This study aimed to compare the effects of different biotinylation methods on the performance characteristics of allergen-specific IgE detection.
Materials and methodsThe Gal d 6 gene was cloned into the pAN6/pAC6 vector, resulting in rGal d 6-Bio/Bio-rGal d 6 vector. The fusion protein was expressed in Escherichia coli AVB101 and simultaneously biotinylated in a site-specific manner. The Gal d 6 gene was amplified via PCR and cloned into the pET-28a vector and transformed into E. coli BL21 and purified via Ni-NTA, followed by chemical biotinylation using Sulfo-NHS-LC-Biotin. Twenty-eight patients allergic to hen's egg white were examined for sensitization against egg yolk. An antigen-capture enzyme-linked immunosorbent assay (AC-ELISA) was developed to detect allergen-specific IgE.
ResultsrGal d 6, Bio-rGal d 6, and rGal d 6 were prepared using different biotin binding modes to detect allergen-specific IgE. rGal d 6-Bio (Kd=0.6154) and Bio-rGal d 6 (Kd=0.6698) had a markedly better detection performance than rGal d 6 (Kd=28.93), and the rGal d 6-Bio had a better detection performance in small-volume serum samples.
ConclusionsrGal d 6-Bio improved the sensitivity for the detection of allergen-specific IgE.
Serological examination based on specific IgE (sIgE) is a predominant diagnostic test for IgE-mediated food allergies.1 Among all types of sIgE-detection methods, the most widely applied approach is the enzyme-linked immunosorbent assay (ELISA).2 ELISA is a sensitive and specific analytical method that does not require sophisticated or expensive equipment.3 ELISA requires high-purity antigens, a potential solution to this is indirect encapsulation. Moreover, complex samples may be analyzed because proteins can be purified with a tag through the cleaning step.4 Furthermore, antigen-capture enzyme-linked immunosorbent assay (AC-ELISA) has been used to detect numerous food allergens.5–7
Biotinylation is one of the mostly commonly used methods of tagging proteins for easy detection, immobilization, and purification. Biotin can be used as a marker owing to its interaction with avidin and/or streptavidin, which has high affinity and specificity.8,9 The conventional coupling method involves chemical modification of functional side chains of proteins.10–12 Owing to the lack of clear binding sites, stochastic chemical conjugation methods for in vitro biotinylation have resulted in heterogeneous modifications in both regional chemistry and stoichiometry. Therefore, the random heterogeneity of binding sites often negatively affects the specificity, sensitivity, and stability of the results.13–15
Hen's egg allergy is the second most common food allergy, affecting up to 9% of neonates, followed by other major food allergies, including those to peanuts (3%) and sesame (0.8%).16 Currently, egg allergy is usually equivalent to egg white allergy, because egg yolk proteins have not been detected. However, 9.1% of children diagnosed with egg allergy have been sensitized to heated egg yolk slightly contaminated with egg white via an oral food challenge (OFC).17 Nonetheless, clinical information regarding egg yolk allergy is very limited, and there is no definite diagnostic reagent for egg yolk allergy. Owing to the high nutritional and genetic correlation, component resolved diagnosis of egg yolk has become a pressing problem. Gal d 6, a 35-kDa fragment of the vitellogenin-1 precursor known as the YGP42 protein, reportedly increases the sensitivity to egg allergy. This protein is heat-stable and reduction-stable, that being one of the characteristics of major allergens. Gal d 1 is the primary egg white allergen with the highest diagnostic value.
This study primarily aimed to develop a recombinant version of site-specific biotinylated Bio-rGal d 6 and rGal d 6 with their N-terminal and C-terminal labelled, not requiring purification from the soluble lysate fraction and having improved IgE-detection capacity in comparison with random chemically biotinylated rGal d 6. Furthermore, we selected one allergen with excellent performance to enhance component resolution during diagnosis and improve the diagnostic sensitivity of the anaphylaxis.
Materials and methodsMaterialsReagentsEscherichia coli AVB101, pAN6, and pAC6 were purchased from Avidity LLC (Aurora Colorado, USA). Sulfo-NHS-LC-Biotin was obtained from Thermo Fisher Scientific Inc. (Rockford, IL, USA). SDS lysis buffer and Coomassie brilliant blue R-250 was obtained from Nanjing KeyGen Biotech Co., Ltd. (Nanjing, China). Immobilon™ Western Chemiluminescent HRP substrate was obtained from Merck KGaA (Darmstadt, Germany). HRP-labelled goat anti-human IgE (cat. no. A9667) and HRP-labelled SA (cat. no. A3151) were obtained from Sigma-Aldrich (Shanghai, China).
