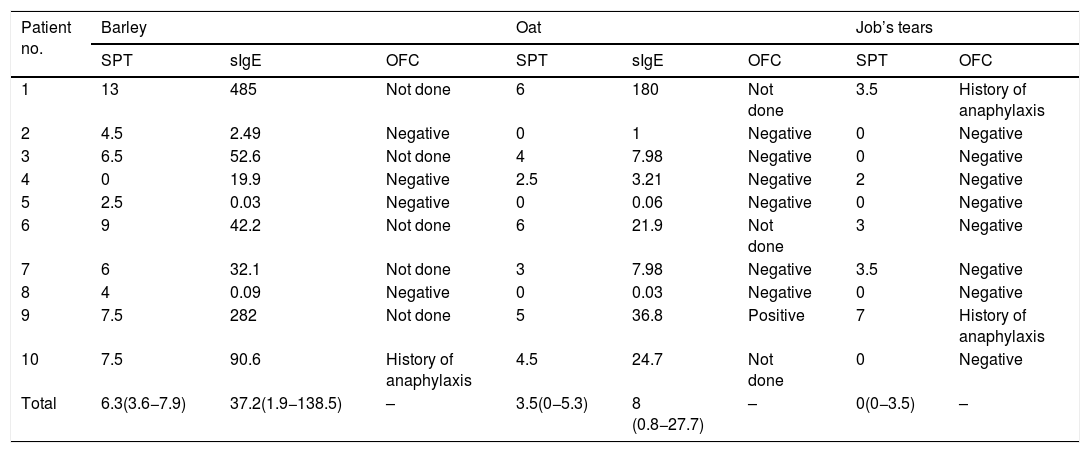
The median size of the SPT and the median level of sIgE to barley was 6.3 mm (IQR: 3.6−7.9) and 37.2 kUA/l (IQR: 1.9−138.5), respectively. All four of the patients with SPT size to barley ≤5 mm had a negative OFC result. Among those with SPT size >5 mm, history of anaphylaxis was reported in one patient, and the other five patients did not undergo OFC (Table 2 and Fig. 1).
MWD of SPT (mm), sIgE level (kUA/l), and the result of OFC to cereal grains.
| Patient no. | Barley | Oat | Job’s tears | |||||
|---|---|---|---|---|---|---|---|---|
| SPT | sIgE | OFC | SPT | sIgE | OFC | SPT | OFC | |
| 1 | 13 | 485 | Not done | 6 | 180 | Not done | 3.5 | History of anaphylaxis |
| 2 | 4.5 | 2.49 | Negative | 0 | 1 | Negative | 0 | Negative |
| 3 | 6.5 | 52.6 | Not done | 4 | 7.98 | Negative | 0 | Negative |
| 4 | 0 | 19.9 | Negative | 2.5 | 3.21 | Negative | 2 | Negative |
| 5 | 2.5 | 0.03 | Negative | 0 | 0.06 | Negative | 0 | Negative |
| 6 | 9 | 42.2 | Not done | 6 | 21.9 | Not done | 3 | Negative |
| 7 | 6 | 32.1 | Not done | 3 | 7.98 | Negative | 3.5 | Negative |
| 8 | 4 | 0.09 | Negative | 0 | 0.03 | Negative | 0 | Negative |
| 9 | 7.5 | 282 | Not done | 5 | 36.8 | Positive | 7 | History of anaphylaxis |
| 10 | 7.5 | 90.6 | History of anaphylaxis | 4.5 | 24.7 | Not done | 0 | Negative |
| Total | 6.3(3.6−7.9) | 37.2(1.9−138.5) | – | 3.5(0−5.3) | 8 (0.8−27.7) | – | 0(0−3.5) | – |
Data presented as median and interquartile range.
Abbreviations: MWD: mean wheal diameter; SPT: skin prick test; OFC: oral food challenge; sIgE: specific IgE.
Regarding oat, the median size of the SPT and the median level of sIgE was 3.5 mm (IQR: 0−5.3) and 8 kUA/l (IQR: 0.8−27.7), respectively. Among the eight patients with SPT size ≤5 mm, seven underwent OFC and one patient with an SPT size of 5 mm developed anaphylaxis. We were not given consent to perform OFC by the parents of the remaining child. OFC was not performed in the other two patients with SPT size >5 mm (Table 2 and Fig. 1).
The median size of the SPT to Job’s tears was 0 mm (IQR: 0−3.5). Among the nine patients with SPT size to Job’s tears ≤5 mm, history of anaphylaxis was reported in one patient and the other eight patients had a negative OFC test result. Only one patient had SPT size >5 mm, and that patient had a history of anaphylaxis to Job’s tears (Table 2 and Fig. 1).
