This meta-analysis aims to access the efficacy of nasal saline irrigation in the treatment of allergic rhinitis (AR) in adults and children.
MethodsTwo authors independently searched databases up to December 2018. Differences in efficacy between saline irrigation and other treatments were compared. Subgroup analyses of discrepancy in effects between children and adults were performed.
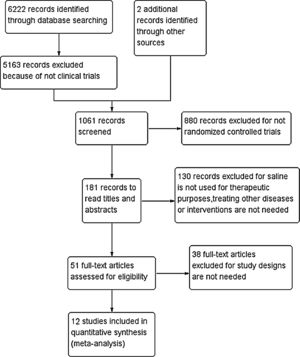
Results(1) Saline irrigation vs. no irrigation, in both children and adults groups, saline irrigation showed significant efficacy. (2) Saline+medication vs. medication, in children group, there was no statistical difference of efficacy between saline+medication and medication; in adults group, efficacy of saline+medicine was superior to that of medication. (3) Saline irrigation vs. medication, in children group, there was no statistical difference between efficacy of saline irrigation and medication; in adults group, efficacy of medication was superior to that of saline irrigation. (4) Hypertonic saline vs. isotonic saline, for children, efficacy of hypertonic saline was superior to that of isotonic saline. Additionally, no adults reported adverse events in all trials. Adverse effects were reported during the first nasal irrigation in 20 children, and one child withdrew due to adverse reactions.
ConclusionsSaline irrigation can significantly improve symptoms of AR in children and adults. Saline irrigation can serve as a safe adjunctive treatment to medication of AR in adults. Saline irrigation can be an alternative therapy for children and pregnant women with AR. Efficacy of hypertonic saline may be better than that of isotonic saline in treating AR of children.
AR is a type I allergic disease of nasal mucosa medicated by IgE (Immunoglobulin E) after exposure to allergens. AR has become a global health issue, mainly manifested as nasal congestion, itching, sneezing, and runny nose. Some patients may be accompanied by uncomfortable symptoms of eyes itching, burning, and so on, seriously affecting patients’ quality of life.1 Nowadays, the primary treatment methods of AR include desensitization, medication, and surgical treatment.2 As a treatment for AR, nasal saline irrigation could wash out the thick mucus, allergens and air contaminant in nasal cavity, increase the hydration of sol layer, enhance mucociliary function, and it has the advantages of safety, convenience and reliability.3,4 However, in the treatment of AR in adults and children, the difference in efficacy of nasal saline irrigation has been less assessed. Whether there are more suitable and individualized treatments to different groups of people has not been discussed. This meta-analysis evaluated the efficacy of nasal saline irrigation in the treatment of AR in adults and children.
Materials and methodsLiterature retrieval: Randomized controlled trials (RCTs) were retrieved in public publications by two evaluators. PubMed, Cochrane Library, EMbase and CNKI (China National Knowledge Infrastructure) were retrieved, and retrieval time was from database establishment until December 2018. At the same time, the references included in the literatures were manually retrieved. All retrieval strategies were determined after multiple pre-searches. If we were unable to obtain enough information from the literature, we would try to obtain them from the authors by letter to maximize the literature included.
Retrieval strategy: allergic rhinitis AND (nasal irrigation OR nasal lavages OR nasal saline OR brine irrigation OR saline rinse).
Inclusion and exclusion criteriaStudies in this meta-analysis had to meet the following inclusion criteria: (1) randomized controlled trials of AR treated by nasal saline irrigation; (2) seasonal and perennial AR patients, regardless of age, gender, ethnicity; (3) nasal saline irrigation as a treatment of AR; (4) Nasal symptom scores: four-symptom score (nasal congestion, itching, sneezing and nasal discharge), eight-symptom score (nasal discharge, itching, sneezing, nasal congestion, tearing, itching of eyes, conjunctival congestion, palpebral edema) and visual analogue scale (VAS) score.5 Exclusion criteria: (1) non-randomized controlled trial; (2) incomplete raw data.
Literature screening and data extractionTwo researchers independently read the literature for screening, and the whole screening process was blind. The title and abstract of the obtained literature was read independently firstly, literature that obviously did not meet the inclusion criteria was removed, and full context reading was conducted for literature that may meet the inclusion criteria, so as to determine whether the document really met the inclusion criteria. Literature that was difficult to determine whether to include during the screening process was discussed or decided by the third researcher. The data was extracted independently by two researchers from trials that met the inclusion criteria, the data extraction form was filled out (Table 1), and the extraction data was cross-checked, the insufficient data was supplemented through reaching out to the corresponding author of the clinical trial.