Patients and serumIgE-mediated hen's egg allergy was diagnosed on the basis of a convincing clinical history and serum sIgE levels (egg-sIgE>0.35kUA/L) measured using the ImmunoCAP System (Thermo Fisher Scientific, Sweden). The characteristics of the positive and negative serum samples are listed in Table 1.
Clinical characteristics of the study subjects.
| N. | Gender | Age | Egg white sIgE (kUA/L) |
|---|---|---|---|
| 1 | F | 18 | 0.78 |
| 2 | M | 56 | 1.42 |
| 3 | M | 35 | 5.52 |
| 4 | F | 39 | 1.27 |
| 5 | M | 8 | 1.03 |
| 6 | F | 7 | 1.84 |
| 7 | M | 3 | 0.91 |
| 8 | M | 3 | 0.91 |
| 9 | M | 71 | 1.42 |
| 10 | F | 40 | 3.63 |
| 11 | F | 5 | 0.49 |
| 12 | F | 38 | 0.60 |
| 13 | M | 4 | 14.71 |
| 14 | M | 3 | 0.78 |
| 15 | F | 3 | 0.60 |
| 16 | M | 4 | 1.27 |
| 17 | F | 3 | 10.35 |
| 18 | F | 4 | 0.44 |
| 19 | F | 2 | 2.11 |
| 20 | F | 3 | 2.20 |
| 21 | M | 10 | 7.00 |
| 22 | F | 16 | 15.60 |
| 23 | F | 24 | 1.71 |
| 24 | M | 19 | 2.58 |
| 25 | M | 32 | 0.56 |
| 26 | F | 3 | 1.20 |
| 27 | M | 5 | 9.07 |
| 28 | F | 11 | 2.01 |
| 29 | F | 29 | <0.35 |
| 30 | M | 4 | <0.35 |
M: male; F: female.
TYH medium containing 10μg/ml chloramphenicol and 100μg/ml ampicillin was seeded with E. coli AVB101 transformed with the plasmid rGal d 6-Bio and Bio-rGal d 6 constructs. The engineering bacteria were incubated overnight at 37°C with agitation. Thereafter, the overnight culture was seeded at a ratio of 1 to 100 in TYH medium supplemented with 100μg/ml ampicillin and 0.5% glucose and incubated at 37°C with agitation until the OD600 approached 0.6. A 1-ml aliquot was extracted as an unconditioned control. rGal d 6-Bio and Bio-rGal d 6 expression was induced with 1.5mM IPTG and biotinylation was carried out with 5mM biotin solution. After 3h, the medium was centrifuged for 10min at 5858×g. The supernatant containing the biotinylated proteins was used directly for SDS-PAGE and immunoblotting for further application.
Generation of rGal d 6 in vitrorGal d 6 was biotinylated by Sulfo-NHS-LC-Biotin in vitro. The antigens were dialyzed in carbonate buffer solution (pH 8.0) and stirred overnight at 4°C. The Sulfo-NHS-LC-Biotin was dissolved in rGal d 6 at molar ratio of 20:1 and stirred at 4°C for 2h. Thereafter, the unconjugated biotin reagent was eliminated via dialysis with 0.1M PBS overnight at 4°C. Samples in dialysis bags were quantitatively stored at −80°C.
AC-ELISA with bacterial lysates containing biotinylated proteinsBio-BSA was diluted in 0.1M PBS at 150μl per well overnight at 4°C. The coated wells were washed thrice with PBST containing 0.05% (v/v) Tween 20, using the ELx50™ Automatic Washing Machine (Bio Tek, USA). The microtiter plate was then blocked with 250μl PBST supplemented with 2% (v/v) polyvinyl alcohol (PVA) and 0.05% glycine (v/v) for 2h at ambient temperature and overnight at 4°C. The liquid was discarded, and the plate was washed twice for 10min each time. Diluted streptavidin was added at 100μl per well, and after allowing it to stand for 1h at ambient temperature, it was washed and dried. The ELISA plates were incubated for 30min at 37°C with bacterial lysates or recombinant protein in 100μl TBST and then washed thrice. Furthermore, 5μl serum diluted 1:20 in PBST was added to the microtiter plate. After incubation for 1h at 37°C, the ELISA plates were washed thrice. Moreover, 1μg/ml of the HRP-labelled goat anti-human IgE in 100μl TBST was incubated for 45min. After five washes with PBST, 3,5′,5,5′-tetramethylbenzidine TMB chromogenic substrate was added and the reaction was terminated with 2M sulfuric acid. Finally, the absorbance was measured at 450nm (OD450) using Synergy2 Multimode Microplate Reader (Bio Tek).
