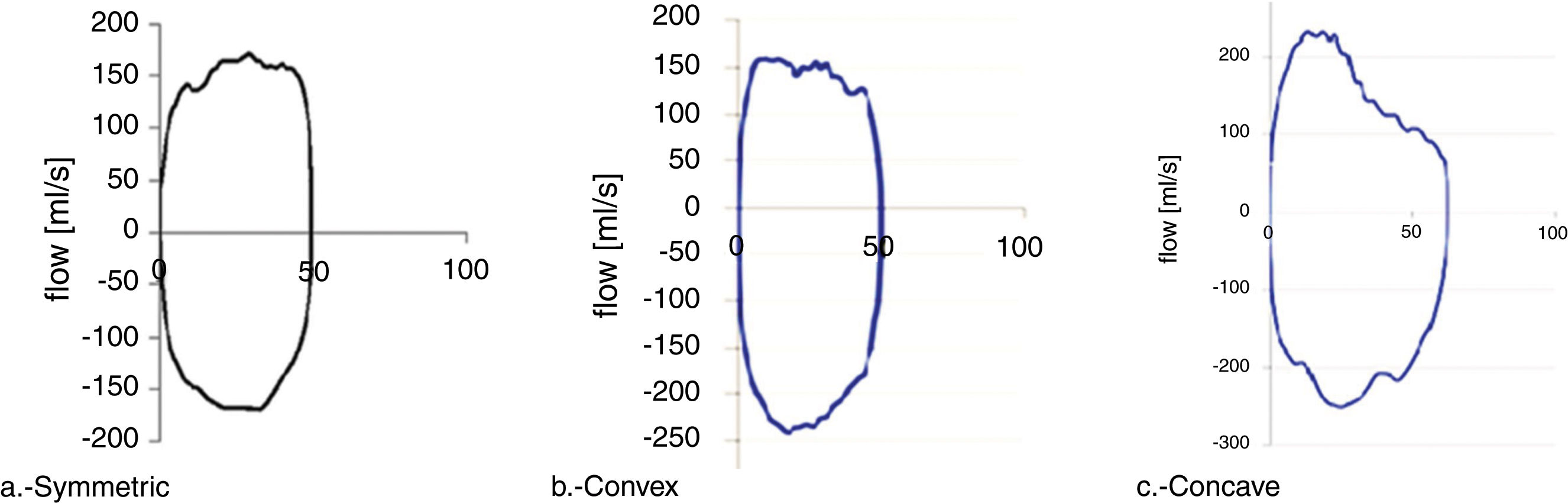
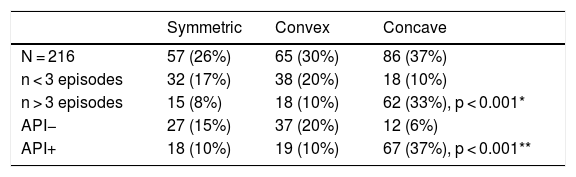
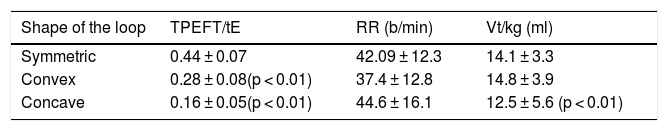
Wheezing (RW) infants with a positive asthma predictive index (API+) have a lower lung function as measured by forced expiratory techniques. Tidal flow-volume loops (TFVL) are easy to perform in infants, and sedation is not necessary.
Materials and methodsA total of 216 wheezing infants were successfully measured, and 183 of them were followed for over a year. TFVL loops were classified into one of three categories depending of their geometric shape (symmetric, convex, and concave). Respiratory rate (Rr), presence of API+, and the number of exacerbations during the following year were also recorded.
ResultsChildren with concave loops had more exacerbations in the following year (OR = 6.8 [IC95% 3.33;13.91]). Infants API + were also significantly more related to concave loops (OR = 10.02 [IC 95% 4.53; 22.15]). Rr was higher in infants with concave loops (44+/−15.5 vs. 36.6 +/−12.6; p < 0.01).
ConclusionInfants with a concave TFVL have a higher probability of experiencing exacerbations in the following year, and are at a higher risk of suffering asthma.
Wheezing in infants is an active area of concern for both pediatricians and respiratory specialists. Currently, it may prove difficult to diagnose and monitor an infant from the detection of the condition to the outcome; while lung studies are feasible in children ages six and over and there are studies that provide spirometric normal values at pre-school ages,1 Pulmonary Function Tests (PFT) on children under three years are difficult to perform and, in some cases, require sedation.2
In earlier studies,3 infants with chronic lung disease showed a typical obstructive pattern when the shape of TBFV was observed. Studies based on partial forced expiratory flow-volume loops obtained using thoraco-abdominal compression helped in characterizing the respiratory disease in obstructive disorders4 as well. The authors have also shown5 that lung function measured by partial forced expiratory flows was lower in API + infants.
