Among chemical products used as additives, sulphites are often used for their antioxidant properties in order to control bacterial growth and prevent discolouration by bleaching agents. They are mostly used with vegetables (i.e. potatoes and green salads) and fresh fruits, where they are added to prevent enzymatic and non-enzymatic browning. They can also be found in soft drinks, wine, beer, and dried foods, where they have antimicrobial effects. In addition, their agents, such as sulphur dioxide and sodium sulphite, are used as sanitisers for containers and equipment.1 Among these agents sodium metabisulphite (SMB) is usually employed as a source of sulphur dioxide in applications where the handling of liquefied gas is inconvenient. It is moreover added to local anaesthetic solutions containing adrenaline, as its antioxidant property prevents oxidation of adrenaline itself, to topical medicaments, and eye drops containing sympathomimetics.1
The ubiquity of sulphites in foods and drugs makes it difficult to pinpoint the relevance of positive patch test reactions.1
Allergy to SMB is known to induce asthma and contact dermatitis, and these reactions have been widely described in adulthood, nevertheless this kind of allergy is rare in childhood and only two studies on SMB-induced asthma have been reported in this age group.2,3 On the other hand, there is no paediatric report on urticaria and anaphylaxis caused by SMB allergy.
Herein the authors report the case of a five-year-old female child, admitted to our Pediatric Acute and Emergency Department for urticaria and anaphylaxis secondary to SMB sensitisation.
A five-year-old female, Caucasian child was admitted to our Pediatric Acute and Emergency Room, Policlinico-Vittorio-Emanuele University Hospital, University of Catania, Italy for daily incontinence after administration of oral cetirizine.
Her familial anamnesis was positive for allergic diseases, as her mother was allergic to common inhalants and to eggs, tomatoes and strawberries, as well as to various antibiotics (penicillin, amoxicillin, most cephalosporines and macrolides). The child's brother was allergic to mite dust antigens and parjetaria.
Since birth the child was affected by atopic dermatitis, secondary to cow's milk protein allergy (CMPA), which was treated with extensive hydrolysed formula. Moreover, she often suffered from bronchial asthma, allergic rhinitis and recurrent episodes of dermatitis. Her mother also referred that the child presented nausea and vomit after ingestion of egg (albumen and yolk), tomato, chocolate and wurstel. The peculiar thing was that the symptoms appeared with the ingestion of preserved food but not with fresh food.
When she was first admitted to our hospital, on November 2012, we performed routine blood analysis that gave a negative result, except for the total IgE dosage that was high (289UI/l, normal value: 70–100UI/l) and skin prick tests were positive for house dust mites and ambrosia, while food skin prick tests were all negative. The child was then discharged with an inhaled corticosteroid therapy and oral anti-leukotriene.
One month later, after the consumption of oral clarithromycin for fever and respiratory infection, the child had an episode of anaphylaxis and she was again admitted to our Paediatric Acute and Emergency Room. On that occasion, her mother referred that the same reaction had previously occurred with the consumption of other antibiotics and in particular with penicillin, amoxicillin and ampicillin, so that antibiotic treatment in this child was difficult to establish. Therefore, the child was hospitalised for four days, during which oral azithromycin was started. During the hospitalisation the child did not manifest any disturbance, but when she was discharged, a second assumption of the same drug provoked another allergic reaction, most likely referable as urticaria. Thus, the child was admitted a third time to our Pediatric Acute and Emergency Unit, and in particular she underwent a Pneumologic and Allergologic consult.
When the child came to our observation, her physical exam showed the presence of skin wheal erythematous manifestations, each of 2–3cm in diameter, spread all over her body, and all over her face and trunk. These manifestations were itching and they appeared as pale red, raised bumps, lasting about one hour and resolving spontaneously. Dermographism was present. She also presented a harsh breath with whistles by the lung, associated with respiratory failure.
We again performed routine blood analyses that revealed high IgE levels (245UI/l) and, on this occasion, considering her multiple food and drug sensitisation, we also performed an extensive patch test for various additives (True Test™, Stallergenes). The patch test was applied to the upper back of the patient and covered with individual square plastic chambers. They were kept in place with special hypoallergenic adhesive tape. After 48h the child returned to the physician and the result was positive for SMB (with a wheal of 8mm of diameter). We also performed the dosage of serum specific IgE for sulphite by ELISA immuno-enzymatic reaction, and they resulted positive for SMB. On the contrary, when we performed the allergological diagnosis for drug allergy, the tests were negative. Therefore, on the basis of her clinical history, as all the drugs she administered contained SMB as addictive, and by the patch test result, a diagnosis of SMB allergy was made, warning her parents to avoid all foods and drugs containing this additive. Moreover an egg, tomato, chocolate and wurstel free-diet was started and it resulted in a sudden improvement of her gastroenteric and cutaneous symptoms.
The peculiarity of this report is the wide cross-reactivity of food and drug allergy present in a child with SMB hypersensitivity. Moreover, in literature, there is no description of allergy caused by SMB in childhood. The only two literature data on SMB hypersensitivity in this age group report two studies on SMB challenge in asthmatic children.2,3 In particular, Boner et al., in 1990, studied 56 children, between 6 and 14 years of age, to evaluate bronchoconstrictive airway responses after ingestion of varying doses of potassium bisulphite, administered in capsule or in solution.2 They found that only six children had a positive bronchoconstrictive response after the ingestion of a maximum dose of potassium bisulphite in capsule.