Egg yolk extractAs previously reported,18 first, yolk was cleaned with distilled water and then freeze-dried. Acetone was used twice, followed by ethanol/ether for degreasing. PBS was used to dissolve the extract for 1h on ice. After centrifugation at 4500×g, the supernatant was dialyzed and quantitatively stored at −80°C.
Dot blotSpots of 2μl of egg yolk extract were placed on a nitrocellulose membrane. After blocking with 5% (w/v) BSA in 0.05% TBST for 2h at ambient temperature, the membranes were washed thrice. Thereafter, 28 positive serum samples and 2 negative serum samples diluted 1:10 were added and incubated for 1h at ambient temperature. After washing thrice, 1μg/ml of the HRP-labelled goat anti-human IgE was incubated for 1h at RT. Finally, 2μl HRP substrate was added.
Immunoblot analysisAfter heating for 5min at 97°C, 5-μl samples were separated via SDS-PAGE and subsequently transferred to a PVDF membrane. After blocking with 5% BSA in TBST for 2h at ambient temperature, the membrane was incubated with HRP-labelled SA/HRP-labelled anti-His for 2h at ambient temperature. After washing five times for 5min with TBST, the signals were detected using Immobilon™ Western Chemiluminescent HRP substrate (Merck KGaA) using the ChemiDoc XRS+imaging system (Bio-Rad Laboratories, Inc.).
Evaluation and screening of rGal d 6-Bio, Bio-rGal d 6, and rGal d 6To evaluate the ability of three antigens to detect sIgE, we selected mixed positive serum samples. Thereafter, sIgE was detected with three antigens, i.e., rGal d 6-Bio, Bio-rGal d 6, and rGal d 6, with different gradient dilutions from 1 to 104. The affinity constants were determined through fitting.
