The first thousand days of life are a critical stage for the development of respiratory and immune systems. Many events in this period may be associated with wheezing in childhood. Objective: This study aimed to investigate the association between early life determinants and wheezing in children aged 6–7 years.
Materials and MethodsPopulation-based case-control study using early-life related questions. We used the International Study of Asthma and Allergies in Childhood questionnaire to assess wheezing symptoms. Multiple logistic regressions were performed according to a hierarchical framework, considering the complex dynamic of wheezing/asthma and potential interaction between different levels of determination.
ResultsA total of 820 children were included, from which 162 reported wheezing symptoms (19.7%). Multivariable analysis identified socioeconomic conditions (OR 2.08, 95% CI 1.08–4.00), family history of asthma (OR 2.28, 95% CI 1.37–3.75), vaginal discharge that required treatment during pregnancy (OR 1.68, 95% CI 1.00–2.83), neonatal hyperbilirubinemia (OR 2.00, 95% CI 1.17–3.42), anemia and intestinal parasitosis in the first two years (OR 2.28, 95% CI 1.22–4.25; OR 1.72, 95% CI 1.02–2.92, respectively) independently associated to wheezing at 6–7 years. Intended pregnancy was associated with reduced wheezing (OR 0.47, 95% CI 0.28–0.77).
ConclusionsSeveral factors were associated with wheezing in childhood. Considering that intended pregnancy reduced wheezing and other associated exposures are considered modifiable, these findings may guide the planning of strategies to decrease the susceptibility to asthma symptoms in childhood.
The first thousand days of life of a child, the period from conception to the end of the second year, has become a core focus to understand the developmental programming of disease predisposition in early life.1 This period is considered as a critical stage because both the respiratory and immune systems are immature at birth and have a prolonged period of postnatal maturation.2,3 In this “window of susceptibility”, some environment exposures may disturb lung growth and delay immune system maturation, increasing the susceptibility to asthma symptoms in childhood.4
Asthma arises in a context of complex interactions between genetic factors, development of the fetal immune system and environmental exposures occurring early in life.5 However, as multiple exposures can influence the development of asthma, a comprehensive analysis of these exposures is required. Wheeze is one of the cardinal manifestations of asthma, and there is a relationship between wheeze and persistent asthma.6 Hence, this study aims to investigate the association between early life determinants and wheezing in children aged 6–7 years from Southern Brazil, according to a predefined hierarchical conceptual framework.
MethodsStudy design and participantsThis is a population-based case-control study. Data were collected between September 2015 and October 2017, in Palhoça – Southern Brazil. The study population consisted of all children born in 2009 and their families, regularly enrolled in the first year of elementary public and private schools in the city. A preliminary survey was conducted in 37 public and 19 private primary schools of Palhoça to locate the study population. The selection of the sample was carried out by means of probabilistic sampling, by conglomerate, according to the geographical distribution of schools in the city.
The following were excluded from the study: (i) children who studied in Palhoça but did not live in the same city; (ii) children whose parents were unable to answer the interview because they did not speak the official language of the country. This study was approved by the Research Ethics Committee of [name of university] (CAAE: 38240114.0.0000.5369). The protocol has been performed in accordance with the Declaration of Helsinki. All parents signed a consent form to indicate their consent to participate as well as their consent for their child’s participation.
Data collectionThe questionnaire, which required about 35 min to be completed, investigated the presence of allergic symptoms in children and the occurrence of specific events during pregnancy, at birth, and during the first two years of life. Trained community health agents administered the questionnaire with the children’s mothers at the family’s home. Cases (children with symptoms of wheezing) were screened by the questions from the International Study of Asthma and Allergies in Childhood (ISAAC). Current wheezing was defined as a positive answer to “Has your child ever had wheezing or whistling in the chest at any time in the past?” and “Has your child had wheezing or whistling in the chest in the past 12 months?”. Thus, children whose parents reported positive answers for both questions were considered as cases. All children who did not meet this condition were considered controls. Severe symptoms of wheezing were defined as ≥4 attacks of wheeze or ≥1 night per week sleep disturbance from wheeze or wheeze affecting speech in the past 12 months.7
Information about children’s sex and ethnicity, older siblings, childcare attendance, number of residents at household, parental education, receiving the benefit from Bolsa Família Program (Brazilian government program that directly transfers income to households living in poverty and extreme poverty), as well family history of asthma, rhinitis symptoms (defined as positive answers to questions “Has your child ever had a problem with sneezing, or a runny, or blocked nose when he/she did not have a cold or the flu?” and “In the past 12 months, has your child had a problem with sneezing, or a runny, or blocked nose when he/she did not have a cold or the flu?”) and eczema symptoms (defined as positive answer to questions “Has your child ever had an itchy rash which was coming and going for at least six months?” and “Has your child had this itchy rash at any time in the last 12 months?”) were extracted from the questionnaire. The following prenatal and birth characteristics were obtained from the questionnaire: (i) mother’s height and ethnicity, (ii) presence of maternal urogynecological infections during pregnancy, (iii) intended pregnancy, (iv) mother’s age at child’s birth, (v) maternal weight gain during pregnancy, (vi) tobacco smoke during pregnancy, (vii) illicit drug and/or alcohol use during pregnancy, (viii) pet ownership, (ix) doctor visits, (x) gestational age, (xi) mode of delivery, (xii) birth weight, (xiii) APGAR scores in the first and fifth minutes (registered in Brazilian Child Health Handbook), (xiv) neonatal hyperbilirubinemia, (xv) neonatal hospitalization, (xvi) neonatal respiratory infections, and (xvii) breastfeeding. We assessed the presence of the following diseases during the first two years of life: (i) chickenpox, (ii) anemia, (iii) lower respiratory tract infections, (iv) gastroesophageal reflux, (v) intestinal parasitosis, (vi) otitis media, tonsillitis, (vii) antibiotics consumption, and (viii) vaccination.