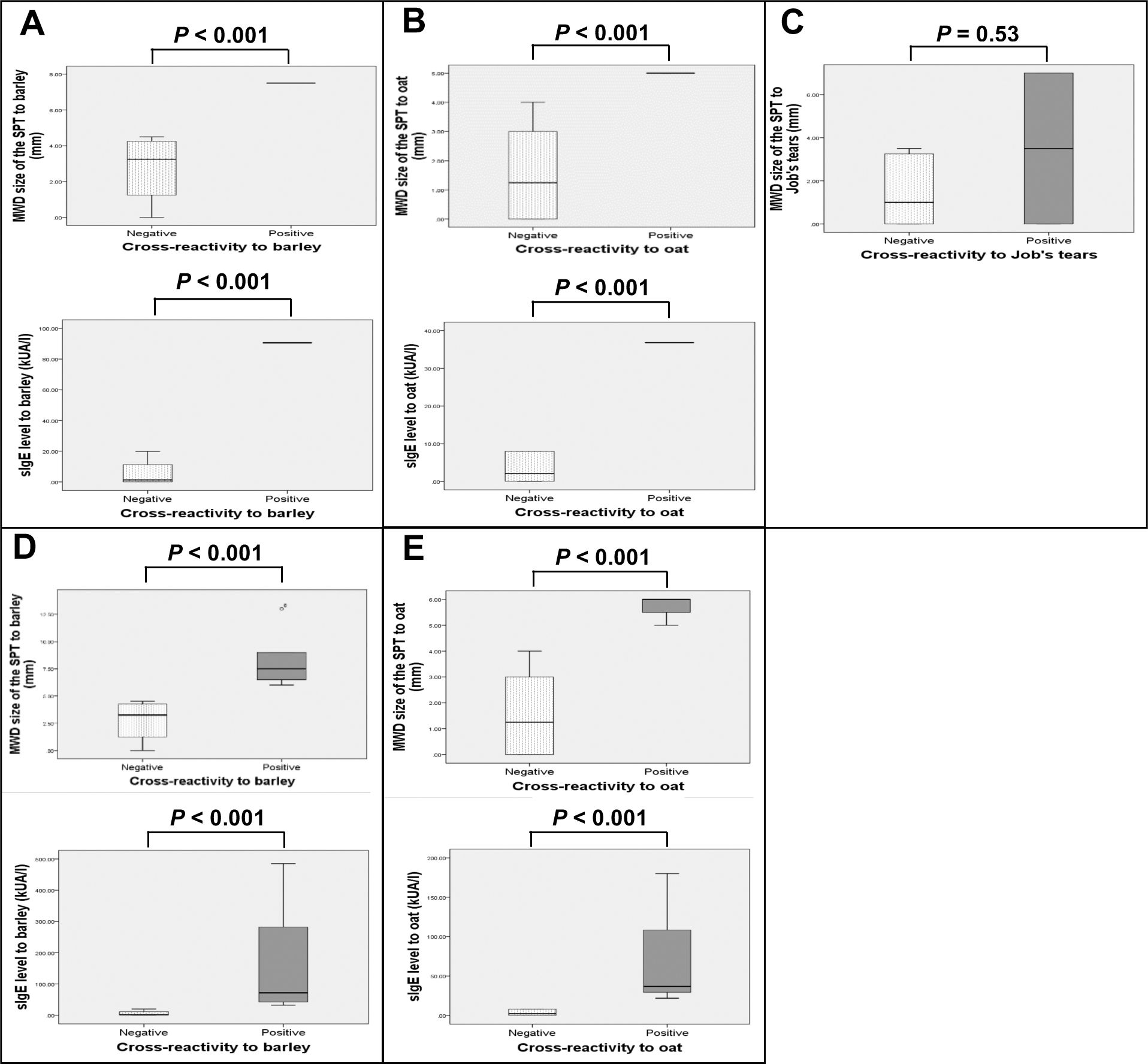
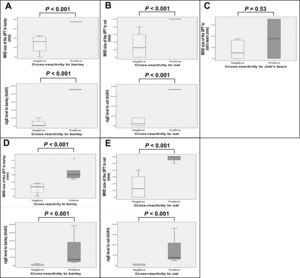
Boxplots of SPT size and sIgE level to each grain were generated and compared between patients with and without cross-reactivity (Fig. 2). Analysis was performed either by including only patients who underwent OFC and those with history of anaphylaxis from barley (Fig. 2A), oat (Fig. 2B), or Job’s tears (Fig. 2C), or by the same number of previous patients in addition to those with a SPT size >5 mm and a likelihood of having positive cross-reactivity to barley (Fig. 2D) or oat (Fig. 2E). Larger SPT size and higher level of sIgE were found to be statistically significant (P < 0.001) for both analyses among patients with positive cross-reactivity to barley or oat, as compared to the negative group. However, only a trend toward larger SPT size for Job’s tears was observed (P = 0.53).