Through prior screening, the serum pools containing low levels of sIgE for recombinant Gal d 6 in egg yolk were obtained, and different serum titres were used to screen for the most optimal raw materials.
Statistical analysisELISA results were analyzed using analysis of variance (ANOVA). Statistical analyses were performed using GraphPad Prism 6.0 software. A P-value of <0.05 was considered statistically significant.
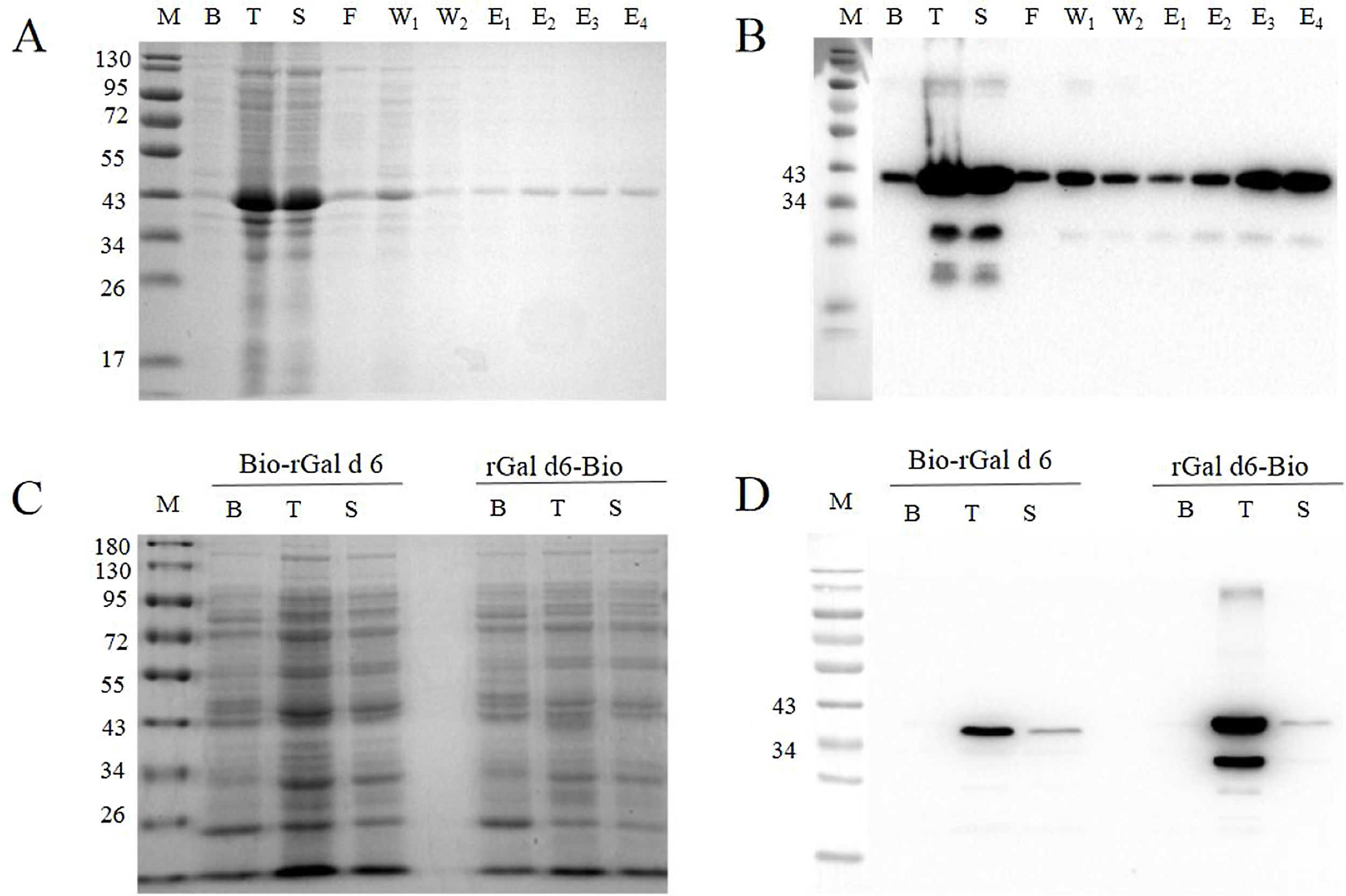
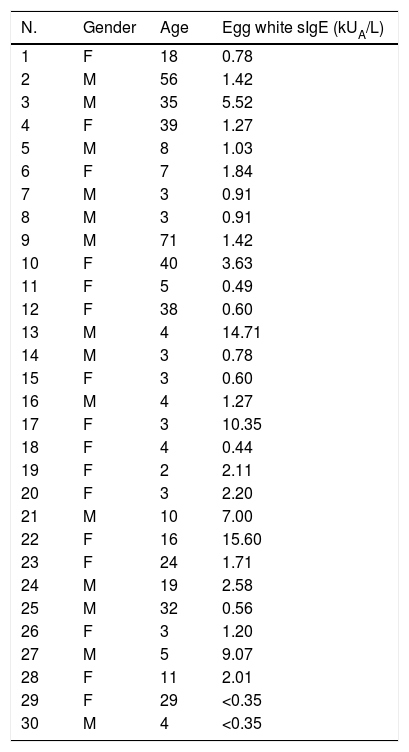
ResultsExpression and identification of rGal d 6, Bio-rGal d 6, and rGal d 6-BiorGal d 6 was expressed in E. coli BL21 as a His-tag fusion protein. Sequence alignment analysis revealed that the sequence of rGal d 6 was consistent with that of the NCBI. High purity recombinant protein rGal d 6 was obtained through Ni-NTA affinity column chromatography. We demonstrated the process and protein purification through SDS-PAGE (Fig. 1A). Resolution through SDS-PAGE and immunoblotting analysis yielded a band of appropriate size (lane 3) of the expected molecular weight (Fig. 1B).
Avi-Gal d 6/rGal d 6-Bio constructs were transformed into chemically competent E. coli AVB101 and expression was induced with IPTG with simultaneous biotinylation in vivo. Protein bands were observed upon SDS-PAGE (Fig. 1C). The two site-specific biotinylated proteins were then immunoblotted with SA-HRP (Fig. 1D), revealing two biotinylated proteins in vivo.
rGal d 6, Bio-rGal d 6, and rGal d 6-Bio were successfully generated. The results are shown in Fig. 1.