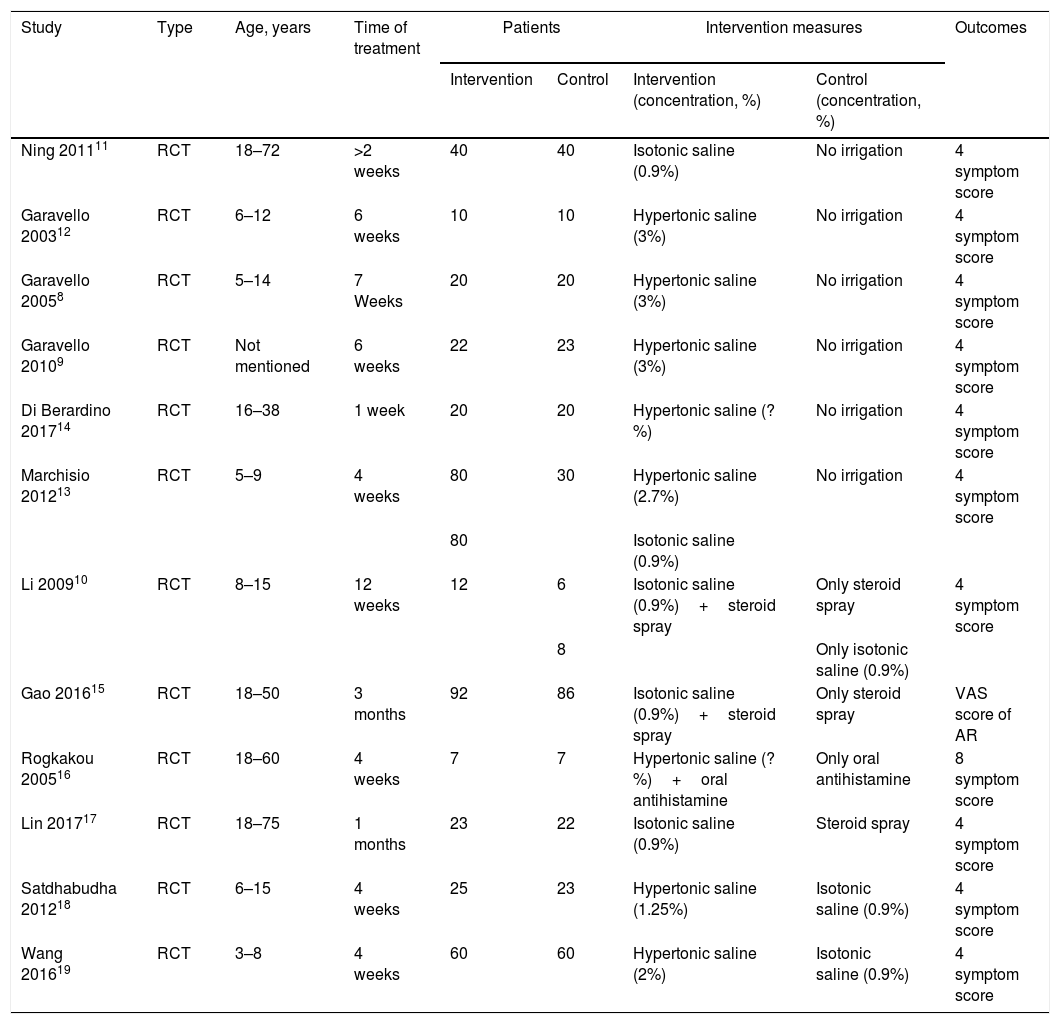
Basic characteristics of included studies.