Sample sizeThe sample size was calculated in OpenEpi version 3 calculator, using 95% confidence level, power of 80%, 1:4 ratio (case: control), and family history of asthma as the exposure variable. Thus, considering 20% as a percentage of exposed controls, and estimating OR at 1.8, the estimated sample size was 140 cases for 557 controls.
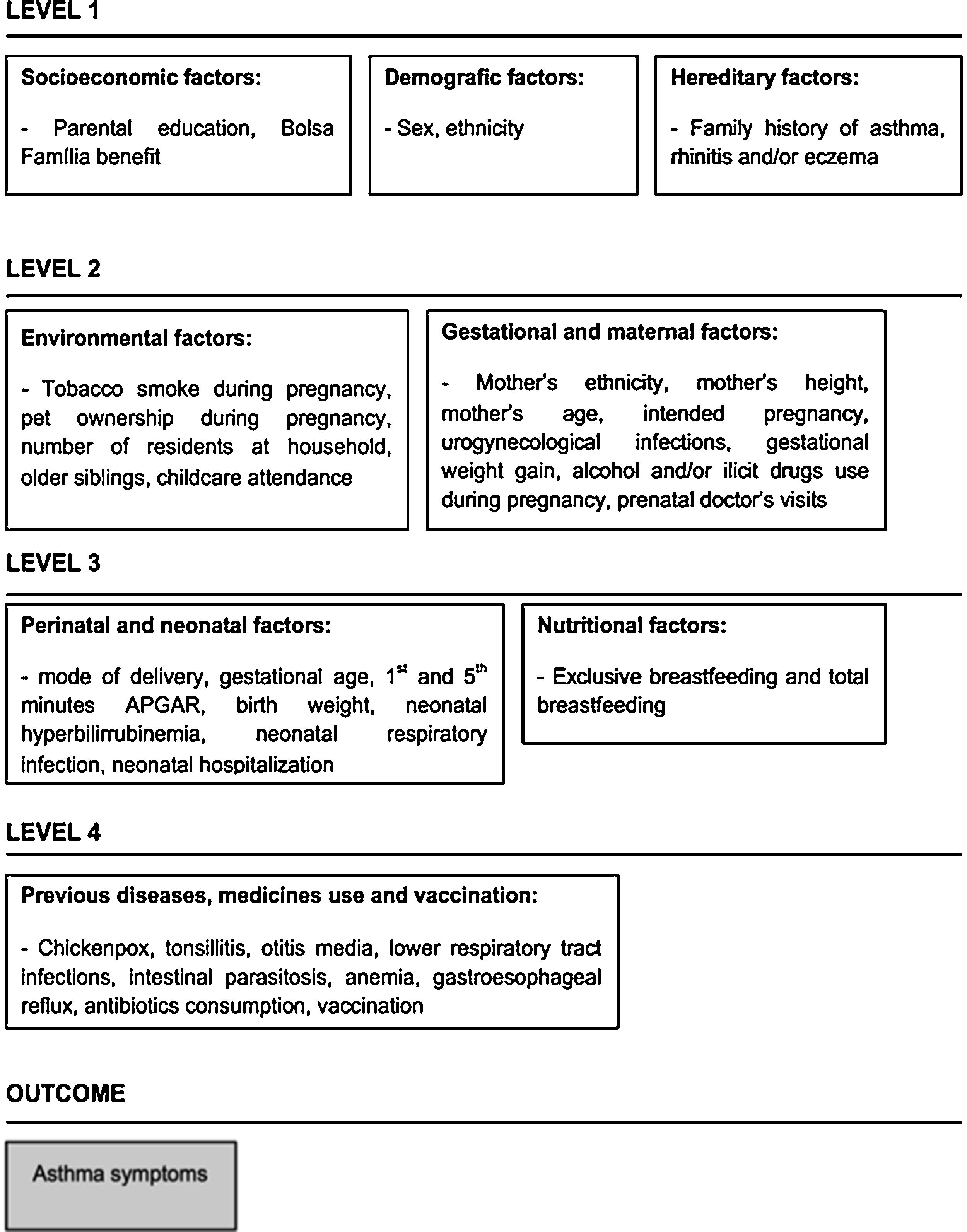
Statistical analysisBivariate logistic regression analysis (unadjusted analysis) was used to assess the association between each variable and groups with wheezing (cases) and without wheezing (controls). Variables were further analyzed by multiple logistic regressions. Hierarchical framework was built considering the complex dynamic of asthma symptoms and the potential interaction between different levels of determination (Fig. 1). Variables in the theoretical framework were organized into four levels. At the distal level, we included demographic and socioeconomic variables, as well as family history of allergic diseases. The environmental variables were placed in the intermediate levels. Health-related variables were located in the proximate level. The multiple logistic regressions included the variables with p value ≤0.20 in the unadjusted analysis. In each level of the multivariate analysis, the variables that remained associated with p ≤ 0.20 moved to the next stage. In the final model, variables that remained associated with outcomes were adjusted for a personal history of symptoms of rhinitis and/or eczema. We used the Hosmer-Lemshow test to assess the model’s goodness-of-fit. The level of significance adopted was p < 0.05. All analyses were performed using Stata® version 15.0 (Stata Corporation, College Station, TX, USA).