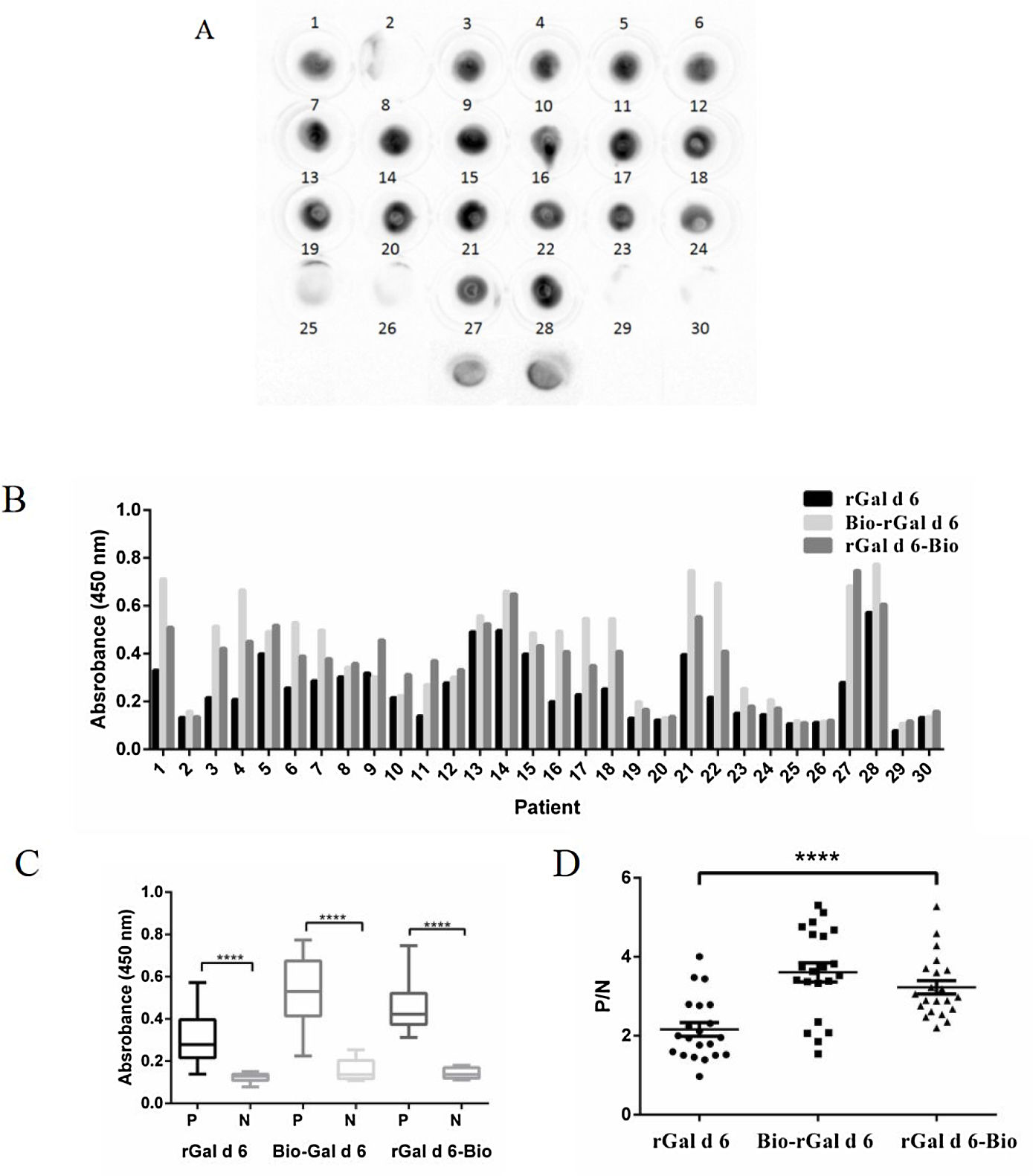
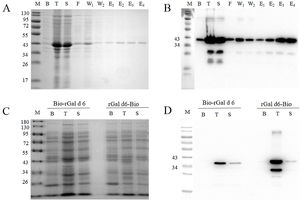
Immunologic characterization and IgE-binding of rGal d 6, Bio-rGal d 6, and rGal d 6-BioEgg yolk extracts were blotted onto nitrocellulose membranes and tested against allergic sera and non-allergic sera. Twenty-eight positive serum samples were subjected to dot-blot analysis to examine IgE binding to nGal d 6. Consequently, 75% (21 out of 28) of egg-allergy-diagnosed individuals were sensitive to nGal d 6. Compared with the control and other subjects sensitive to nGal d 6, 7, 8, 9, 11, 15, and 22 displayed strong IgE response (Fig. 2A).
IgE reactivity of human sera against rGal d 6, Bio-rGal d 6, and rGal d 6-Bio. (A) IgE reactivity to nGal d 6 in patients sensitized to hen's egg white was assessed via the dot blot assay. (B) A comparison of the reactivity of rGal d 6, Bio-rGal d 6, and rGal d 6-Bio with 30 serum samples via AC-ELISA. (C) P/N indicates the positive to negative ratios. (D) Comparison of the median integrated density values in the three biotinylated proteins. Box plots show the median integrated density values and the 5th and 75th interquartile ranges. Vertical bars indicate the 5–95% range. P: patient sera; N: non-sensitive individuals.
sIgE levels towards rGal d 6-Bio, Bio-rGal d 6, and rGal d 6 allergens were quantified via AC-ELISA using patient sera with or without nGal d 6 sensitivity previously identified. For most patient serum samples, the most prominent IgE binding was observed with natural and recombinant Gal d 6, while lesser sIgE binding was observed for other sensitive patients (Fig. 2B). Biotinylated Gal d 6 in vivo had higher values of P/N than the chemically biotinylated protein. The rGal d 6-Bio group seemed more “concentration” than Bio-rGal d 6 group (Fig. 2C). Fig. 2D shows different sIgE heterogeneity in different serum samples.