Respiratory measurements performed obtaining TBFV have been used in the past for epidemiological studies, due to their feasibility.6 Some of the findings indicate that the ratio between the time from peak expiratory flow to the total expiratory time (tPEF/tE) is reduced in infants with acute respiratory obstruction, including conditions such as bronchiolitis7 and asthma.8 Decreased tPEF/tE has also been associated with recurrent wheezing during the first three years of life,9 and is present in newborn infants exposed to maternal smoking during pregnancy compared to those not exposed.10 tPEF/tE has been shown to be significantly decreased in young infants even before their first wheezing episode.11 However, the association of time parameters with the impairment of small airways is still speculative.
The assessment of lung function in conjunction with the timing of the first wheezing episode has also helped in classifying different groups of wheezing children early in life and studying their lung function behavior over time.12 Longitudinal follow-ups on the different groups according to their background and lung-function measurement have helped in describing the progression of wheezing disorders.
Clinical evaluation based mainly on auscultation has been shown to be a weak tool for assessing airway narrowing.13 However, lung function studies are difficult to perform, as most of these patients are infants two years old or younger. A simple and non-invasive method to evaluate the respiratory system at this age is needed.
Some of the available studies that have assessed the response of wheezing disorders to inhaled corticosteroids14 are mainly based on thoraco-abdominal compression techniques on sedated children; however, this approach is not practical in daily medical practice.
While the tPEF/tE ratio has been used to detect airway obstruction together with respiratory rate (RR),6,12,15 with a degree of success, the association of this parameter with a small airway caliber could not be demonstrated,16,17 possibly because parametric measurements exclude some valuable information expressed by the whole expiratory limb. As a result, the discriminating power of the TBFV loops has been underestimated in the past; the tPEF/tE ratio is the mathematical expression of the flow-volume loop during tidal breathing, but we feel that the shape of the loop includes a great deal more information, as the sequence of instant flows along the expiratory part of the loop may depend on the degree of obstruction of the airways, regardless of the value of the aforementioned ratio and thus may determine different loop morphologies.
Different shapes and analyses of TBFV loops have been studied in infants, including convex and concave expiratory limbs, with good results.18,19 Concave expiratory limbs were nearly exclusively noted in infants with chronic lung disease and the highest RR.18 For asthmatic children, an index of the convexity of the overall shape of the expiratory limb correlated well with expired flows.19 A different type of shape analysis such as concavity indexes, taken along with symptoms, was also introduced for diagnostic support based on different tidal breathing patterns.20 The goal of the present study is to determine if the shape of the flow-volume curve assessed with the flow-volume technique at tidal volumes is related to the outcome of the disease and/or to the presence of risk factors of asthma in infants after their first wheezing episode.
MethodsWe enrolled 267 infants (male = 176; 66%), between ages two and 30 months, with a median age of seven months, during or after their first wheezing episode visiting the Paediatric Clinic at Hospital Quirón Palmaplanas, Palma de Mallorca, Spain. Enrollment was done during a 38-month period spanning January 2013 to April 2016. The wheezing episode was defined as an acute respiratory episode mostly caused by an infection. All infants were otherwise healthy, with no chronic respiratory disease (bronchopulmonary dysplasia or cystic fibrosis), and none of them had been born pre-term.
Each infant was booked for a study including lung-function testing a minimum of two weeks after their first wheezing episode. The infants were all asymptomatic during the lung-function study visit, and auscultation was normal in all cases. Patients were also scheduled for control visits every three months for the following year. During these visits, parents were asked if the child had had any wheezing exacerbations during that period, and if it had happened in the context of an infection (cold, fever, cough, runny nose). The occurrence of respiratory exacerbations was recorded, as was the treatment applied. The etiology of the infections was not studied.
An asthma positive index (API) was considered when at least one of the parents had asthma or the child had eczema.21 Those episodes that needed treatment with oral corticosteroids were considered as severe exacerbations. Lung function was obtained applying a face mask attached to a pneumotachometer (Hans-Rudolph non-heated 0−35 l/min. Shawnee, USA). Measurements of the flow-volume loop shape were performed using a custom-built system5 and following the ATS/ERS Working Group of Standardization of Infant Pulmonary Tests. Potential leaks around the mask were monitored by observing the non-existence of drift to either side of the x axis and a stable end expiratory level.