ResultsBetween September 2015 and October 2017, we identified 1437 eligible children; 820 children of whom lived and studied in Palhoça and were thus included in this study. Of these, 84.6% (n = 694) were enrolled in public schools and 51.3% (n = 420) were males. Regarding socioeconomic status, 12.9% of the sample received Bolsa Família. In this sample, 162 children (19.7%) had asthma symptoms and were classified as “cases”. Regarding the severity of wheezing, 112 children (69.1%) had severe symptoms, and approximately 28% of the children had parents, mothers and / or siblings with asthma.
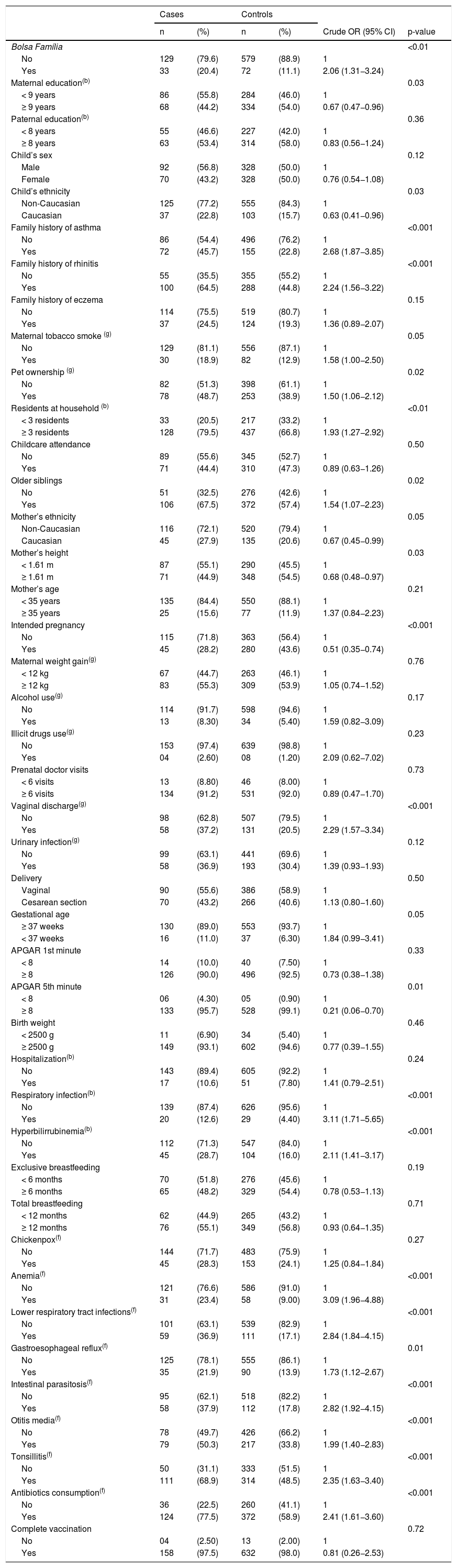
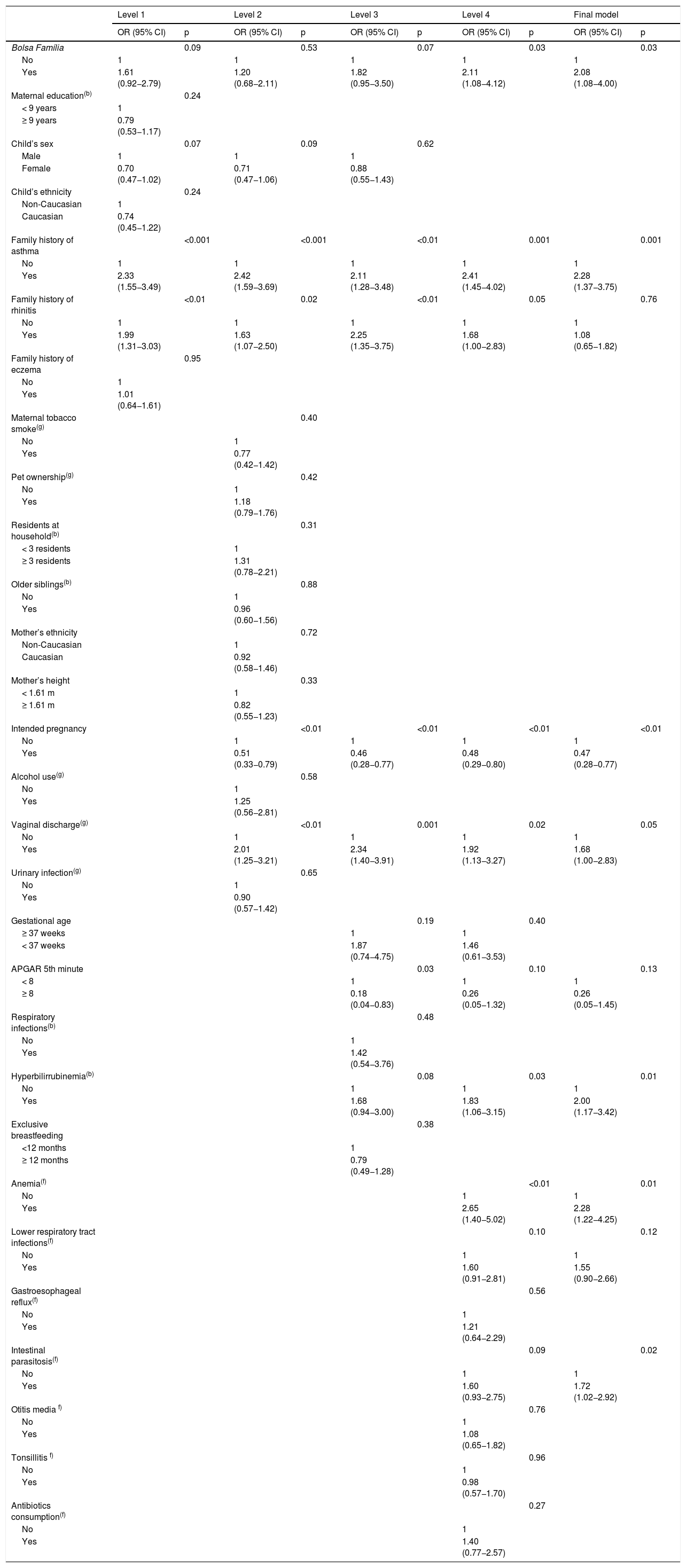
Table 1 shows the association of each independent variable with case and control groups. In the multiple logistic regressions according to the hierarchical theoretical model, most of the associations found in the bivariate analysis lost statistical significance. Although Bolsa Família presented p-value ≥0.20 in the second level, it was maintained in the subsequent levels because that variable was considered a proxy for the socioeconomic status and has an effect on the variables positioned distally in the conceptual framework. In the final model, after adjustment for symptoms of rhinitis and eczema at 6–7 years, the odd ratios of wheezing increased with worse socioeconomic conditions (OR = 2.08, 95% CI 1.08–4.00), family history of asthma (OR = 2.28, 95% CI 1.37–3.75), vaginal discharge which required treatment during pregnancy (OR = 1.68, 95% CI 1.00–2.83), and neonatal hyperbilirubinemia (OR = 2.00, 95% CI 1.17–3.42). Similarly, during the first two years of life, children who had anemia were 2.28 times more likely to have wheezing (95% CI 1.22–4.25) and those who had intestinal parasitosis had a 1.72-fold increase (95% CI 1.02–2.92). In contrast, having an intended pregnancy reduced the chance of wheezing at 6–7 years by 53% (Table 2).
Associations between wheezing at ages 6–7 and early life exposures, demographics and family history of allergic diseases.
| Cases | Controls | |||||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | Crude OR (95% CI) | p-value | |
| Bolsa Família | <0.01 | |||||
| No | 129 | (79.6) | 579 | (88.9) | 1 | |
| Yes | 33 | (20.4) | 72 | (11.1) | 2.06 (1.31−3.24) | |
| Maternal education(b) | 0.03 | |||||
| < 9 years | 86 | (55.8) | 284 | (46.0) | 1 | |
| ≥ 9 years | 68 | (44.2) | 334 | (54.0) | 0.67 (0.47−0.96) | |
| Paternal education(b) | 0.36 | |||||
| < 8 years | 55 | (46.6) | 227 | (42.0) | 1 | |
| ≥ 8 years | 63 | (53.4) | 314 | (58.0) | 0.83 (0.56−1.24) | |
| Child’s sex | 0.12 | |||||
| Male | 92 | (56.8) | 328 | (50.0) | 1 | |
| Female | 70 | (43.2) | 328 | (50.0) | 0.76 (0.54−1.08) | |
| Child’s ethnicity | 0.03 | |||||
| Non-Caucasian | 125 | (77.2) | 555 | (84.3) | 1 | |
| Caucasian | 37 | (22.8) | 103 | (15.7) | 0.63 (0.41−0.96) | |
| Family history of asthma | <0.001 | |||||
| No | 86 | (54.4) | 496 | (76.2) | 1 | |
| Yes | 72 | (45.7) | 155 | (22.8) | 2.68 (1.87−3.85) | |
| Family history of rhinitis | <0.001 | |||||
| No | 55 | (35.5) | 355 | (55.2) | 1 | |
| Yes | 100 | (64.5) | 288 | (44.8) | 2.24 (1.56−3.22) | |