| Study | Type | Age, years | Time of treatment | Patients | Intervention measures | Outcomes | ||
|---|---|---|---|---|---|---|---|---|
| Intervention | Control | Intervention (concentration, %) | Control (concentration, %) | |||||
| Ning 201111 | RCT | 18–72 | >2 weeks | 40 | 40 | Isotonic saline (0.9%) | No irrigation | 4 symptom score |
| Garavello 200312 | RCT | 6–12 | 6 weeks | 10 | 10 | Hypertonic saline (3%) | No irrigation | 4 symptom score |
| Garavello 20058 | RCT | 5–14 | 7 Weeks | 20 | 20 | Hypertonic saline (3%) | No irrigation | 4 symptom score |
| Garavello 20109 | RCT | Not mentioned | 6 weeks | 22 | 23 | Hypertonic saline (3%) | No irrigation | 4 symptom score |
| Di Berardino 201714 | RCT | 16–38 | 1 week | 20 | 20 | Hypertonic saline (?%) | No irrigation | 4 symptom score |
| Marchisio 201213 | RCT | 5–9 | 4 weeks | 80 | 30 | Hypertonic saline (2.7%) | No irrigation | 4 symptom score |
| 80 | Isotonic saline (0.9%) | |||||||
| Li 200910 | RCT | 8–15 | 12 weeks | 12 | 6 | Isotonic saline (0.9%)+steroid spray | Only steroid spray | 4 symptom score |
| 8 | Only isotonic saline (0.9%) | |||||||
| Gao 201615 | RCT | 18–50 | 3 months | 92 | 86 | Isotonic saline (0.9%)+steroid spray | Only steroid spray | VAS score of AR |
| Rogkakou 200516 | RCT | 18–60 | 4 weeks | 7 | 7 | Hypertonic saline (?%)+oral antihistamine | Only oral antihistamine | 8 symptom score |
| Lin 201717 | RCT | 18–75 | 1 months | 23 | 22 | Isotonic saline (0.9%) | Steroid spray | 4 symptom score |
| Satdhabudha 201218 | RCT | 6–15 | 4 weeks | 25 | 23 | Hypertonic saline (1.25%) | Isotonic saline (0.9%) | 4 symptom score |
| Wang 201619 | RCT | 3–8 | 4 weeks | 60 | 60 | Hypertonic saline (2%) | Isotonic saline (0.9%) | 4 symptom score |
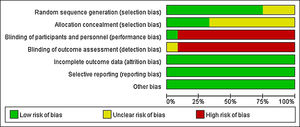
The quality of included RCTs was independently evaluated by two reviewers in accordance with the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. The inconsistencies were agreed upon through the third evaluator's intervention and discussion. The following aspects were evaluated respectively: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias. “Low risk” indicated low bias risk, “High risk” meant high bias risk, “Unclear risk” showed the literature did not offer sufficient or certain information to bias assessment.
Statistical methodsThe included literature was analyzed by RevMan 5.3 software from Cochrane Collaboration, the weighted mean difference (WMD) or standardized mean difference (SMD) were used to measure the data, and both were expressed as 95% confidence intervals (95% CI). The heterogeneity between the studies was evaluated, if P≥0.1, I2≤50%, indicating that the possibility of heterogeneity among studies is small, the fixed effect model shall be used; if P<0.1, I2>50%, showing that there is heterogeneity between studies, and the random effect model should be applied for analysis.
