The pomegranate, the fruit of Punica granatum, belonging to Lythraceae family and commonly cultivated in the Mediterranean area, has been involved in some immediate reactions,1,2 including severe symptoms such as anaphylactic shock and laryngeal oedema.3,4 Cases of hypersensitivity reactions to pomegranate have been reported and the implication of 29-kDa1,2,4 and 9-kDa3 protein allergens has been described. Subsequent characterisation of the 9-kDa allergen demonstrated its belonging to the lipid transfer protein (LTP) family, a family widely distributed in fruits, vegetables and nuts, which has been suggested as being responsible for immunological cross-reactivity between fruits, nuts and/or pollens. Allergy cases involving LTPs from pomegranate and peach, hazelnut and peanut have been published.1–4 Furthermore, a study of LTPs in pomegranate identified and isolated two LTP isoforms with different IgE binding capacities [LTP1a (9-kDa) and 1b (7-kDa)].5
We describe the case of an 18-year-old women, without history of allergic disease, who suffered from angio-oedema, generalised urticaria, glottis oedema, vomiting, abdominal pain and malaise five to ten minutes after ingesting a pomegranate. Three years before she had two episodes of angio-oedema and urticaria after apple ingestion and one episode of urticaria, abdominal pain and angio-oedema after drinking pear juice. The patient did not develop any kind of allergic symptoms after performing a Rosaceae and pomegranate free diet.
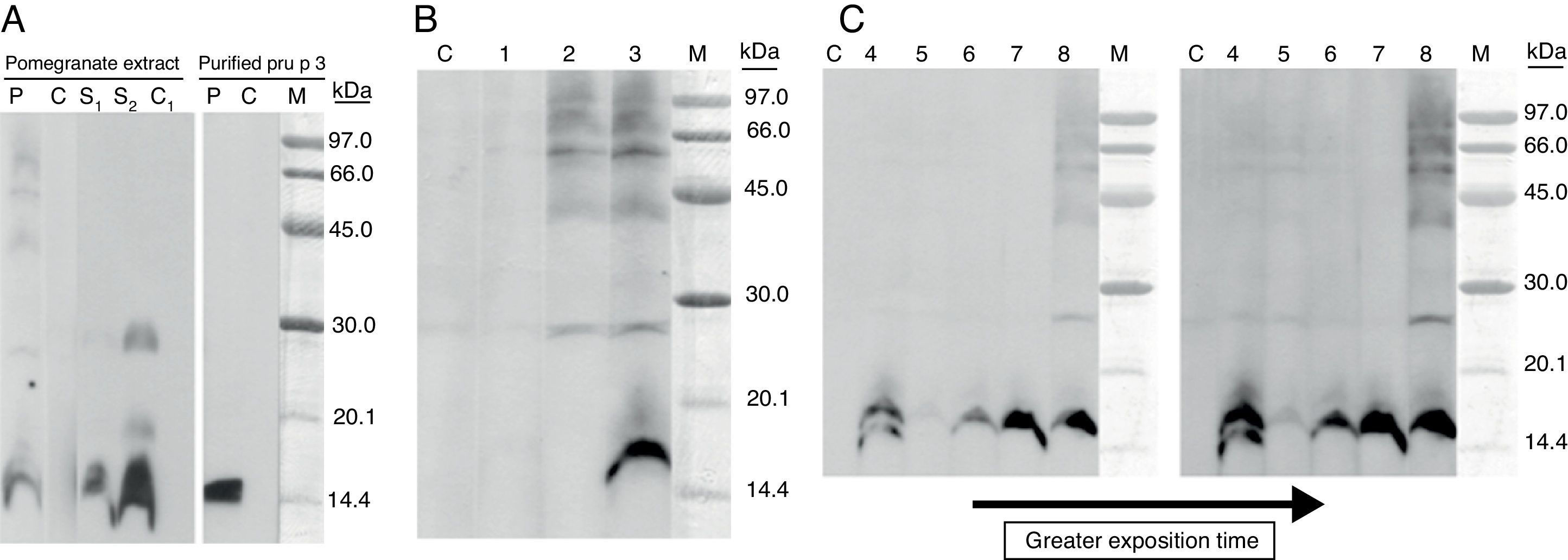
The patient underwent skin prick tests with a commercial extract panel of common inhalants, fruits, vegetables and nuts allergens (Leti laboratories, Spain) and the results were positive to Dermatophagoides pteronyssinus, grass pollen, mugwort pollen, peach, apple, pomegranate, strawberry, cherry, almond, peanut, tomato, bell pepper, green beans, black bean and soybean seed. Prick test with profilin and LTP extract (Alk Abelló, Madrid, Spain) were positive. Prick-prick test with fresh pomegranate was positive. Specific IgE, performed with UniCAP® method, was positive to peach (10.3kU/L), apple (8.45kU/L), pear (6.61kU/L), cherry (9.79kU/L) and green bean (3.94kU/L). Immuno solid-phase allergen chip (ISAC®) microarray was positive to nPru p 3 (12ISU) and nSal k1 (2.5ISU). Because of the systemic reactions and the positive test results with pomegranate, peach, apple and peanut, a possible involvement of LTPs and IgE-mediated allergy was expected. We performed a SDS-PAGE immunoblotting assay in non-reducing electrophoresis conditions (without 2-mercaptoetanol) with pomegranate extract in solid phase and using the patient serum and an anti-Pru p 3 serum from rabbit. Both sera revealed a clear 16kDa-IgE reactive protein and some others faint ones with higher molecular masses. Identical assay, carried out with