| Family history of eczema | 0.15 | |||||
| No | 114 | (75.5) | 519 | (80.7) | 1 | |
| Yes | 37 | (24.5) | 124 | (19.3) | 1.36 (0.89−2.07) | |
| Maternal tobacco smoke (g) | 0.05 | |||||
| No | 129 | (81.1) | 556 | (87.1) | 1 | |
| Yes | 30 | (18.9) | 82 | (12.9) | 1.58 (1.00−2.50) | |
| Pet ownership (g) | 0.02 | |||||
| No | 82 | (51.3) | 398 | (61.1) | 1 | |
| Yes | 78 | (48.7) | 253 | (38.9) | 1.50 (1.06−2.12) | |
| Residents at household (b) | <0.01 | |||||
| < 3 residents | 33 | (20.5) | 217 | (33.2) | 1 | |
| ≥ 3 residents | 128 | (79.5) | 437 | (66.8) | 1.93 (1.27−2.92) | |
| Childcare attendance | 0.50 | |||||
| No | 89 | (55.6) | 345 | (52.7) | 1 | |
| Yes | 71 | (44.4) | 310 | (47.3) | 0.89 (0.63−1.26) | |
| Older siblings | 0.02 | |||||
| No | 51 | (32.5) | 276 | (42.6) | 1 | |
| Yes | 106 | (67.5) | 372 | (57.4) | 1.54 (1.07−2.23) | |
| Mother’s ethnicity | 0.05 | |||||
| Non-Caucasian | 116 | (72.1) | 520 | (79.4) | 1 | |
| Caucasian | 45 | (27.9) | 135 | (20.6) | 0.67 (0.45−0.99) | |
| Mother’s height | 0.03 | |||||
| < 1.61 m | 87 | (55.1) | 290 | (45.5) | 1 | |
| ≥ 1.61 m | 71 | (44.9) | 348 | (54.5) | 0.68 (0.48−0.97) | |
| Mother’s age | 0.21 | |||||
| < 35 years | 135 | (84.4) | 550 | (88.1) | 1 | |
| ≥ 35 years | 25 | (15.6) | 77 | (11.9) | 1.37 (0.84−2.23) | |
| Intended pregnancy | <0.001 | |||||
| No | 115 | (71.8) | 363 | (56.4) | 1 | |
| Yes | 45 | (28.2) | 280 | (43.6) | 0.51 (0.35−0.74) | |
| Maternal weight gain(g) | 0.76 | |||||
| < 12 kg | 67 | (44.7) | 263 | (46.1) | 1 | |
| ≥ 12 kg | 83 | (55.3) | 309 | (53.9) | 1.05 (0.74−1.52) | |
| Alcohol use(g) | 0.17 | |||||
| No | 114 | (91.7) | 598 | (94.6) | 1 | |
| Yes | 13 | (8.30) | 34 | (5.40) | 1.59 (0.82−3.09) | |
| Illicit drugs use(g) | 0.23 | |||||
| No | 153 | (97.4) | 639 | (98.8) | 1 | |
| Yes | 04 | (2.60) | 08 | (1.20) | 2.09 (0.62−7.02) | |
| Prenatal doctor visits | 0.73 | |||||
| < 6 visits | 13 | (8.80) | 46 | (8.00) | 1 | |
| ≥ 6 visits | 134 | (91.2) | 531 | (92.0) | 0.89 (0.47−1.70) | |
| Vaginal discharge(g) | <0.001 | |||||
| No | 98 | (62.8) | 507 | (79.5) | 1 | |
| Yes | 58 | (37.2) | 131 | (20.5) | 2.29 (1.57−3.34) | |
| Urinary infection(g) | 0.12 | |||||
| No | 99 | (63.1) | 441 | (69.6) | 1 | |
| Yes | 58 | (36.9) | 193 | (30.4) | 1.39 (0.93−1.93) | |
| Delivery | 0.50 | |||||
| Vaginal | 90 | (55.6) | 386 | (58.9) | 1 | |
| Cesarean section | 70 | (43.2) | 266 | (40.6) | 1.13 (0.80−1.60) | |
| Gestational age | 0.05 | |||||
| ≥ 37 weeks | 130 | (89.0) | 553 | (93.7) | 1 | |
| < 37 weeks | 16 | (11.0) | 37 | (6.30) | 1.84 (0.99−3.41) | |
| APGAR 1st minute | 0.33 | |||||
| < 8 | 14 | (10.0) | 40 | (7.50) | 1 | |
| ≥ 8 | 126 | (90.0) | 496 | (92.5) | 0.73 (0.38−1.38) | |
| APGAR 5th minute | 0.01 | |||||
| < 8 | 06 | (4.30) | 05 | (0.90) | 1 | |
| ≥ 8 | 133 | (95.7) | 528 | (99.1) | 0.21 (0.06−0.70) | |
| Birth weight | 0.46 | |||||
| < 2500 g | 11 | (6.90) | 34 | (5.40) | 1 | |
| ≥ 2500 g | 149 | (93.1) | 602 | (94.6) | 0.77 (0.39−1.55) | |
| Hospitalization(b) | 0.24 | |||||
| No | 143 | (89.4) | 605 | (92.2) | 1 | |
| Yes | 17 | (10.6) | 51 | (7.80) | 1.41 (0.79−2.51) | |
| Respiratory infection(b) | <0.001 | |||||
| No | 139 | (87.4) | 626 | (95.6) | 1 | |
