Fatty acid synthetase (Fas)/Fas ligand (FasL)-dependent apoptotic pathways have been reported as being involved in the pathogenesis of drug-induced maculopapular rashes.
ObjectiveWe investigated serum soluble FasL (sFasL) levels and peripheral blood lymphocyte subtypes to discriminate maculopapular drug eruptions (MPDE) from viral exanthema (VE).
Patients/methodsChildren with confirmed MPDE (group I), VE (group II), and drug rashes with eosinophilia and systemic symptoms (DRESS) or drug-induced hypersensitivity syndrome (DIHS) (group III) were included. Serum sFasL levels and peripheral blood lymphocyte subtypes were analyzed in groups I–III on admission, and repeated twice (only once for group IV – controls).
ResultsThere were no significant serum soluble FasL level differences among the groups for all the samples. In the initial samples, CD19+ cell numbers in group II were significantly higher than in group IV, and the CD4+/CD8+ ratio was higher than groups I and IV. In the second samples, CD4+ and CD19+ cell numbers were significantly higher in group II than group I. In the final samples, CD4+ cell numbers in group II were significantly higher than group I and group III. CD19+ cells numbers in group III were significantly lower than the other groups for all samples.
ConclusionSerum sFasL levels were not found to be useful in discriminating viral exanthemas from other drug rashes. The significant differences between MPDE, VE, and DRESS were high CD4+ and CD19+ cell-count numbers in VE but low B-cell numbers in DRESS. This might be important for discriminating VE from DRESS, and the low B-cell count in early symptoms might be a useful predictor of DRESS development.
Skin manifestations are the most common findings related to drug allergy.1–3 They can be observed in forms varying from maculopapular drug eruptions (MPDE) to more severe reactions, such as Stevens-Johnson Syndrome (SJS)/toxic epidermal necrolysis (TEN), or can develop into drug rash with eosinophilia and systemic symptoms (DRESS) or drug-induced hypersensitivity syndrome (DIHS).2,3
In children, the major difficulty in the diagnosis of drug reactions is differentiating MPDE from viral exanthema (VE), commonly observed in this age group. MPDE usually develops within 7–14 days after the onset of treatment and resolves completely within 1–2 weeks after discontinuing the putative drug.3,4
Clinically, these exanthems usually appear as erythematous macular or papular eruptions often starting on the trunk and subsequently spreading to the extremities in a symmetrical fashion.3 Severe pruritus and resolution of the lesions with desquamation tend to lead to an exanthematous drug eruption, whereas enanthema, palm and sole involvement, fever, and lymphadenopathies are fundamental—though not exclusive—indicators of infectious etiology.5
Although similar clinical entities may be induced by viruses and drug reactions, immune responses to viruses are different from immune mechanisms underlying various forms of drug-induced exanthems. Viral infections are generally followed by immunological protection, but in drug reactions re-exposure can induce the reaction again.6,7 On the other hand, underlying viral infections may also act as cofactors in susceptible individuals.2,8 In children, aminopenicillins may induce an exanthema in patients with Epstein-Barr virus infection.9,10 Human herpesvirus (HHV)-6 reactivation may be a cofactor in DRESS.2,11,12
Because of similarities in clinical manifestations of viral infections and MPDE, correct identification of the etiological agent is necessary for the management of the disease. Skin tests and controlled administration of the culprit drug have proved useful but should ideally be performed 1–6 months after complete recovery of the initial reaction.2 Additionally, intradermal tests (IDT) and drug provocation tests (DPT) are contraindicated in cases of severe reactions.13–15
Recently, fatty acid synthetase (Fas)/Fas ligand (FasL)-dependent apoptotic pathways have been reported as being involved in the pathogenesis of drug-induced maculopapular rashes.16 The present study investigated serum soluble FasL (sFasL) levels to discriminate drug-induced skin reactions from other clinically resembling skin diseases, such as exanthematous viral infections. In addition, we aimed to investigate the role of different subsets of peripheral blood lymphocytes in various drug-induced diseases to evaluate which subtypes of T cells are effective in apopitotic mechanisms.