purified Pru p 3 and patient serum, showed an IgE binding band with the same molecular mass. The molecular weight of binding band protein detected is higher than that usually described for LTPs (7–9kDa).5 This apparent molecular weight difference is probably explained by the electrophoretic mobility shift that occurs under non-reducing conditions, as has already been shown by Zoccatelli.5 We decided to perform the electrophoresis assay in non-reducing conditions in order to preserve the conformational structure of epitopes LTP and consequently their IgE-binding capacities. Cross-reactivity between the pomegranate IgE binding protein and Pru p 3 (peach LTP) was demonstrated and was then confirmed by immunoblotting inhibition assay.
Despite the absence of clinical inhalant allergy as positive skin prick tests results were detected with extracts from grass pollen, mugwort pollen and plane tree pollen, the possible cross-reactivity between pollen and pomegranate extract was studied. A very high level of IgE-binding inhibition on pomegranate band was detected when Artemisia vulgaris pollen extract was used as inhibitor, whereas partial inhibition was observed with Olea europaea pollen extract (Fig. 1).
(A) SDS-PAGE IgE-immunoblotting of pomegranate extract (under non-reducing conditions). Lane P: patient serum; Lane C: control serum (pool from non-atopic subject sera); Lane S1 and S2: Anti-Pru p 3 rabbit serum (dilution 1/10,000–1/5000); Lane C1: serum from non-immunised rabbit (Dilution 1/5000); M, molecular mass markers. (B) SDS-PAGE IgE-immunoblotting-inhibition with pomegranate extract in solid phase. Lane 1: patient serum pre-incubated with pomegranate extract; Lane 2: patient serum pre-incubated with Pru p 3 (150¿g/ml); Lane 3: patient serum pre-incubated with BSA (150¿g/ml). (C) SDS-PAGE immunoblotting-inhibition with pomegranate in solid phase and pollen extracts as inhibitors: Lolium perenne (Lane 4), Artemisia vulgaris (Lane 5), Olea europaea (Lane 6), Parietaria judaica (Lane 7), lamb as negative control (Lane 8), molecular mass markers (Lane M).
The clinical history, the skin tests and the immunoblotting results led us to conclude that the allergy symptoms were probably caused by the presence of specific IgE for the 15/16-kDa protein completely inhibited by Pru p 3, and suggest that the primary sensitisation must be due to Rosaceae LTPs, greatly consumed in southern European countries. The inhalation of pollen LTP could have contributed to maintain the specific LTP IgE levels in patient serum. LTP appears to be the main factor associated with cross-reactivity with clinical allergy between pomegranate and botanically unrelated food from different families, such as nuts, namely hazelnut, and peanut.
Ethical disclosuresConfidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors have no conflict of interest to declare.