| Yes | 20 | (12.6) | 29 | (4.40) | 3.11 (1.71−5.65) | |
| Hyperbilirrubinemia(b) | <0.001 | |||||
| No | 112 | (71.3) | 547 | (84.0) | 1 | |
| Yes | 45 | (28.7) | 104 | (16.0) | 2.11 (1.41−3.17) | |
| Exclusive breastfeeding | 0.19 | |||||
| < 6 months | 70 | (51.8) | 276 | (45.6) | 1 | |
| ≥ 6 months | 65 | (48.2) | 329 | (54.4) | 0.78 (0.53−1.13) | |
| Total breastfeeding | 0.71 | |||||
| < 12 months | 62 | (44.9) | 265 | (43.2) | 1 | |
| ≥ 12 months | 76 | (55.1) | 349 | (56.8) | 0.93 (0.64−1.35) | |
| Chickenpox(f) | 0.27 | |||||
| No | 144 | (71.7) | 483 | (75.9) | 1 | |
| Yes | 45 | (28.3) | 153 | (24.1) | 1.25 (0.84−1.84) | |
| Anemia(f) | <0.001 | |||||
| No | 121 | (76.6) | 586 | (91.0) | 1 | |
| Yes | 31 | (23.4) | 58 | (9.00) | 3.09 (1.96−4.88) | |
| Lower respiratory tract infections(f) | <0.001 | |||||
| No | 101 | (63.1) | 539 | (82.9) | 1 | |
| Yes | 59 | (36.9) | 111 | (17.1) | 2.84 (1.84−4.15) | |
| Gastroesophageal reflux(f) | 0.01 | |||||
| No | 125 | (78.1) | 555 | (86.1) | 1 | |
| Yes | 35 | (21.9) | 90 | (13.9) | 1.73 (1.12−2.67) | |
| Intestinal parasitosis(f) | <0.001 | |||||
| No | 95 | (62.1) | 518 | (82.2) | 1 | |
| Yes | 58 | (37.9) | 112 | (17.8) | 2.82 (1.92−4.15) | |
| Otitis media(f) | <0.001 | |||||
| No | 78 | (49.7) | 426 | (66.2) | 1 | |
| Yes | 79 | (50.3) | 217 | (33.8) | 1.99 (1.40−2.83) | |
| Tonsillitis(f) | <0.001 | |||||
| No | 50 | (31.1) | 333 | (51.5) | 1 | |
| Yes | 111 | (68.9) | 314 | (48.5) | 2.35 (1.63−3.40) | |
| Antibiotics consumption(f) | <0.001 | |||||
| No | 36 | (22.5) | 260 | (41.1) | 1 | |
| Yes | 124 | (77.5) | 372 | (58.9) | 2.41 (1.61−3.60) | |
| Complete vaccination | 0.72 | |||||
| No | 04 | (2.50) | 13 | (2.00) | 1 | |
| Yes | 158 | (97.5) | 632 | (98.0) | 0.81 (0.26−2.53) | |
OR: odds ratio; 95%CI: 95% confidence interval; Bolsa Família: Brazilian government program that directly transfers income to households living in poverty and extreme poverty; Parental education is expressed as years of formal education; (g): during the gestational period; (b): at birth or in the neonatal period; (f): during the first two years of life.
Associations between wheezing at ages 6–7 adjusted according variables of the hierarchical framework.
| Level 1 | Level 2 | Level 3 | Level 4 | Final model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Bolsa Família | 0.09 | 0.53 | 0.07 | 0.03 | 0.03 | |||||
| No | 1 | 1 | 1 | 1 | 1 | |||||
| Yes | 1.61 (0.92−2.79) | 1.20 (0.68−2.11) | 1.82 (0.95−3.50) | 2.11 (1.08−4.12) | 2.08 (1.08−4.00) | |||||
| Maternal education(b) | 0.24 | |||||||||
| < 9 years | 1 | |||||||||
| ≥ 9 years | 0.79 (0.53−1.17) | |||||||||
| Child’s sex | 0.07 | 0.09 | 0.62 | |||||||
| Male | 1 | 1 | 1 | |||||||
| Female | 0.70 (0.47−1.02) | 0.71 (0.47−1.06) | 0.88 (0.55−1.43) | |||||||
| Child’s ethnicity | 0.24 | |||||||||
| Non-Caucasian | 1 | |||||||||
| Caucasian | 0.74 (0.45−1.22) | |||||||||
| Family history of asthma | <0.001 | <0.001 | <0.01 | 0.001 | 0.001 | |||||
| No | 1 | 1 | 1 | 1 | 1 | |||||
| Yes | 2.33 (1.55−3.49) | 2.42 (1.59−3.69) | 2.11 (1.28−3.48) | 2.41 (1.45−4.02) | 2.28 (1.37−3.75) | |||||
| Family history of rhinitis | <0.01 | 0.02 | <0.01 | 0.05 | 0.76 | |||||