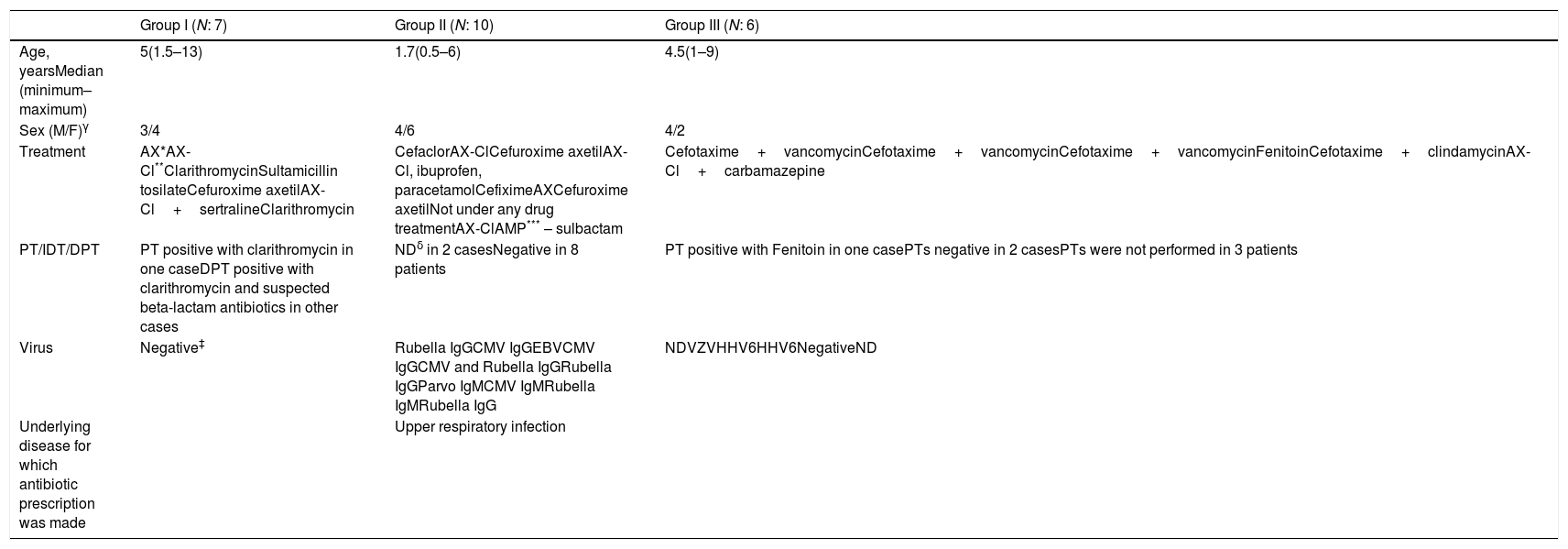
Patients and methodsPatientsTwenty-three children admitted to our Pediatric Allergy Immunology outpatient clinic with a maculopapular/morbilliform eruption, or who had developed a maculopapular eruption during hospital treatment, and presented to our department between August 2010 and June 2011 were eligible for inclusion. The study included seven children with confirmed MPDE (group I: 3 males [M], four females [F]; median age five years), 10 children with VE (group II: 4M, 6F; median age 1.7 years), and six children with DRESS/DIHS (group III: 4M, 2F; median age 4.5 years) (Table 1), who were identified by a detailed history, physical examination, routine laboratory tests, viral serology, skin testing, and DPT when required.
Clinical data of the patients with viral serology and drug allergy workup.
| Group I (N: 7) | Group II (N: 10) | Group III (N: 6) | |
|---|---|---|---|
| Age, yearsMedian (minimum–maximum) | 5(1.5–13) | 1.7(0.5–6) | 4.5(1–9) |
| Sex (M/F)γ | 3/4 | 4/6 | 4/2 |
| Treatment | AX*AX-Cl**ClarithromycinSultamicillin tosilateCefuroxime axetilAX-Cl+sertralineClarithromycin | CefaclorAX-ClCefuroxime axetilAX-Cl, ibuprofen, paracetamolCefiximeAXCefuroxime axetilNot under any drug treatmentAX-ClAMP*** – sulbactam | Cefotaxime+vancomycinCefotaxime+vancomycinCefotaxime+vancomycinFenitoinCefotaxime+clindamycinAX-Cl+carbamazepine |
| PT/IDT/DPT | PT positive with clarithromycin in one caseDPT positive with clarithromycin and suspected beta-lactam antibiotics in other cases | NDδ in 2 casesNegative in 8 patients | PT positive with Fenitoin in one casePTs negative in 2 casesPTs were not performed in 3 patients |
| Virus | Negative‡ | Rubella IgGCMV IgGEBVCMV IgGCMV and Rubella IgGRubella IgGParvo IgMCMV IgMRubella IgMRubella IgG | NDVZVHHV6HHV6NegativeND |
| Underlying disease for which antibiotic prescription was made | Upper respiratory infection |
γM: male, F: female.