At least 10 stable and breathing cycles were selected for each study, then, one of the pre-established shapes described below was assigned. Infants of age one and over were measured sitting in the upright position on the caregiver’s lap, whilst younger patients were set on their backs. In all cases, the head and neck of the infant were positioned to achieve the strongest and most reliable signal. The tests were performed in a quiet environment, and the person performing the measurement waited until the baby was calm.
In the few cases that the children were not calm enough, they were not included in the study. In all cases, the reason for the study was explained to the caregivers, and consent was obtained before the procedure. No ethics approval was considered due to the simplicity and non-invasiveness of the study. However, there was written approval by the medical directory board.
All measured curves were classified into one of three curve geometric shapes: symmetric (Fig. 1a), convex, having peak expiratory flow before 50% of tidal volume and a convex expiratory limb (Fig. 1b), and concave, having the peak expiratory flow before 50% of tidal volume and concave expiratory limb (Fig. 1c).
Other respiratory parameters were compared between groups as well: respiratory rate (RR), tPTEF/tE, and tidal volume per kilogram (Vt/kg).
Statistical analysisA two-way table of frequencies with chi-square and residuals analysis was performed to assess the association between qualitative variables. In order to understand whether the shape of the loop was associated with recurrent wheezing, a logistic regression analysis was carried out considering recurrent wheeze (three or more episodes) as the dependent variable; and respiratory rate, age, and gender, as independent variables.
To assess whether Concave loops were associated with risk factors of asthma, a second logistic regression analysis was performed considering the concave loop pattern (yes or no) as the dependent variable, and having risk factors (asthma, rhinitis, eczema), respiratory rate, age, and gender, as independent variables.
The difference between groups for RR, tPTEF/tE and Vt/kg was calculated by Student’s t-test.
ResultsWe obtained reliable measurements in 216 out of 267 infants at the first study visit. Fifty-one out of 267 patients did not complete the first visit for different reasons (dropout, still wheezing, or under treatment for other reason. There was no statistical difference between patients studied at the first visit and those who were not.
The group that completed the first visit had a median weight of 7753 g (range 3800-13800gr), and a median age of seven months (range 2–30). One hundred and eighty-three out of 216 cases were followed for more than one year; 104 out of 216 (48%) had a higher risk of developing asthma (API+) and 95 out of 183 (52%) patients followed during one year had more than three exacerbations (Table 1).
Characteristics of patients enrolled who completed first visit.
| Patients studied at first visit (n) | 216 |
| Gender (male) | 176/216 |
| Birth weight (gr) | 3286 ± 354.4 |
| Weight at study visit (gr) | 7753 (3800/13800) |
| Age at study visit (months) | 7 (2/30) |
| Patients followed one year | 183/216 |
| Patients API+ | 104/216 |
| Patients who suffered >3 episodes one year | 95/216 |
During the study we counted a total of 705 exacerbation episodes of which 496 were suffered by API + children. Four hundred and twenty-seven exacerbations were treated with oral corticosteroids, and 137 episodes not related to a cold were informed. Six patients were hospitalized due to a severe exacerbation.
Fifty-seven out of 216 infants (26%) showed a symmetric loop shape, 65 (30%) a convex loop shape and 86 (37%) a concave loop shape. Eight patients (3%) had loops with an undefined shape (Table 2).
Number of episodes and patients with higher risk for each shape of Tidal F/V loop.
| Symmetric | Convex | Concave | |
|---|---|---|---|
| N = 216 | 57 (26%) | 65 (30%) | 86 (37%) |
| n < 3 episodes | 32 (17%) | 38 (20%) | 18 (10%) |
| n > 3 episodes | 15 (8%) | 18 (10%) | 62 (33%), p < 0.001* |
| API− | 27 (15%) | 37 (20%) | 12 (6%) |
| API+ | 18 (10%) | 19 (10%) | 67 (37%), p < 0.001** |
Patients having concave loops (OR = 6.80 [IC95% 3.33; 13.91]) showed a much higher and statistically significant risk of having more than three wheezing episodes in the following year according to a logistic regression analysis adjusted by gender, respiratory rate, and age (Table 2).
Similarly, the second logistic regression analysis indicated that infants with an API + were significantly more likely to have a concave loop (OR = 10.02 [IC95% 4.53; 22.15]) (Table 2).
tPTEF/tE was significantly lower in the cases presenting convex and concave loops. There was no difference between respiratory rates in the three groups. Vt/kg was significantly lower for the concave (Table 3).