| No | 1 | 1 | 1 | 1 | 1 | |||||
| Yes | 1.99 (1.31−3.03) | 1.63 (1.07−2.50) | 2.25 (1.35−3.75) | 1.68 (1.00−2.83) | 1.08 (0.65−1.82) | |||||
| Family history of eczema | 0.95 | |||||||||
| No | 1 | |||||||||
| Yes | 1.01 (0.64−1.61) | |||||||||
| Maternal tobacco smoke(g) | 0.40 | |||||||||
| No | 1 | |||||||||
| Yes | 0.77 (0.42−1.42) | |||||||||
| Pet ownership(g) | 0.42 | |||||||||
| No | 1 | |||||||||
| Yes | 1.18 (0.79−1.76) | |||||||||
| Residents at household(b) | 0.31 | |||||||||
| < 3 residents | 1 | |||||||||
| ≥ 3 residents | 1.31 (0.78−2.21) | |||||||||
| Older siblings(b) | 0.88 | |||||||||
| No | 1 | |||||||||
| Yes | 0.96 (0.60−1.56) | |||||||||
| Mother’s ethnicity | 0.72 | |||||||||
| Non-Caucasian | 1 | |||||||||
| Caucasian | 0.92 (0.58−1.46) | |||||||||
| Mother’s height | 0.33 | |||||||||
| < 1.61 m | 1 | |||||||||
| ≥ 1.61 m | 0.82 (0.55−1.23) | |||||||||
| Intended pregnancy | <0.01 | <0.01 | <0.01 | <0.01 | ||||||
| No | 1 | 1 | 1 | 1 | ||||||
| Yes | 0.51 (0.33−0.79) | 0.46 (0.28−0.77) | 0.48 (0.29−0.80) | 0.47 (0.28−0.77) | ||||||
| Alcohol use(g) | 0.58 | |||||||||
| No | 1 | |||||||||
| Yes | 1.25 (0.56−2.81) | |||||||||
| Vaginal discharge(g) | <0.01 | 0.001 | 0.02 | 0.05 | ||||||
| No | 1 | 1 | 1 | 1 | ||||||
| Yes | 2.01 (1.25−3.21) | 2.34 (1.40−3.91) | 1.92 (1.13−3.27) | 1.68 (1.00−2.83) | ||||||
| Urinary infection(g) | 0.65 | |||||||||
| No | 1 | |||||||||
| Yes | 0.90 (0.57−1.42) | |||||||||
| Gestational age | 0.19 | 0.40 | ||||||||
| ≥ 37 weeks | 1 | 1 | ||||||||
| < 37 weeks | 1.87 (0.74−4.75) | 1.46 (0.61−3.53) | ||||||||
| APGAR 5th minute | 0.03 | 0.10 | 0.13 | |||||||
| < 8 | 1 | 1 | 1 | |||||||
| ≥ 8 | 0.18 (0.04−0.83) | 0.26 (0.05−1.32) | 0.26 (0.05−1.45) | |||||||
| Respiratory infections(b) | 0.48 | |||||||||
| No | 1 | |||||||||
| Yes | 1.42 (0.54−3.76) | |||||||||
| Hyperbilirrubinemia(b) | 0.08 | 0.03 | 0.01 | |||||||
| No | 1 | 1 | 1 | |||||||
| Yes | 1.68 (0.94−3.00) | 1.83 (1.06−3.15) | 2.00 (1.17−3.42) | |||||||
| Exclusive breastfeeding | 0.38 | |||||||||
| <12 months | 1 | |||||||||
| ≥ 12 months | 0.79 (0.49−1.28) | |||||||||
| Anemia(f) | <0.01 | 0.01 | ||||||||
| No | 1 | 1 | ||||||||
| Yes | 2.65 (1.40−5.02) | 2.28 (1.22−4.25) | ||||||||
| Lower respiratory tract infections(f) | 0.10 | 0.12 | ||||||||
| No | 1 | 1 | ||||||||
| Yes | 1.60 (0.91−2.81) | 1.55 (0.90−2.66) | ||||||||
| Gastroesophageal reflux(f) | 0.56 | |||||||||
| No | 1 | |||||||||
| Yes | 1.21 (0.64−2.29) | |||||||||
| Intestinal parasitosis(f) | 0.09 | 0.02 | ||||||||
| No | 1 | 1 | ||||||||
| Yes | 1.60 (0.93−2.75) | 1.72 (1.02−2.92) | ||||||||
| Otitis media f) | 0.76 | |||||||||
| No | 1 | |||||||||
| Yes | 1.08 (0.65−1.82) | |||||||||
| Tonsillitis f) | 0.96 | |||||||||
| No | 1 | |||||||||
| Yes | 0.98 (0.57−1.70) | |||||||||
| Antibiotics consumption(f) | 0.27 | |||||||||
| No | 1 | |||||||||
| Yes | 1.40 (0.77−2.57) | |||||||||
OR: odds ratio; 95%CI: 95% confidence interval; Bolsa Família: Brazilian government program that directly transfers income to households living in poverty and extreme poverty; Parental education is expressed as years of formal education; (g): during the gestational period; (b): at birth or in the neonatal period; (f): during the first two years of life. Final model: p = 0.29 for Hosmer-Lemeshow test.