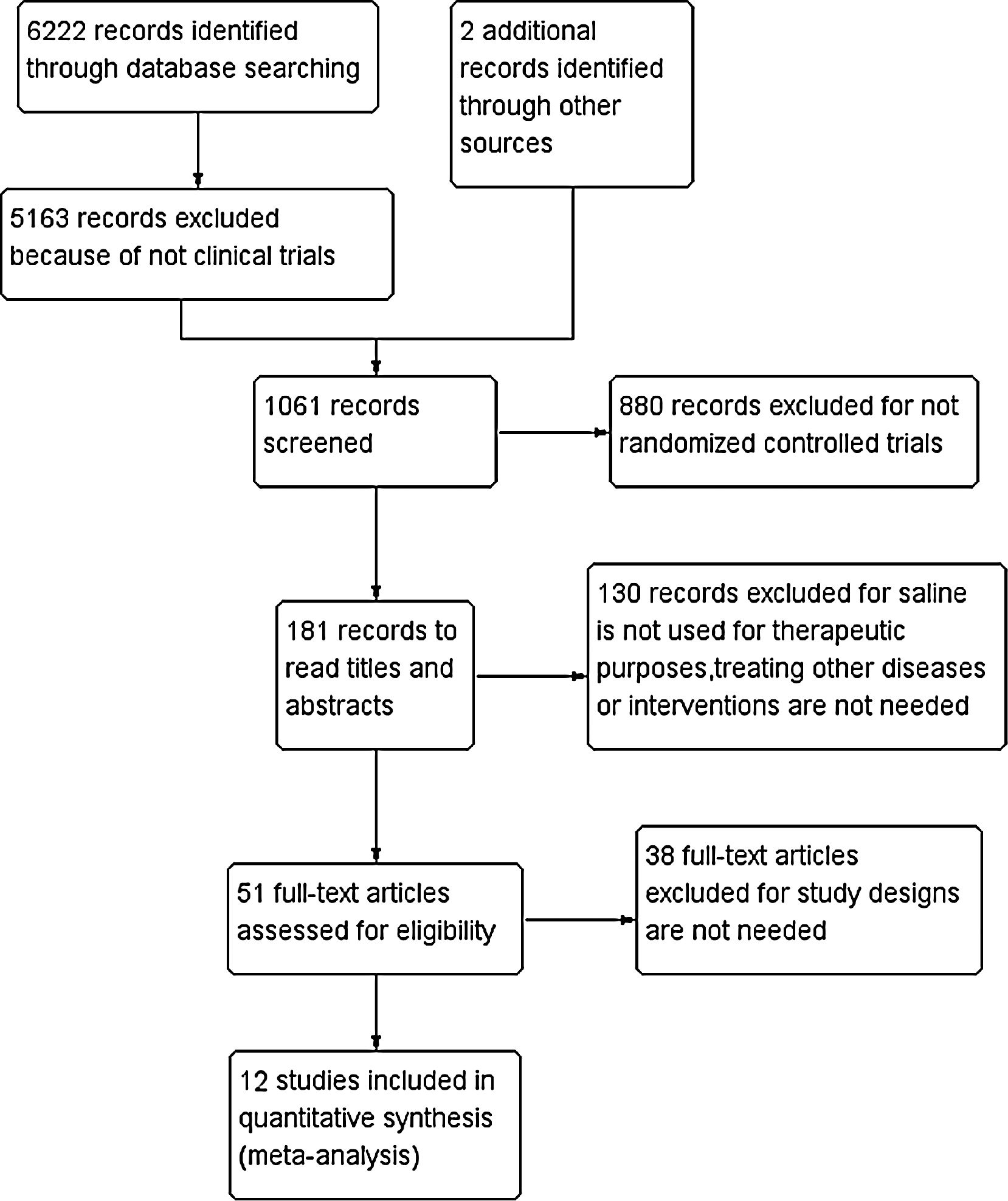
ResultsThe included literature and its basic characteristicsIn the preliminary retrieval, a total of 6222 references were obtained from Chinese and English electronic databases, as well as two references from other sources. With stepwise selection of inclusion, exclusion criteria, and research needs, 12 articles totaling 819 patients were included in the study (Fig. 1). There were 10 English literatures, two in Chinese, six for adults (≥16 years old, a total of 342 cases), and six for children (≤15 years old, 477 cases). The basic characteristics of the included studies are shown in Table 1.
Adverse effectsStudies have shown that nasal saline irrigation rarely produces adverse effects, such as local burning, tearing, epistaxis, earache, headache, etc.6,7 Adverse effects have not been well reported in these studies, and the quality of evidence is low. Three studies only reported no adverse effects in the saline group, but did not describe the types of adverse effects (Garavello 2005,8 40 children; Garavello 2010,9 45 adults; li 2009,10 26 children). The other three studies reported no adverse effects in both active groups and control groups, but did not describe the specific types of adverse effects (Ning 2011,11 20 adults; Garavello 2003,12 20 children; Marchisio 2012,13 190 children). None of the three studies mentioned adverse effects associated with nasal saline irrigation (Di Berardino 2017,14 40 adults; Gao 2016,15 178 adults; Rogkakou 2005,16 14 adults). One study showed that no adverse events were reported in the nasal irrigation group, and adverse events in the steroids group was 27.3%, with the main symptom being sore throat (Lin 2017,17 45 adults). One study reported nasal irritation and burning in 35% (17 children) of patients after the first nasal irrigation, but the frequency of this complaint was decreased in the second and third visits, and one participant (2%) withdrew due to adverse effects without reasons being described in detail (Satdhabudha 2012,18 48 children). One study indicated transient and mild ear pain, headache, or epistaxis in three children with only the first irrigation, and that none of these symptoms reappeared in subsequent treatment (Wang 2016,19 120 children). Spray was used to irrigate in three studies (Ning 2011,11 Garavello 2005,8 Di Berardino 201714). Disposable syringe was used in three studies (Garavello 2003,12 Garavello 2010,9 Satdhabudha 201218). Positive-pressure nasal irrigation applicator was used in two studies (li 2009,10 Gao 201615). Bulb syringe was used in one study (Marchisio 201213). Infusion apparatus was used in one study (Lin 201717). Two studies did not describe how to irrigate (Rogkakou 2005,16 Wang 201619).