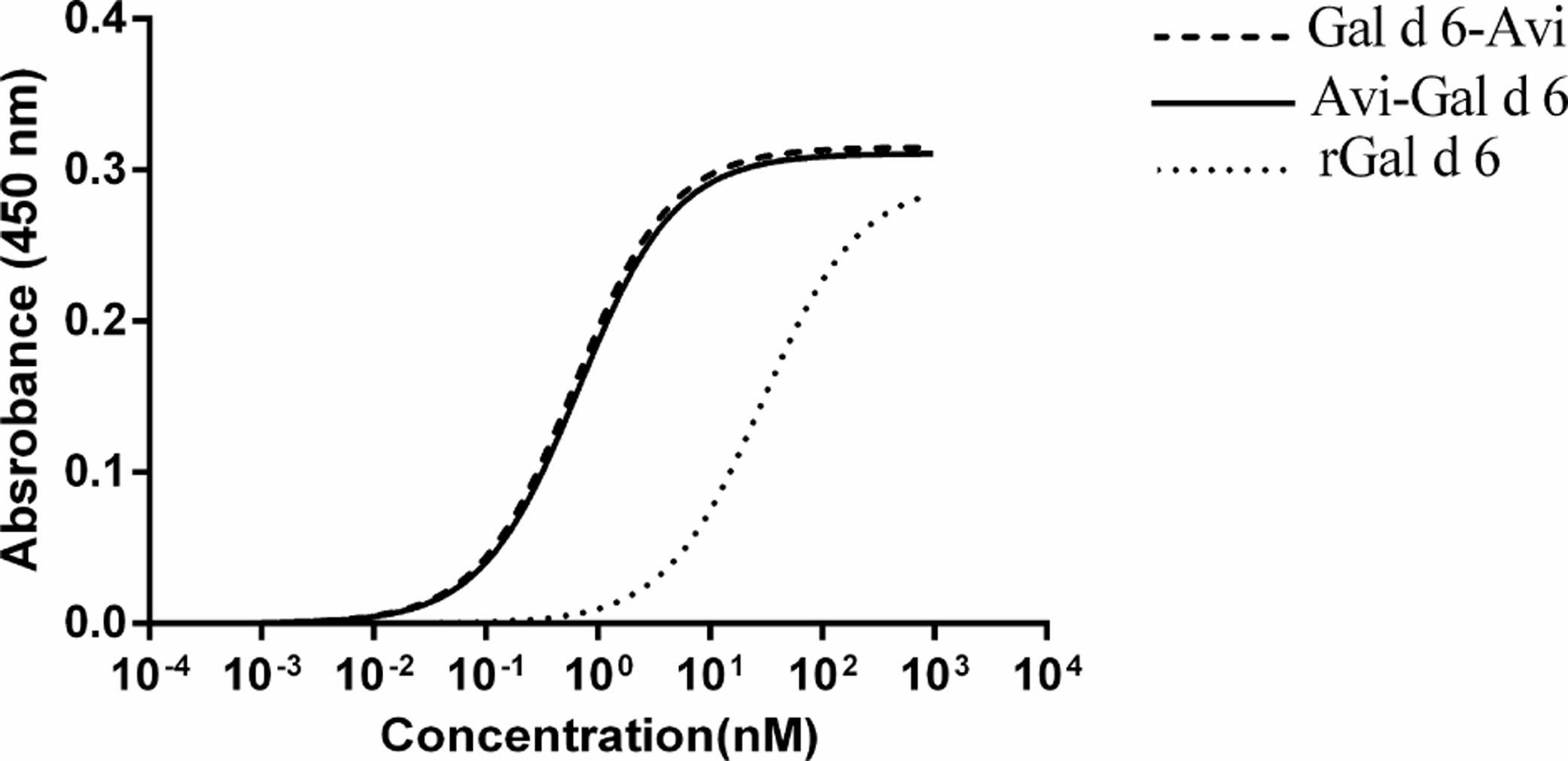
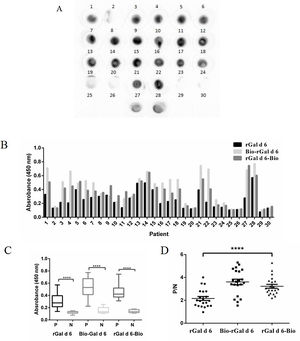
Comparation of performance characteristics among rGal d 6-Bio, Bio-rGal d 6, and rGal d 6 to detect allergen-specific IgEThe concentrations of rGal d 6-Bio, Bio-rGal d 6, and rGal d 6 were reduced by 1 to 104 fold. The dissociation constant (Kd) was determined through equilibrium binding analysis. The Kd of rGal d 6-Bio (Kd=0.6154) and Bio-rGal d 6 (Kd=0.6698) was markedly greater than that of rGal d 6 (Kd=28.93), indicating increased affinity at site-specific biotinylated Gal d 6. When the antigen concentrations were 1nM, the random chemical conjugation products had nearly not bound to sIgE. When the antigen concentrations were 10nM, site-specific biotinylated products completely bound to sIgE (Fig. 3).