Boxplots of the MWD size of the SPT (mm), and sIgE level (kUA/l) to barley (A†,D‡), oat (B§,E¶), and Job’s tears (C#) among wheat-allergic patients with positive cross-reactivity compared to those without cross-reactivity.
Analyses included the following number and clinical types of patients: †: 5 patients (n = 1 history of anaphylaxis, n = 4 negative OFC), ‡:10 patients (n = 1 history of anaphylaxis, n = 5 with SPT size >5 mm and likely to have positive OFC, n = 4 negative OFC), §: 7 patients (n = 1 positive OFC, n = 6 negative OFC), ¶: 9 patients (n = 1 positive OFC, n = 2 with SPT size >5 mm and likely to have positive OFC, n = 6 negative OFC), and #: 10 patients (n = 2 history of anaphylaxis, n = 8 negative OFC)
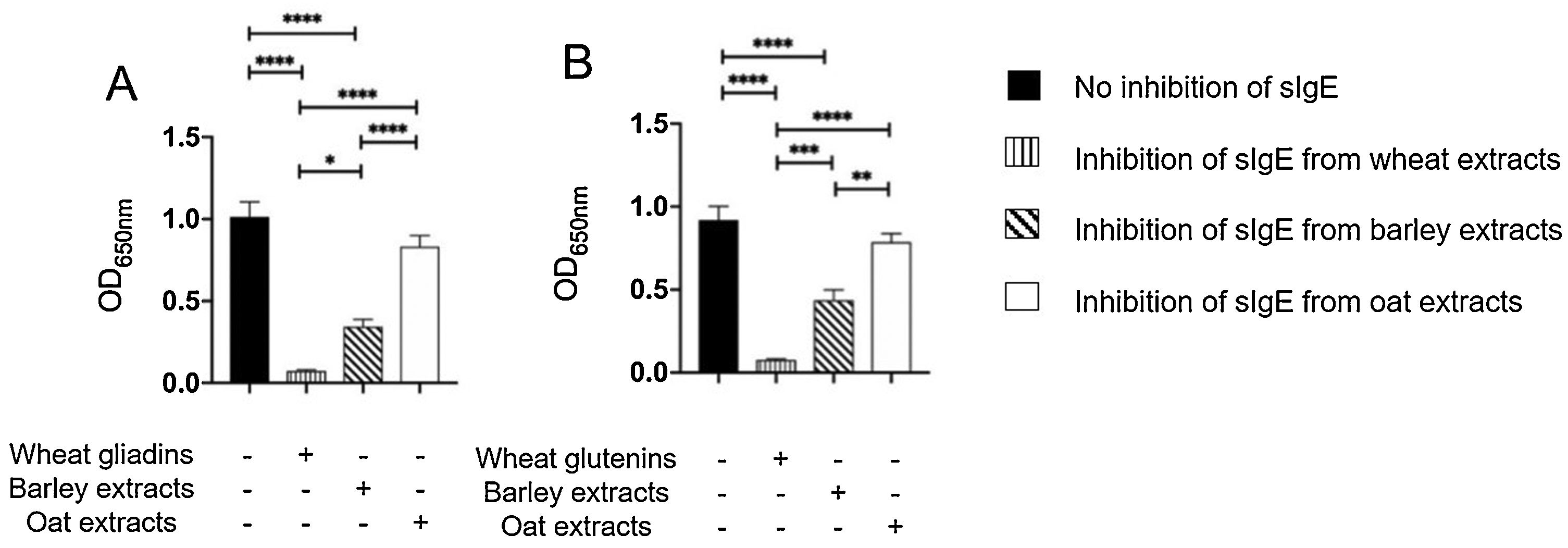
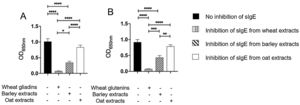
The results of immunoblot showed that all ten patients had sIgE bound to gliadins and glutenins of wheat proteins with MWD of 30–35 and 45−48 kDa, (Supplementary Fig. 2A). Moreover, nine of ten patients had sIgE bound to 50 and 64 kDa of barley (Supplementary Fig. 2B), and sIgE bound to 34 kDa oat protein (Supplementary Fig. 2C). The results of inhibition of IgE ELISA showed that extract of barley proteins inhibited 66% of sIgE bound to wheat gliadins, whereas extract of oat proteins inhibited only 18% of sIgE bound to wheat gliadins (Fig. 3A). Furthermore, extract of barley proteins inhibited 53% of sIgE bound to wheat glutenins, whereas extract of oat proteins inhibited only 15% of sIgE bound to wheat glutenins (Fig. 3B).