DRESS diagnosis was based on Kardaun et al.’s criteria (17) in the RegiSCAR study group and included at least three of the following five criteria: acute skin rash, fever (>38°C), enlarged lymph nodes in at least two sites, involvement of at least one internal organ, and blood-count abnormalities (lymphocyte level above or below laboratory limits, eosinophil level above laboratory limits [by percentage or absolute count], and platelet level below laboratory limits). Eosinophilia was defined as more than 10% of total white blood cells (WBC) (if leukocytes<4000/mm3) or >700/mm3.17
Twelve healthy children (4M, 8F; median age eight years) taking the same drugs as children with drug-induced rashes, but with good tolerance and viral serology not indicating acute or reactivated infection, were selected as controls (group IV). None of these controls had previous histories of adverse drug reactions or cutaneous or immunological disease when selected.
Written informed consent was obtained from the parents before all diagnostic procedures. Approval for the study was obtained from the local ethics committee and the study was supported by the Research Council of Trakya University (TUBAP-2009/157) (Edirne, Turkey).
Standard laboratory testsA full blood count (FBC) with WBC differential was performed on all the patients using automated hematology analyzers (Beckman Coulter LH 780 Analyzer; Beckman Coulter, Galway, Ireland). Other laboratory tests, such as blood urea nitrogen, serum creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and γ-glutamyl transpeptidase (GGT) were also performed.
Serum soluble FasL levelsSerum sFasL levels were analyzed in all of the cases (groups I–III) on admission (T1) and repeated twice: 3–5 days after T1 (T2) and 6–10 days after T1 (T3) (Human sFas-L Platinum ELISA kit, Bender MedSystems, Vienna, Austria). The limit of detection of human sFas-L was determined to be 0.07ng/ml.
Flow cytometryLymphocyte subsets were identified by fluorescence-assisted cell sorting (FACS) by using a three-color Epics XL Coulter Flow Cytometer with System II software. Three milliliters of blood were inserted into EDTA tubes for FACS analysis. In groups I–III, the first blood samples were obtained on admission (T1) and repeated twice (T2 and T3) with the same intervals as the serum soluble FasL analyses. In group IV, this analysis was performed only once.
Viral serologyVirological investigations were performed to examine the possibility of viral infections. Samples of the patients’ sera were tested on admission for viral hepatitis (types A, B, and C), the Ebstein-Barr virus (EBV), rubella, parvovirus B19, cytomegalovirus, herpes simplex virus (HSV)-1, and varicella-zoster virus (VZV). Human herpesvirus (HHV)-6 DNA was analyzed by polymerase chain reaction (PCR) in peripheral blood mononuclear cells only in cases diagnosed with DRESS. Anti-CMV IgG, anti-rubella IgG, and anti-parvovirus B19 IgG antibodies were tested four weeks later to evaluate the seroconversion of IgG titers if needed.
Skin tests and drug provocation testsPatch tests (PT) were performed in all of the cases in group I, with the suspected drug if negative to proceed to IDT. If skin tests resulted negative, then DPT was performed to establish or exclude drug hypersensitivity. In group II, since most of the patients (9/10) were under treatment with an antibiotic alone or an antipyretic drug together with an antibiotic, skin tests and/or DPT were also performed in all using any medication with the suspected drug—except for one, whose viral serology was positive and whose parents declined additional testing (case no. 10 in group II). With antibiotics available as oral preparations (cefaclor and cefixime) only PT and DPT were performed. Nonsteroidal anti-inflammatory drug hypersensitivity (ibuprofen and paracetamol) was also tested with PT and DPT. In group III, PT was performed only in three from six cases. In control cases (group IV), skin tests, and DPT were not performed (Table 1). Skin tests and DPT were performed with an antibiotic, antipyretic, or anticonvulsant drug according to current guidelines.15,18,19
For patch testing, cefixime and cefaclor diluted 30% in water and in petrolatum, paracetamol and ibuprofen diluted 10% in petrolatum, and a drop of amoxicillin (20mg/ml), ampicillin (20mg/ml), cefotaxime (10mg/ml), cefuroxime (10mg/ml), clarithromycin (0.5mg/ml), vancomycin (50mg/ml), phenytoin (50mg/ml), and normal saline and petrolatum (as negative controls) were applied to normal skin on the child's upper back using Finn Chambers on adhesive tape (12mm diameter). The occlusion time was 48h; results were read 15min after removing the cups, then again at 48 and 72h.20
IDTs were performed using intravenous (parenteral) preparations of the drug. Non-irritating intradermal skin-test concentrations were used as stock solutions for the antibiotics (amoxicillin: 20mg/ml, ampicillin: 20mg/ml, clarithromycin: 0.5mg/ml, cefuroxime: 10mg/ml, cefotaxime: 10mg/ml).21–23 Then, sequential dilutions (1/100 and 1/10) of stock solutions in a 0.9% saline solution were prepared. IDT was performed using increasing drug concentrations (1/100, 1/10, and full-strength concentrations) at intervals of 20min. Reconstitution and further dilutions were carried out under sterile conditions no more than two hours before administration.