Asthma involves multiple, complex and interacting factors, and for this reason the present study used hierarchical conceptual framework in the statistical analysis. Asthma (or wheezing illnesses in children) is caused by the interaction of multiple genes, some having a protective effect and others contributing to the pathogenesis of the disease.8,9 In this study, family history of asthma was the variable most strongly associated with asthma symptoms in childhood. Despite the genetic component, asthma is influenced by environmental factors and therefore, its risk seems to be greater when both genetic and environmental risk factors are present simultaneously.10
In the present study, children who received Bolsa Família, a benefit granted to families living in poverty and extreme poverty, were more likely to develop asthma symptoms. This association is consistent with findings from a systematic review, which showed that people with low socioeconomic status were 1.11 times more likely to have asthma than people with better socioeconomic status (95% CI 1.09–1.14).11 During the data collection period, Brazil had high unemployment rates (10–13%), affecting all occupational sectors and workers of different educational levels. For that reason, it is believed that Bolsa Família was more sensitive than maternal education to assess socioeconomic status. Lower socioeconomic status could also contribute to infantile diseases. In this study, intestinal parasitosis and anemia in the first two years of life were associated with asthma symptoms at ages 6–7. The role of intestinal parasite infections and the subsequent development of allergic diseases are not yet completely clear. A meta-analysis demonstrated that infection by any intestinal parasite was associated with a small and non-significant increase in the risk of asthma in the general population (OR = 1.24; 95%CI 0.98–1.57), while infections caused specifically by Ascaris lumbricoides were associated with a significant increase in the odd ratios of asthma (OR = 1.34, 95%CI 1.05–1.71).12 These findings suggest that the odd ratios of asthma symptoms could be more related to some specific parasites. Regarding anemia, the mechanism that connects it to asthma symptoms remains unknown. Drury, Schaeffer & Silverberg showed an increase in the odd ratios of asthma and eczema in children with microcytic anemia, suggesting a possible association between iron deficiency and the development of allergic diseases.13 Additionally, an inverse association was reported between umbilical cord blood iron levels and wheezing in children of 3.5 years of age.14 Some experimental studies have indicated that iron administration is able to reduce eosinophil infiltration, mast cell granules, IgE-mediated degranulation, inflammatory cytokine production, as well as decrease hyperreactivity of the airways.15,16 Thus, these findings suggest a possible relationship between the absence of this mineral in the early life and the subsequent development of asthma, which is a possible explanation for the findings of this study.