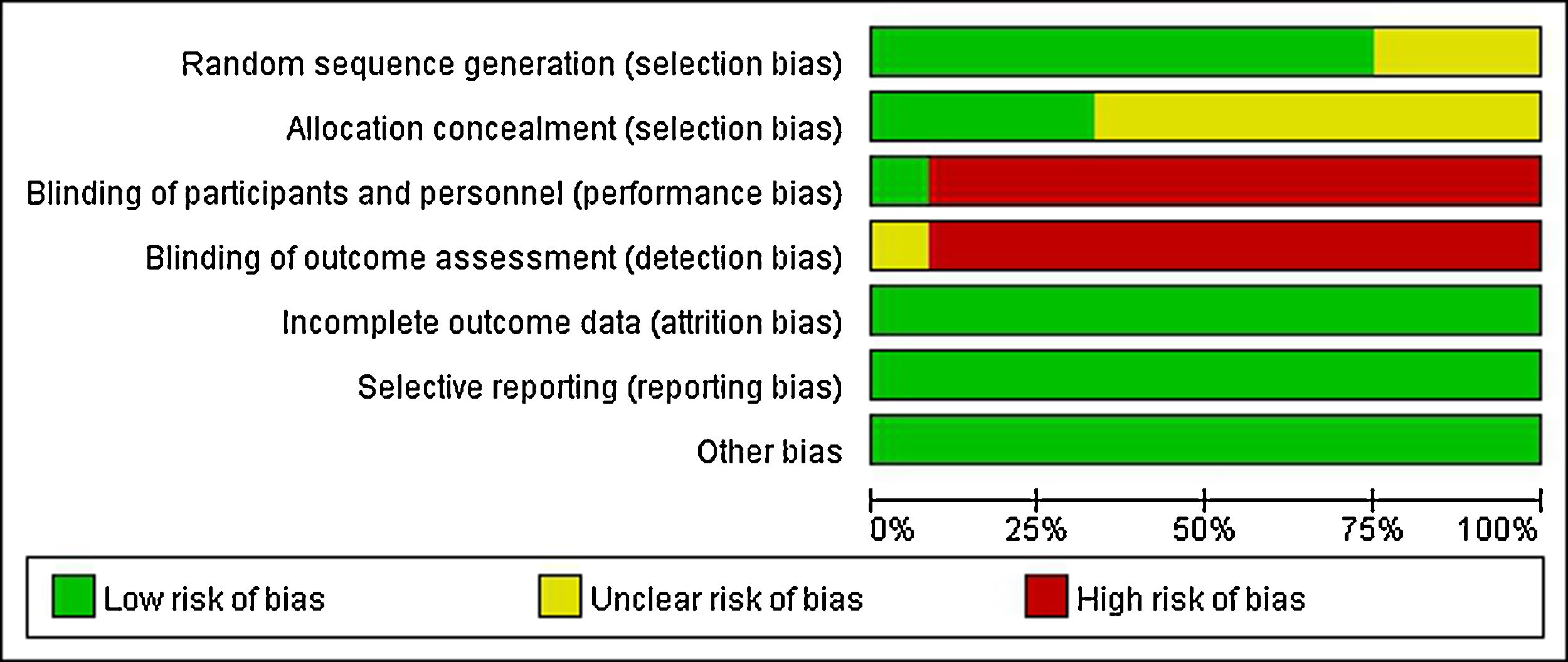
Quality evaluation for included literaturesA clear and appropriate random grouping method was applied in nine studies, and three studies referred random allocation, while there was no elaboration on the random allocation approach. Only four studies adopted an allocation sequence method that clinician and subjects could not foresee, while the remaining eight studies had no explanation of allocation concealment. As the intervention was therapeutic, almost no participants or therapist were double blind or single blind, there was only one study showing that “Patients and the investigator were blinded to solution allocation”. As almost all researchers knew exactly which treatment and control groups were involved, therefore, the blind method of outcome evaluation could not be achieved. All studies were able to ensure the integrity of result data. There were no obvious selective reports and other bias in all studies. In general, the level of evidence was relatively low (Fig. 2).