DPT was performed in six patients in group I and nine cases in group II, according to Messaad et al.’s protocol.24 Increased doses of the drugs were administered every 30min, under close observation.
All challenge phases were performed in a specialized hospital unit, where appropriate medication and resuscitation equipment was directly available. None of the patients were under any other treatment that might have modified results. Epinephrine, antihistamines, and potential transfer to the Emergency Department were immediately available on site in case there was any suspicion of a systemic reaction.
The clinical and laboratory characters of the cases in all the groups in the initial and subsequent blood samples are given in Tables 1–3.
StatisticsDescriptive statistics are expressed as mean±SD, median, and range. Comparison of values among the four groups was done using the Kruskal–Wallis test, then the Mann–Whitney U test was used for multiple comparisons when significant results were obtained.
ResultsThe mean ages of the children in groups I–IV were 5.67±4.17, 2.25±1.74, 4.77±3.38, and 6.85±4.31 years, respectively. There were no statistically significant differences among the groups according to age (p=0.059). There was also no difference among the groups with regard to gender (p=0.601) (Table 1).
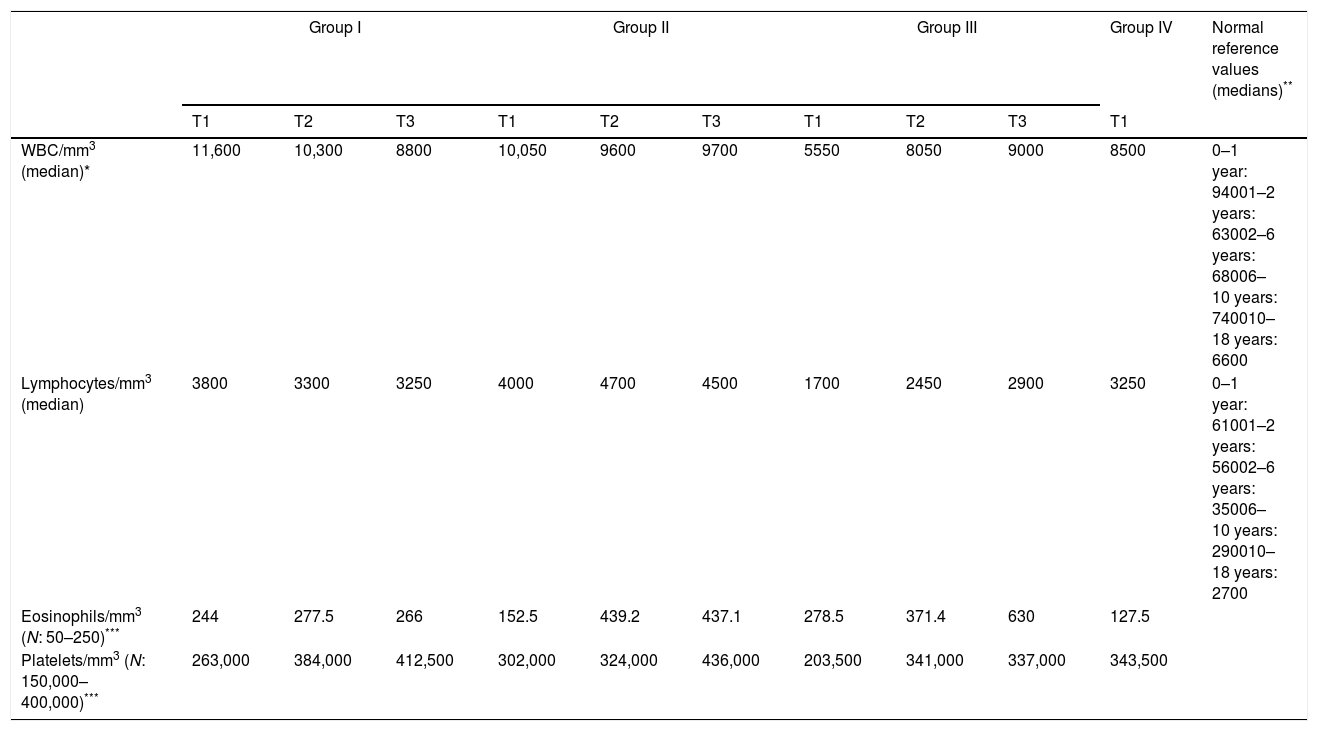
WBC numbers, absolute numbers of peripheral blood lymphocytes and eosinophils, and platelet counts (median values) in the first (T1), second (T2), and final blood samples (T3) are shown in Table 2. There were no statistically significant differences among the groups in the first and the second samples. In the final blood samples (T3), a significant difference was only observed in the absolute numbers of lymphocytes between groups II and III (p=0.009).