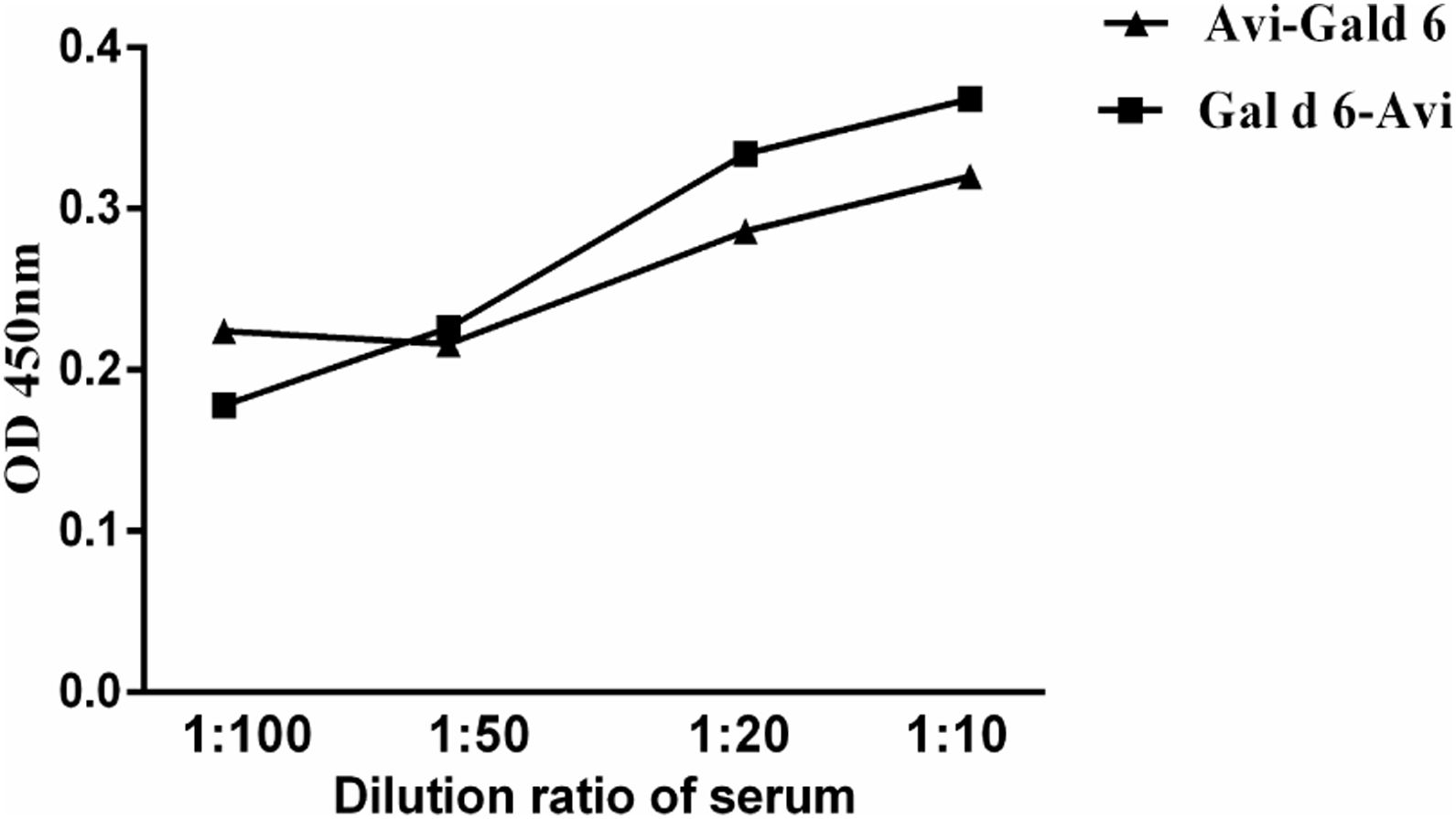
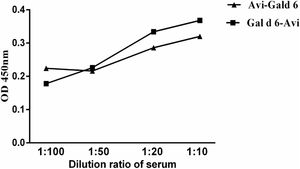
To compare the two site-specific biotinylated proteins in low-region samples, we selected low-value mixed serum samples. The sensitivity was compared through different serum titres. The assay sensitivity of the rGal d 6-Bio is shown in Fig. 4. Based on previous studies, we selected two site-specific biotinylated proteins for subsequent studies. Low-concentration serum samples were serially diluted to 20-fold. The difference in the OD450 between the rGal d 6-Bio and Bio-rGal d 6 indicated the high sensitivity of rGal d 6-Bio for detecting allergen-specific IgE in low-region samples.