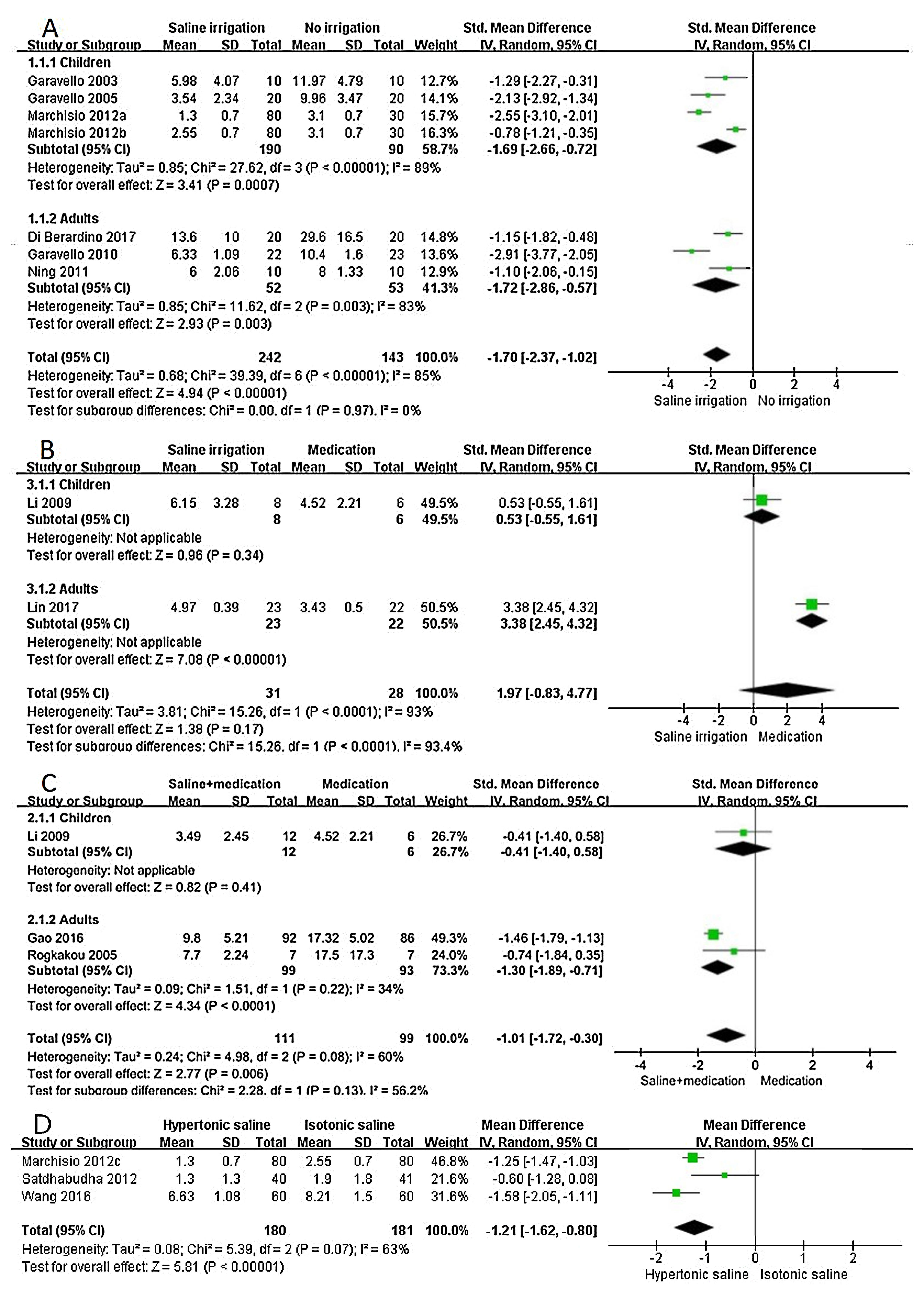
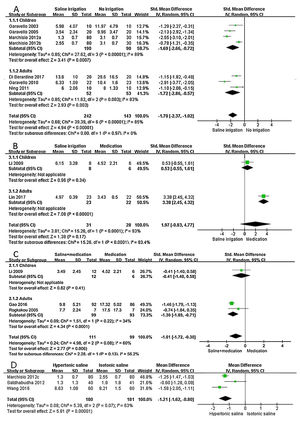
Saline irrigation versus no irrigationIn children group, there was heterogeneity among the included studies (P<0.1, I2=89%), the difference of symptom score was statistically significant between saline irrigation and no irrigation (SMD=−1.69, 95%CI −2.66 to −0.72), indicating that the efficacy of saline irrigation was superior to that of no saline irrigation. In the adults group, there was heterogeneity among the included studies (P<0.1, I2=83%), the difference of symptom score was statistically significant between saline irrigation and no irrigation (SMD=−1.72, 95%CI −2.86 to −0.57), suggesting that efficacy of saline irrigation was better than that of no irrigation (Fig. 3A).