Descriptive data on FBC with white blood cell differential on admission (T1) in all groups and during the following period (T2–T3) in groups I–III.
| Group I | Group II | Group III | Group IV | Normal reference values (medians)** | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | T1 | ||
| WBC/mm3 (median)* | 11,600 | 10,300 | 8800 | 10,050 | 9600 | 9700 | 5550 | 8050 | 9000 | 8500 | 0–1 year: 94001–2 years: 63002–6 years: 68006–10 years: 740010–18 years: 6600 |
| Lymphocytes/mm3 (median) | 3800 | 3300 | 3250 | 4000 | 4700 | 4500 | 1700 | 2450 | 2900 | 3250 | 0–1 year: 61001–2 years: 56002–6 years: 35006–10 years: 290010–18 years: 2700 |
| Eosinophils/mm3 (N: 50–250)*** | 244 | 277.5 | 266 | 152.5 | 439.2 | 437.1 | 278.5 | 371.4 | 630 | 127.5 | |
| Platelets/mm3 (N: 150,000–400,000)*** | 263,000 | 384,000 | 412,500 | 302,000 | 324,000 | 436,000 | 203,500 | 341,000 | 337,000 | 343,500 | |
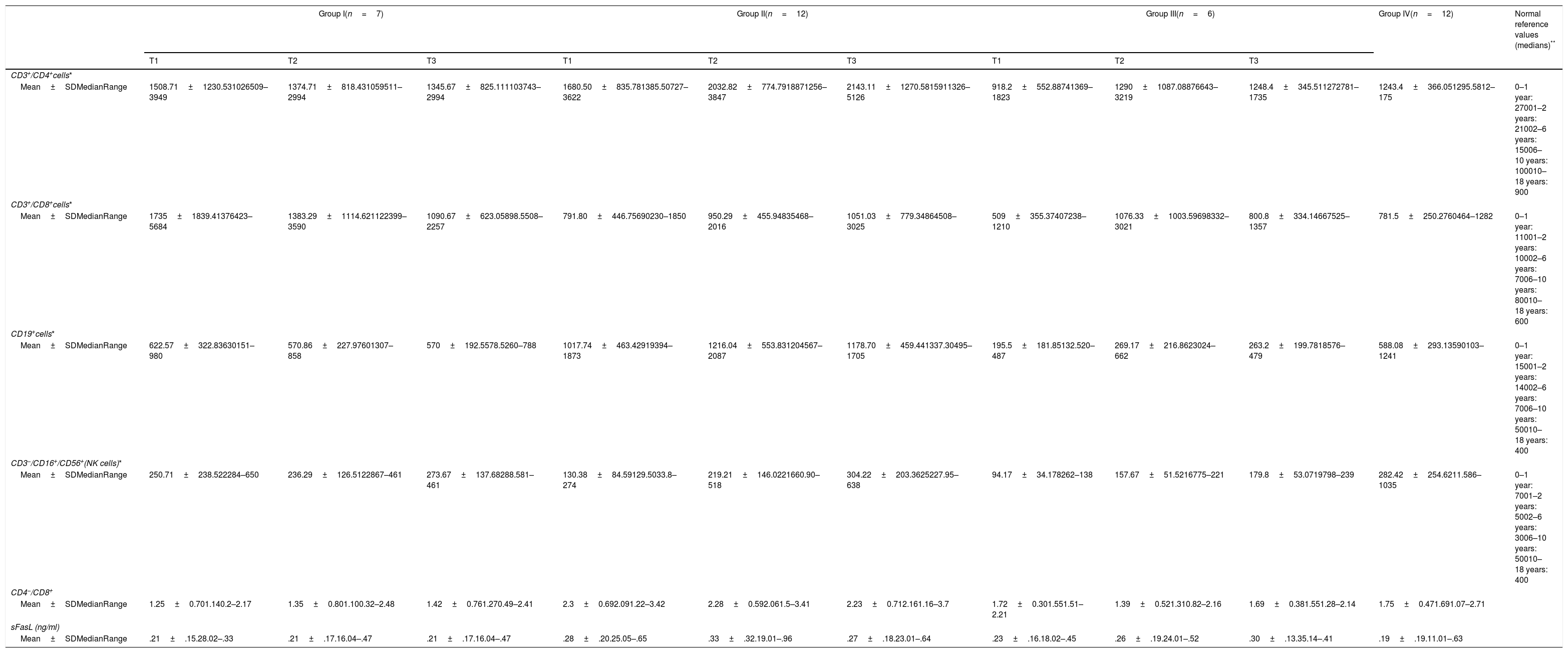
The median values for serum sFasL levels in groups I, II, III, and IV in the initial and subsequent samples are presented in Table 3. There were no statistically significant differences among the groups in the initial and subsequent samples (p=0.513, 0.852, and 0.853, respectively).