Values (mean+/−sd) for TPEFT/tE, Respiratory Rate and Vt/kg for each shape of the loop.
| Shape of the loop | TPEFT/tE | RR (b/min) | Vt/kg (ml) |
|---|---|---|---|
| Symmetric | 0.44 ± 0.07 | 42.09 ± 12.3 | 14.1 ± 3.3 |
| Convex | 0.28 ± 0.08(p < 0.01) | 37.4 ± 12.8 | 14.8 ± 3.9 |
| Concave | 0.16 ± 0.05(p < 0.01) | 44.6 ± 16.1 | 12.5 ± 5.6 (p < 0.01) |
Infant lung-function testing has enabled the study of the pathophysiology of different breathing obstructive disorders. The studies, however, have been used predominantly to classify different groups of infants at early ages according to their level of airway patency, and the likelihood of following different wheezing paths.11 The use of these tests in very young children has only been included in epidemiological studies to recognize the early changes of lung function in different respiratory diseases4,6 but has no significant role in deciding case-specific treatment strategies. At the clinical level, most pediatricians and pediatric respiratory specialists still rely solely on clinical parameters to diagnose respiratory obstruction in infants at early ages.
Forced expiratory flow-volume loops in pre-school children have also helped to gather data about normal lung function at early ages. However, it is not possible to obtain voluntary forced expiratory maneuvers in the first two years of life and performing lung function tests using sedation is not feasible in everyday practice.
In contrast, acquiring a TFVL in an infant, or even in a newborn, is relatively simple and non- invasive. When TFVL techniques are used, a high proportion of wheezing infants have shown low lung function prior to their first acute episode.12 Flow ratios have been used to characterize the lung function of large cohorts; however, in the case of individual observations, the presence of a low ratio would only detect an early expiratory peak flow.
The early expiratory peak flow is indirect evidence of airway obstruction; however, the shape of the limb after the PEF can deliver valuable information about the patency of the airways, information that is lost if only the ratio is considered. However, the visual recognition of the shape can be useful in itself: A concave limb indicates more obstruction than a convex one,18,20 due to a higher flow limitation.
Morris and Lane24 demonstrated that in older children and adults, the values of tPTEF/tE were significantly lower in subjects with obstructive airway disease.
When we compared tPTEF/tE between groups, we found that it is significantly lower in asymmetric loops and even much lower in the concave group. Tidal volume relative to body weight is lower in the concave group also. However, there was no difference in respiratory rate between groups.
These results emphasize the good correlation between respiratory obstruction, early tPTEF and an asymmetric shape of the TFVL. As respiratory rates are not different between groups, we can assume that higher frequencies did not affect the ability to obtain a consistent expiratory loop; nor did they influence the shape of the loop.
ConclusionsWe have characterized the shape of the tidal flow-volume loops (TFVL) in a group of infants that had had a first wheezing episode. Within the group, those patients that showed an asymmetric TFVL with a concave expiratory limb were statistically more likely to suffer exacerbations during the following year. Moreover, the presence of risk factors of suffering asthma (API+) was associated to concave loops as well.
None of the infants studied was wheezing at the moment of the examination, and most of them had a convex or concave TFVL. Further, using TFVL, we could identify those who ended up having a worse outcome during the following months, according to the recurrence of the exacerbations.
Therefore, obtaining a simple tidal flow volume test could be useful to anticipate the evolution of the wheezing disorder or decide on a type of treatment.
In case of an airway narrowing secondary to a viral injury identifying asymmetric concave TFVL could also help to quantify the magnitude of the impact, and be useful to predict a higher risk of future exacerbations.
We also found that the presence of a concave loop and a higher incidence of an API+ are related. The presence of factors related to atopy increase the risk of impairment of lung function, as we showed earlier in infants with positive API and lower lung function.5
We were not able to gather data from a control group of healthy infants with no history of a wheezing episode. However, we have been able to characterize three different groups in children with previous disease, and identify those with a higher probability to suffer repeated exacerbations in the future. Moreover, there is a clear difference between the group with symmetric loops and the rest as the incidence of exacerbations amongst them was much lower. tPTEF/tE ratio in those children was found to be in the normal range (0.44+/−0.07), as observed in previous studies.25
The level of lung function at early ages is strongly related to respiratory health at older ages.22,23 The response to treatment would need to be approached as closely as it is commonly done also at older ages. A lung function assessment by means of a simple test could help both to confirm the state of airway patency and to assess the response to treatment.
Longitudinal controlled studies in infants with wheezing disorders using lung-function testing should be performed to evaluate the response to treatment and the outcome of the disease.
Mrs. Stella Papalexiou for database management.
We assessed the lung function of wheezing infants by means of the shape of tidal flow/volume loops (TFVL). A concave TFVL predicted more severe excacerbations in a one-year follow up.