Forest plot of subgroup analyses using the random-effects model. (A) Saline irrigation vs. no irrigation (a: Hypertonic saline vs. No irrigation; b: Isotonic saline vs. No irrigation); (B) Saline irrigation vs. medication; (C) saline+medication vs. medication; (D) hypertonic saline vs. isotonic saline (c: Hypertonic saline vs. Isotonic saline).
In the children group, the difference of symptom score was not statistically significant in treatment of saline irrigation and medication (SMD=0.53, 95%CI −0.55 to 1.61), indicating that there was no statistical difference between therapies of saline irrigation and medication. In the adults group, there was statistical difference between treatment of saline irrigation and medication (SMD=3.38, 95%CI 2.45 to 4.32), suggesting the efficacy of medication therapy was superior to that of saline irrigation (Fig. 3B).
Saline+medication versus medication (steroid nasal spray or oral antihistamine)In the children group, the difference of symptom score was not statistically significant in the treatment of saline+medication and only medication (SMD=-0.41, 95%CI −1.40 to 0.58), indicating that there was no statistical difference between therapies of saline+medication and only medication. In the adults group, there was statistical difference between the treatment of saline+medication and only medication (SMD=−1.30, 95%CI −1.89 to −0.71), suggesting that the efficacy of saline+medication therapy was superior to that of only the medication (Fig. 3C).
Hypertonic saline versus isotonic salineThere was heterogeneity among the three studies (P<0.1, I2=63%). There was statistical difference between treatment of hypertonic saline and isotonic saline irrigation (MD=−1.21, 95%CI −1.62 to −0.80), suggesting that the efficacy of hypertonic saline was superior to that of isotonic saline (Fig. 3D).
DiscussionSaline irrigation versus no irrigationThis study revealed that nasal saline irrigation can significantly improve the local symptoms of AR in adults and children. Its possible mechanism is that nasal saline irrigation has the function of mechanical scouring and clearing, which is conducive to discharge nasal secretions, and reduces the concentration of local inflammatory factors in nasal cavity, alleviates the congestion and edema of the nasal mucosa, and improves the function of mucociliary oscillation in nasal cavity.20,21
Saline irrigation versus medication (steroid nasal spray)There was no statistical difference between efficacy of nasal saline irrigation and steroid spray in the children group, while the efficacy of medication was superior to that of saline irrigation in the adults group. This may be related to young children have greater difficulty in the application of nasal irrigation and nasal spray than adults, which leads to inadequate treatment. Furthermore, there is only one study in each group, totaling 59 patients, so adding that the research scale is small, the conclusion is not convincing enough. The efficacy difference between brine irrigation and steroid nasal spray, the difference in adult and children groups, need to be further explored.
Saline+medication versus medication (steroid nasal spray or oral antihistamine)In the children group, there was no significant difference in the efficacy of saline+medication therapy and medication therapy, while for the adults group, the efficacy of saline+medication was superior to that of medication alone. The results suggest that saline irrigation can enhance the efficacy of adults AR as an adjunct to medication. After the comparison of simply steroid nasal spray and steroid spray combined with normal saline irrigation in the treatment of AR patients, Nguyen et al.22 found that the combination therapy significantly improved the adults patients’ quality of life and nasal ventilation volume. The study suggested that nasal saline irrigation has the effect of clearing secretions in nasal cavity, then steroid hormone spray can be directly sprinkled on the nasal mucosa, completely absorbed without being blocked or diluted by the secretions; the spray could form a higher drug concentration on lesion mucosa, and act more directly on the lesion target cells, which further allow the steroid hormone to play a powerful anti-inflammation and detumescence role in the nasal cavity.22 Compared with only medication, saline+medication showed no further improvement in the symptoms of AR children. In the study of Li 2009,10 although the local symptoms of AR patients were significantly improved after the treatment of saline+medication and medication only, there was no statistical difference in the efficacy of the two groups. This may relate to the narrower nasal cavity in children or poor irrigation skills, causing the irrigation of the nasal cavity to not be as sufficient as adults.23 In addition, as the sample size of the children group and their weights were small, the evidence that supported the conclusion was insufficient and studies of larger samples were required to conduct the demonstration.