Peripheral blood lymphocyte subtypes, CD4+/CD8+ ratio, and serum soluble FasL levels in initial (T1), second (T2), and final samples (T3).
| Group I(n=7) | Group II(n=12) | Group III(n=6) | Group IV(n=12) | Normal reference values (medians)** | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | T1 | T2 | T3 | T1 | T2 | T3 | |||
| CD3+/CD4+cells* | |||||||||||
| Mean±SDMedianRange | 1508.71±1230.531026509–3949 | 1374.71±818.431059511–2994 | 1345.67±825.111103743–2994 | 1680.50±835.781385.50727–3622 | 2032.82±774.7918871256–3847 | 2143.11±1270.5815911326–5126 | 918.2±552.88741369–1823 | 1290±1087.08876643–3219 | 1248.4±345.511272781–1735 | 1243.4±366.051295.5812–175 | 0–1 year: 27001–2 years: 21002–6 years: 15006–10 years: 100010–18 years: 900 |
| CD3+/CD8+cells* | |||||||||||
| Mean±SDMedianRange | 1735±1839.41376423–5684 | 1383.29±1114.621122399–3590 | 1090.67±623.05898.5508–2257 | 791.80±446.75690230–1850 | 950.29±455.94835468–2016 | 1051.03±779.34864508–3025 | 509±355.37407238–1210 | 1076.33±1003.59698332–3021 | 800.8±334.14667525–1357 | 781.5±250.2760464–1282 | 0–1 year: 11001–2 years: 10002–6 years: 7006–10 years: 80010–18 years: 600 |
| CD19+cells* | |||||||||||
| Mean±SDMedianRange | 622.57±322.83630151–980 | 570.86±227.97601307–858 | 570±192.5578.5260–788 | 1017.74±463.42919394–1873 | 1216.04±553.831204567–2087 | 1178.70±459.441337.30495–1705 | 195.5±181.85132.520–487 | 269.17±216.8623024–662 | 263.2±199.7818576–479 | 588.08±293.13590103–1241 | 0–1 year: 15001–2 years: 14002–6 years: 7006–10 years: 50010–18 years: 400 |
| CD3−/CD16+/CD56+(NK cells)* | |||||||||||
| Mean±SDMedianRange | 250.71±238.522284–650 | 236.29±126.5122867–461 | 273.67±137.68288.581–461 | 130.38±84.59129.5033.8–274 | 219.21±146.0221660.90–518 | 304.22±203.3625227.95–638 | 94.17±34.178262–138 | 157.67±51.5216775–221 | 179.8±53.0719798–239 | 282.42±254.6211.586–1035 | 0–1 year: 7001–2 years: 5002–6 years: 3006–10 years: 50010–18 years: 400 |
| CD4−/CD8+ | |||||||||||
| Mean±SDMedianRange | 1.25±0.701.140.2–2.17 | 1.35±0.801.100.32–2.48 | 1.42±0.761.270.49–2.41 | 2.3±0.692.091.22–3.42 | 2.28±0.592.061.5–3.41 | 2.23±0.712.161.16–3.7 | 1.72±0.301.551.51–2.21 | 1.39±0.521.310.82–2.16 | 1.69±0.381.551.28–2.14 | 1.75±0.471.691.07–2.71 | |
| sFasL (ng/ml) | |||||||||||
| Mean±SDMedianRange | .21±.15.28.02–.33 | .21±.17.16.04–.47 | .21±.17.16.04–.47 | .28±.20.25.05–.65 | .33±.32.19.01–.96 | .27±.18.23.01–.64 | .23±.16.18.02–.45 | .26±.19.24.01–.52 | .30±.13.35.14–.41 | .19±.19.11.01–.63 | |
Absolute numbers of peripheral blood lymphocyte subsets in healthy Turkish children (medians) (ref. no. 25).
CD4/CD8 normal ratio: >1.