Inhibition of IgE ELISA showed that A) extracts of barley and oat proteins could inhibit 66% and 18%, respectively, of sIgE bound to wheat gliadins, and that B) extracts of barley and oat proteins could inhibit 53% and 15%, respectively, of sIgE bound to wheat glutenins, as compared to negative control (no inhibition of sIgE) and positive control (inhibition of sIgE from wheat extracts).
* P < 0.05, ** P < 0.001, *** P < 0.0001, **** P < 0.00001
This study shows that cross-reactivity of cereal grains is quite low for oat and Job’s tears when compared to barley, even in patients with wheat anaphylaxis. Jones et al.13 conducted OFC to assess the degree of intra-botanical cross-reactivity between cereal grains and found that among 26 patients with wheat hypersensitivity reaction, only 20% had positive challenge responses to one or more of the other cereal grains, including barley, oat, rye, and corn.
In this study, we found that no reactions occurred among the four patients with SPT size ≤5 mm that underwent OFC to barley. However, one patient with SPT size result >5 mm to barley reported severe clinical reactivity after consuming these grains. The cross-reactivity rate among these five patients was 20% (1/5 patients). Moreover, the other five patients who did not undergo OFC showed a significantly larger size of SPT and a higher level of sIgE to barley, which suggests that these patients had a high probability of developing a clinical reaction and this would increase the cross-reactivity rate to 60%. This is similar to the study from Pourpak et al.14 which found that 55% of patients with IgE-mediated wheat allergy had a positive barley challenge test result.
Positive reaction to oat developed in one of seven patients who underwent OFC for a cross-reactivity rate of 14.3% (1/7 patients). However, another two patients with an SPT size result to oat >5 mm had a high probability of developing a clinical reaction; thus, the cross-reactivity rate would be 33.3% (3/9 patients). This rate is higher than the rate reported by Varjonen E et al.15 who evaluated clinical cross-reactivity between oat and wheat by OFC among children with severe atopic dermatitis (AD). They found that 23.8% of patients with severe AD in conjunction with IgE-mediated wheat allergy developed a positive OFC result to oat. Although their study included patients with severe AD, all included patients were confirmed to have an IgE-mediated reaction to wheat by OFC.
The cross-reactivity rate for Job’s tears was 20% (2/10 patients), and these two patients also showed a positive SPT result (3.5 and 7 mm). Clinical cross-reactivity to Job’s tears was observed previously among our wheat-allergic patients. However, we did not perform any in vitro testing of Job’s tears in this study.
Significantly larger SPT size and higher sIgE level to barley and oat, and only a trend toward larger SPT size to Job’s tears were observed in patients with positive cross-reactivity, as compared to patients without cross-reactivity. This finding may be another tool for predicting cross-reactivity in addition to OFC test.
Interestingly, the sIgE-inhibition ELISA results showed that allergens in barley extracts share some similarity with allergens in wheat gliadin and glutenin extracts. In contrast, allergens in oat extracts may be different from allergens in wheat gliadin and glutenin extracts. These findings support the outcome of our clinical reactivity analysis that IgE-mediated wheat-allergic patients would have positive responses to barley during the food challenge test, while IgE-mediated oat allergy may be caused by allergens independent from the allergens in wheat gliadin and glutenin extracts.
This is the first study to demonstrate both clinical and laboratory cross-reactivity of cereal grains, including Job’s tears, among patients with severe wheat allergy. The limitations of this study include its small sample size and the fact that all patients were recruited from a single center. In addition, some of our patients did not undergo OFC, particularly those with higher SPT size to cereal grains due to concerns about patient safety; however, the result of sIgE-inhibition ELISA could support our clinical cross-reactivity outcome. This pilot study will be followed by a further prospective study in a larger study population that will give our study the statistical power needed to identify all significant associations and differences. Lastly, the non-concordance of our study outcome relative to rate and type of cereal grain cross-reactivity compared to other studies may be due to the fact that patients with different severity of allergic reaction to wheat were enrolled, and that the OFC protocols used varied among studies.
In conclusion, the cross-reactivity rate was quite low for oat and Job’s tears when compared with that of barley; therefore, avoidance of all cereal grains may be unnecessary in patients with severe wheat allergy. SPT size and sIgE level to barley and oat may be helpful for determining cross-reactivity.
Clinical trial registrationThis study was registered with the Thai Clinical Trials Registry (reg. no. TCTR20160628001).
Conflict of interest declarationAll authors declare no personal or professional conflicts of interest, and no financial support from the companies that produce and/or distribute the drugs, devices, or materials described in this report.
Funding disclosureThis study was funded by a Siriraj Grant for Research Development, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand (grant number 006/2559).
The authors gratefully acknowledge the patients that participated in this study and Ms. Julaporn Pooliam of the Division of Clinical Epidemiology, Department of Research and Development, Faculty of Medicine Siriraj Hospital, Mahidol University for assistance with statistical analysis.