Hypertonic saline versus isotonic salineThree studies on children suggested that hypertonic saline may be superior to isotonic saline in improving local symptoms of AR. However, there was heterogeneity between the three studies, so we need to be cautious about the outcome. Marchisio 2012 believed that hypertonic saline can not only relieve the nasal symptoms, but also has good therapeutic effect on some complications of AR (such as otitis media, gland enlargement).13 Furthermore, Robinson's study has shown that hyperosmotic solution significantly increased the mucociliary clearance, while physiological saline had no such effect.24 However, it is noteworthy that the concentration of hypertonic saline should not exceed 3%, since a higher concentration may cause some severe mucosal irritation and other adverse reactions.25,26
The therapeutic medicine of allergic rhinitis includes nasal steroid hormone, antihistamine, antileukotriene, which bring certain therapeutic effects. However, these medicines may have stimulative effects and adverse reactions, with a relatively expensive price, and the efficacy is declined after long-term usage. Although nasal glucocorticoid therapy has been widely recognized by people, many patients still have fear and resistance to steroid hormone, especially for children, pregnant and lactating women.
In these studies, few adverse effects associated with nasal saline irrigation have been reported. No adults were reported to have had adverse reactions during saline irrigation in all the included studies. Adverse reactions were reported only in the study of saline irrigation for the treatment of AR in children. Almost all of the adverse effects occurred just at the first nasal rinse, and only one child in all the studies withdrew due to adverse events. So nasal saline irrigation can be considered as a recommendable treatment for children AR. Nasal saline irrigation may be more easily accepted by children's parents, because that helps to dispel parents’ concerns about using steroid hormone in children. Cases with adverse reactions were operated with disposable syringes (Satdhabudha 2012,18 48 children). This may be due to the difficulty of adjusting the pressure of disposable syringes or the difficulty for children to master the use of syringes. However, no adverse reactions were reported in another study using disposable syringes (Garavello 2003,12 20 children). This may be related to the small sample size, and suitable irrigation method for children needs further study. Moreover, no adverse events were reported in 22 pregnant women who underwent nasal hypertonic saline lavage in the study of Garavello 2010.9 In 2016, the expert panel from the United States recommended that nasal saline irrigation can be served as a suitable maintenance treatment of nasosinusitis during pregnancy, which also supports the good safety of nasal saline irrigation.27 Hermelingmeier et al. reviewed the allergic rhinitis cases treated by nasal irrigation with isotonic saline from 1994 to 2010.28 Symptoms of all patients were improved, and nasal irrigation was able to reduce the dosage and side effects of glucocorticoid, accelerate nasal mucociliary clearance and enhance the quality of life, so isotonic saline irrigation was recommended as a complementary therapy of AR.
ConclusionIn summary, saline irrigation can significantly improve local symptoms of AR in children and adults. For adults with AR, steroid nasal spray alone is more effective than saline lavage, and the efficacy of saline+medication is superior to that of only medication, so we suggest nasal saline irrigation as an adjuvant therapy to medication treatment for adult AR. In addition, saline irrigation can be recommended as a safe and effective alternative treatment for children and pregnant women. Additionally, hypertonic saline may be more effective in improving AR symptoms than isotonic saline for children. This study supports the standpoint that nasal saline irrigation is a safe, effective and well-tolerated method in adjuvant treatment of AR. However, the heterogeneity of the included trials in this study was large, the bias risk was relatively high, and the level of evidence is low. Therefore, it is necessary to be cautious about the conclusions. Scientifically designed, randomized controlled trials and blind method should be carried out in future clinical research, and multi-center large sample studies should be conducted if necessary.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
FundingThis work was supported by the National Science Foundation of China (grant no. 8187040043), Western Medicine Guide Project of Shanghai City (grant no. 17411970500), Shenkang Medical Development Center Clinical Science and Technology Innovation Project of Shanghai City (grant no. SHDC 12019X07), and Health Commission Advanced Technology Promotion Project of Shanghai City (grant no. 2019SY071).
Conflict of interestThe authors have no conflict of interest to declare.