Descriptive data for absolute numbers of CD3+/CD4+, CD3+/CD8+, CD19+, and CD3−CD16+/CD56+ (NK cells), and the CD4+/CD8+ ratio in the initial and subsequent samples are shown in Table 3.
In the initial samples (T1), a significant difference was observed in CD19+ cell numbers and the CD4+/CD8+ ratio among the four groups (p=0.002 and 0.03, respectively). CD19+ cell numbers in group III were significantly lower than in group I (p=0.022), group II (p=0.002), and group IV (p=0.009). CD19+ cell numbers in group II were significantly higher than in group IV (p=0.021). The CD4+/CD8+ ratio in group II was higher than in group I (p=0.015) and IV (p=0.049).
In the second samples (T2), significant differences were observed in CD4+, CD19+ cell numbers, and the CD4+/CD8+ ratio among the groups (p=0.046, 0.002, and 0.045, respectively). CD4+ cell numbers in group II were significantly higher than group I (p=0.039). CD19+ cell numbers in group II were significantly higher than group I (p=0.03). CD19+ cell numbers in group III were significantly lower than group I (p=0.032) and group II (p=0.002). The CD4+/CD8+ ratio in group II was higher than in group I (p=0.039) and group III (p=0.039).
In the final samples (T3), significant differences were observed in CD4+ and CD19+ cell numbers among the groups (p=0.022 and 0.002, respectively). CD4+ cell numbers in group II were significantly higher than group I (p=0.018) and group III (p=0.039). CD19+ cell numbers in group II were significantly higher than group I (p=0.034), and CD19+ cell numbers in group III were significantly lower than group I (p=0.028) and group II (p=0.003).
DiscussionMPDE are the most common skin manifestations of drug allergy2,3 but can frequently be confused with VE, especially in children. The disappearance of the lesion after withdrawal of a suspected drug increases the probability of a causative association; failure to resolve after withdrawal is against diagnosis. However, non-drug-induced skin lesions can resolve coincidentally after withdrawal of a drug, and drug-induced lesions can persist despite drug withdrawal.27 Additionally, this information of improvement after drug withdrawal is not always available from the clinical history. Controlled administration of the culprit drug has proved to be useful in the diagnosis of urticaria or exanthematic reactions,6 this is, however, contraindicated in cases of severe reaction.6,13–15
Although eosinophilia is often suggestive of a drug-induced allergic reaction, most patients with drug-induced allergic reactions do not have eosinophilia and thus this absence clearly does not exclude a drug-induced allergic cause.28 Comparisons of peripheral blood eosinophil counts among the four groups (MPDE, VE, DRESS, and controls) in the first sample (T1) showed no significant differences. Comparisons of peripheral blood eosinophil counts among the three groups (MPDE, VE, and DRESS) in the other two samples (T2, T3) showed no significant differences. Similar results have been reported in previous studies.29,30
The only significant difference in the FBC among the groups was in the absolute numbers of lymphocytes in the third blood samples (T3). Peripheral blood lymphocyte counts were lower in group III than group II (p=0.009). The low number of peripheral blood lymphocytes in DRESS might be important in discriminating viral rashes from DRESS in which viral reactivation plays a role in the pathogenesis.
Recently, Fas/FasL-dependent apoptotic pathways have been reported as being involved in the pathogenesis of drug-induced maculopapular rashes (MPRs).16 Stur et al. demonstrated that the determination of sFasL serum levels can be a most valuable tool in discriminating drug-induced skin reactions from other clinically resembling skin diseases, such as exanthematous viral infections. Elevated levels of FasL were detected not only in TEN patients but also in sera and the lesional skin of patients with MPR. However, in our study, serum sFasL levels were not found to be useful in discriminating viral exanthemas from other drug-induced rashes and the results were not found to be different on repeated evaluations.
Viard et al. first reported increased sFasL concentrations in the sera of patients with TEN. However, they could not detect increased sFasL concentrations in patients with drug-induced maculopapular rash.31 In another study, Abe et al. reported increased sFasL concentration in the sera of patients with SJS/TEN, while the levels were not increased in erythema multiforme-like drug eruptions.32 More recently, Murata et al. investigated whether sFasL could be a useful marker of SJS/TEN at the early stage when patients have no skin detachment or mucosal lesions. They showed significantly increased sFasL levels in sera of patients with SJS/TEN before disease onset. They suggested that early diagnosis of SJS might be made by monitoring serum sFasL levels in patients with cutaneous adverse drug reactions suspected of progressing to SJS/TEN.33 In another study, Bellini et al.5 investigated the immunohistochemical expression of a cytokine panel including FasL in skin biopsy specimens obtained from drug-induced exanthema and virus- or bacteria-induced exanthema patients. They observed a FasL expression in suprabasal and basal keratinocytes, particularly in subjects with a maculopapular eruption after intaking amoxicillin. They suggested that the FasL expression might be connected with the causative drug (amoxicillin).