DiscussionEgg is one of the most well-known sources of allergens including egg white and egg yolk. Egg white has been extensively assessed and egg white allergy is an indication of an egg allergy. However, individuals allergic to egg white may derive nutrients from egg yolk. Therefore, herein, we selected Gal d 6, an important yolk allergen, as the target protein for single-component resolution for the diagnosis of this anaphylaxis and improvement of the diagnostic sensitivity.
Recombinant allergens have been used in studies on allergies including those on the diagnosis and management of allergies. Herein, recombinant allergens were commonly employed assessed in various immunoassays including Western blotting and ELISA. For experimental purposes, we designed and expressed three types of yolk allergen proteins including rGal d 6, Bio-rGal d 6, and rGal d 6-Bio. rGal d 6 was chemically coupled with biotin in vitro. The other two proteins were expressed and simultaneously biotinylated in a site-specific manner. Full-length cDNA of Gal d 6 was cloned and expressed as previously reported. Furthermore, SDS-PAGE and Western blotting indicated appropriate expression of target proteins.
Gal d 6-specific IgE was detected using sera of patients diagnosed with egg white allergy and those without a clinical diagnosis of egg yolk allergy. The immunoassays confirmed that rGal d 6 produced herein was similar to nGal d 6 based on IgE-binding reactivity.
For the analysis, a sensitive AC-ELISA was developed to specifically detect bio-antigens, serving as a valuable tool for the detection of allergen-specific IgE. Biotin-avidin binding is the strongest noncovalent interaction known in nature in comparison with any other affinity tag. Therefore, the antibiotic–biotin interaction is considered a molecular tool to facilitate protein assembly or immobilization.19 Biotinylation has been previously performed using chemical reagents lacking site specificity, which can inactivate some biological molecules. This issue was initially addressed using fusion of domains of naturally biotinylated proteins to establish a generic method of labelling chimeric proteins with biotin at a single site. This specificity was possible because of the extreme selectivity of biotin holoenzyme synthetases, also known as biotin ligases, which catalyze the covalent attachment of biotin to the e amino group of particular lysine residues in target proteins. A 15-amino-acid sequence, termed the biotin acceptor peptide (BAP), can covalently attach a biotin molecule to the lysine residue of the Avitag, in vivo or in vitro.20,21 The recombinant protein can be biotinylated in vivo by endogenous BirA. However, it usually overexpresses BirA in conjunction with BAP-labelled proteins of interest and is amplified cells in cells cultured in medium supplemented with biotin to improve the efficiency of biotin production.22,23 When producing biotinylated proteins, site-specific biotinylation of affinity tags is more advantageous than random chemical binding. Thus far, BirA-mediated biotinylation of affinity label fusion protein has been used for protein purification, immobilization, and detection.24–26 In vivo biotinylation markers allow site-specific biotinylation of proteins, which not only promotes immobilization, but also makes biotinylated proteins more widely applicable in nano-assembly formation and intracellular sensitive detection.27,22
On comparing the performance characteristics of rGal d 6-Bio, Bio-rGal d 6, and rGal d 6 for the detection of allergen-specific IgE, we found that rGal d 6-Bio had the best “affinity” and sensitivity. In conclusion, the present results indicate that the definite-site specificity is superior to that of random biotinylated proteins. Furthermore, considering the amino acid sequence, C-terminal biotinylation of Gal d 6 improved its performance characteristics for detection of allergen-specific IgE. The allergen dose-risk relationship may predict the sensitivity to low-dose allergic foods.
Conflict of interestThe authors have no conflict of interest to declare.
This study was funded by the National Natural Science Foundation of China (# 8177225).