Previously, in 1984, Tonnes et al.3 led a challenge on 29 children with SMB in capsule, and to all children with a positive response, a diet that excluded foods containing SMB was prescribed. After three months on these restricted diets, the children were reassessed to determine any therapeutic response. The authors found a 66% of incidence of positive challenge with SMB, and none of the children reacted to SMB in capsule form (maximum dose=100mg), but 19/29 reacted to SMB in solution with 30mL of 0.5% citric acid.
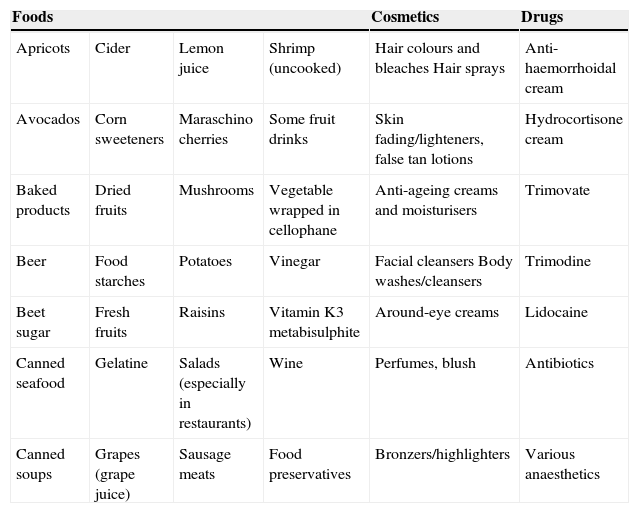
SMB is recognised as a potential cause of airway irritation and possibly occupational asthma. As a matter of fact, in adulthood there are several reports on occupational hypersensitivity to SMB, described in a photographer technician,4 a radiographer,5 and in patients working in the fish processing industry.6 Moreover SBM has also been reported as the cause of a great percentage of drug allergy in adulthood, and in particular for contact dermatitis after usage of an anti-haemorrhoidal cream,7 hydrocortisone cream,8 lidocaine,9 and even fresh fish6 and beer.10 The most common foods, cosmetics and drugs containing SMB are listed in Table 1.
Sulphite-containing foods and drugs.
| Foods | Cosmetics | Drugs | |||
|---|---|---|---|---|---|
| Apricots | Cider | Lemon juice | Shrimp (uncooked) | Hair colours and bleaches Hair sprays | Anti-haemorrhoidal cream |
| Avocados | Corn sweeteners | Maraschino cherries | Some fruit drinks | Skin fading/lighteners, false tan lotions | Hydrocortisone cream |
| Baked products | Dried fruits | Mushrooms | Vegetable wrapped in cellophane | Anti-ageing creams and moisturisers | Trimovate |
| Beer | Food starches | Potatoes | Vinegar | Facial cleansers Body washes/cleansers | Trimodine |
| Beet sugar | Fresh fruits | Raisins | Vitamin K3 metabisulphite | Around-eye creams | Lidocaine |
| Canned seafood | Gelatine | Salads (especially in restaurants) | Wine | Perfumes, blush | Antibiotics |
| Canned soups | Grapes (grape juice) | Sausage meats | Food preservatives | Bronzers/highlighters | Various anaesthetics |
Even if SMB allergic reactions have never been described in childhood, it seems that SMB exposure in the diet is likely to occur from a young age and such exposure can be referred as a mechanism of later tolerance to contact allergy. Recently, While et al.11 speculated that application of contact allergens (preservatives, additives, perfumes and antioxidants) in baby skin care products could favour the development of immune tolerance, anticipating the later acquisition of specific tolerance on “natural” dietary exposure after weaning.
The real problem to face in case of SMB allergy is the treatment, above all on how to treat anaphylaxis since adrenaline and epinephrine products contain SMB. In addition reports of bronchospasm, urticaria, angio-oedema, nausea, abdominal pain, diarrhoea, seizures, and death, anaphylactic and anaphylactoid reactions to sulphites have been documented.12 Therefore, in patients with a definitive history consistent with anaphylactic or anaphylactoid reactions to sulphites, avoidance of medications, including epinephrine, which contain metabisulphites as preservatives might be indicated. On the other hand, medically preferred agents during these occasions are: airway support, fluid replacement with colloids and vasoconstrictors agents such as methoxamine, whose role is supported by recent publications of Breslin and McBrien.13 It is interesting to note that glucocorticoids and colloids, used in the treatment of anaphylaxis, have also been implicated as causative agents for anaphylaxis and should be avoided in sensitised patients. Nevertheless, as the manageability of methoxamine in childhood is still a topic of debate,14 steroids, bronchodilators, and anti-histamine H1 and H2 blockers are to be considered as the first line to treat SMB atopic children in case of anaphylaxis.
The importance of this case report is the knowledge of the possibility of a SMB allergy also in childhood. The underlying mechanism remains unknown, because to our knowledge, our report is the first case ever described in literature in such early childhood. Nevertheless, it is useful to know its entity because the prevalence of allergic disease in childhood is deeply increasing and the access to Pediatric Acute and Emergency Units is equally raised, so that in case of urticaria and anaphylactic episodes it is important to perform a good allergic anamnesis, in order to start the correct treatment and to alert physicians on the cautious use of drugs.
Ethical disclosureProtection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.