In our study, skin biopsies were not obtained, and cytokine expressions were not investigated by immunohistochemistry. However, our data do not support the role of Fas/FasL-dependent apoptotic pathway in the pathogenesis of drug-induced MPRs, concordant with the above-mentioned reports. It seems more probable that sFasL-mediated keratinocyte apoptosis might correlate with the severity of the disease.
Immunological mechanisms taking part in viral infections involve T-and B-cell responses, generating specific lymphocytes and antibodies, whereas most drugs involved in non-immediate drug-induced cutaneous reactions do not induce humoral responses.6
In maculopapular rashes, a delayed cell-mediated immune mechanism involving drug-specific T cells is thought to be important.3,34 Pichler et al.35 subdivided type IV delayed hypersensitivity reactions into four groups according to the clinical presentation and the involvement of different types of drug-responsive T-cells. Morbilliform or maculopapular eruptions showing prominent eosinophil infiltration in biopsies are probably mediated by type IVb T-cell responses.
On the other hand, cytotoxic functions by either CD4+ or CD8+ T cells (type IVc) seem to participate in multiple types of drug-induced delayed hypersensitivity reactions, such as contact dermatitis, maculopapular, and bullous drug eruptions, and drug-induced hepatitis.35
The present study also evaluated the role of different subsets of peripheral blood lymphocytes in drug-induced eruptions and VE to assess whether immunological response could be useful in determining precise etiology.
CD4+ cells were significantly higher in group II than group I at T2 and T3, and the difference was more prominent at T2. At T1, the CD4+/CD8+ ratio was significantly higher in group II than group I and group IV. CD19+ cell counts were significantly higher in group II than group IV at T1. Additionally, the difference in CD19+ cell counts was also significant between group II and group I in other samples (T2 and T3). Our results support the importance of T- and B-cell responses in immunological mechanisms taking part in viral infections.6 CD4+ and CD19+ immune responses are more prominent in patients with VE. B cells and CD8+ T are well-accepted components of the adaptive immune response to viruses; however, recent reports provide new evidence for CD4+ T cells as direct effectors in antiviral immunity.36
The other most prominent and consistent finding was significantly lower numbers of CD19+ cells in group III than the other groups in all the samples. Although recent studies have suggested the role of viral infections in the development of DRESS,8 in our study, unlike viral induced exanthema, B-cell numbers were found to be low in DRESS. This would be useful for early detection. The exact pathogenesis of DRESS remains unknown. The metabolism of the parent drug to chemically reactive metabolites is thought to be important in initiating the cascade of events leading to DRESS.37 Recently, the role of HHV-6 reactivation as a possible etiologic factor in DRESS has been suggested.11,12,38,39 Reactivation of HHV-6 from latently infected peripheral blood mononuclear cells (PBMCs) requires T-cell activation. Tohyama et al.12 suggested T-cell activation caused by reactive metabolites as the primary step for the development of DRESS. However, many adverse drug reactions via T-cell activation do not always develop into DRESS. Alternatively, Kano et al.40 stated that drug (metabolites)-induced hypogammaglobulinemia and B-cell depletion lead to an environment in which HHV-6 reactivates. Recently, Inaoka41 proposed that the intrinsic dysfunction of NK cells to express CD122 is primarily responsible for the drug-induced decrease in B cells and the subsequent reactivation of HHV-6. Additionally, Blanca et al.42 demonstrated that NK cells could induce the differentiation of B cells into Ig-producing cells. Our results are consistent with our previous reports of cases with DRESS.43,44 However, in the present study, even NK-cell numbers were low in all samples in DRESS, a difference not significant.
In conclusion, in our study, serum sFasL levels were not found to be useful for discriminating viral exanthemas from other drug rashes. Additionally, the results were not found to differ in repeated evaluations. The significant differences between MPDE, VE, and DRESS were high CD4+ and CD19+ cell counts in VE, and low B-cell numbers in DRESS. This difference might be important in discriminating viral rashes from DRESS where viral reactivation has a role in pathogenesis, and the low B-cell numbers in initial symptom days might be a useful predictor of DRESS development.
Conflict of interestThe authors have no conflict of interest to declare.
This study was supported by the Research Council of Trakya University (TUBAP-2009/157) (Edirne, Turkey).