Maternal infections are also reported as an exposure that is associated to asthma in childhood. In the present study, the odd ratios of asthma were higher and statistically significant only in children whose mothers reported vaginal discharge requiring treatment during pregnancy. Collier et al. found that urinary tract infections were associated with a 60% increase in the odd ratios of childhood asthma, but not in other types of maternal infections in the prenatal period.17 Findings from another study indicate that gynecological infections during pregnancy, especially in the third trimester, increased the risk of eczema in childhood (OR = 2.32; 95% CI, 1.73–4.31).18 Colonization of the mother’s cervical and vaginal tract contributes to the formation of the child’s intestinal microbiota. It is possible that children whose mothers showed growth of pathogenic vaginal bacteria are at higher risk of early pathogenic microbial colonization.19 Thus, maternal vaginal dysbiosis could affect the development of the child’s healthy intestinal microbiota, impairing the maturation of the immune system and possibly facilitating the further development of atopic disorders.
Regarding the association between neonatal hyperbilirubinemia and childhood asthma, it has already been reported that both physiological and pathological hyperbilirubinemia increased 4.26 times the odd ratios of asthma in children up to 12 years (95% CI 4.04–4.50).20 There are some biological mechanisms that support this association. At high levels, bilirubin acts as a potent pro-oxidant. In addition, when there is an increase in bilirubin concentrations, there is an increase in the production of the heme group, which also has a pro-oxidant role. Moreover, experimental studies have shown that bilirubin and/or unconjugated bile acids are able to promote an inhibition of the capacity of pulmonary surfactant to reduce the surface tension of the alveoli, as well as inhibiting the growth of intestinal anaerobic bacteria.21 These factors may aid in the induction of airway inflammation and alter the balance of T helper cells, which may lead to the development of allergic diseases later in life.
Interestingly, this study found that having an intended pregnancy was associated with reduced asthma symptoms at 6–7 years. Although causal relationships are difficult to establish, a literature review has shown that unintended pregnancies are associated with negative health outcomes.22 Associations between unintended pregnancies and maternal risk factors, such as alcohol use, illicit drugs and smoking have also been reported.23 In addition, meta-analyses demonstrated the association between unintended pregnancies and adverse outcomes, such as premature birth (OR = 1.31, 95% CI 1.09–1.58) and low birth weight (OR = 1.41, 95% CI 1.31–1.51).24,25 These findings suggest that gestational planning has effects on maternal behavior, with relatively consistent evidence for the negative effect of unintended pregnancy on antenatal care (such as medical visits), breastfeeding, and child nutrition.26 In addition, unexpected events or psychological stress during pregnancy may influence the development of the respiratory system in the uterine period. It is hypothesized that maternal stress generates a rupture of the balance between the autonomic, neuroendocrine and immunological nervous systems, specifically in the hypothalamic-pituitary-adrenal axis. The rupture of this balance seems to increase vulnerability to inflammation and reactivity of the airways, as well as the reduction of lung function in childhood, thus being associated with asthma symptoms.27 Although previous studies have indicated that intended pregnancy has an impact on the health and well-being of families, this seems to be the first study to show the association of this exposure on asthma symptoms in childhood.
This study has some limitations. Only information regarding gestational age, mode of delivery, birth weight and APGAR scores were registered in the Child Health Handbook. Other exposures present in early life were collected retrospectively and the mothers of the children reported them. Besides that, some exposures have not been thoroughly investigated, such as the period of gestation that some events happened, the causal agents of infections, the number of episodes of respiratory infections, type and dose of antibiotics used. Despite the limitations exposed, it is worth mentioning that cases and controls were from a population-based sample. Thus, the typical selection bias of a traditional case-control study was minimized. In addition, this design allowed the analysis of multiple exposures, including some factors little explored previously. However, since most children had severe asthma symptoms, the results of this study may not be generalized to all children with symptoms of the disease.
In summary, several factors present in the first thousand days of life are associated with wheezing in childhood. Since many exposures associated with asthma symptoms are considered modifiable, this knowledge can be used to plan strategies to reduce the impact of these environmental exposures in the subsequent development of asthma. Longitudinal studies are required to confirm some of the findings, mainly potential protective factors, like intended pregnancy.
FundingThis work was supported by Fundação de Amparo à Pesquisa e Inovação do Estado de Santa Catarina (FAPESC), public call 09/2015 – Support to Research Groups of the Institutions of the ACAFE System (process number 2016TR222).
Conflict of interestThe authors have no conflict of interest to declare.
The authors would like to thank the Programa de Suporte à Pós-Graduação de Instituições Comunitárias de Ensino Superior (PROSUC), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES – Brazil) for supporting this study with a PhD scholarship to K.S.