Russula cutefracta (synonymous with Russula cyanoxanthus) is a mushroom of the genus Russula commonly found in parts of Europe and Asia. Although species of Russula have rarely been used for medicinal preparations, extracts of other mushrooms species are used as traditional remedies for various diseases in Asian countries. Recent evidence indicates that the mushroom lectins, polysaccharides, and other molecules have general anti-tumour, anti-inflammatory, and immunodulatory properties. Here, we investigated the effects of R. cutefracta on mast cell degranulation, an important step in allergic reactions.
Mast cells are important for immediate hypersensitivity and the inflammatory response and the rat basophilic leukaemia cell line, RBL-2H3, has proven useful for studying the biology of mast cells.1 Allergy, or immediate (Type I) hypersensitivity, is one of four types of hypersensitivity.2 The binding of antigen to the high affinity IgE receptor (Fc¿RI) on the surface of mast cells and basophils induces the release of histamine, prostaglandin, arachidonic acid metabolites, proteases, heparin sulphate, serotonin, leukotrienes, and proinflammatory cytokines from secretory vesicles called granules.3,4
Basophils and mast cells express high levels of Fc¿RI consisting of an α chain, a β chain, and two disulfide-linked γ chains.3,4 The α chain of Fc¿RI contributes to high affinity IgE binding whereas the β and γ chains, which contain immunoreceptor tyrosine-based activation motifs (ITAMs), initiate the downstream signalling cascade leading to release of preformed and newly synthesised mediators.3,4 The spleen tyrosine kinase (Syk) plays an essential role in the IgE-dependent activation of mast cells. For example, cross-linking of Fc¿RI results in phosphorylation of the ITAMs on the β and γ chains by Lyn, a Src family kinase.5 The phosphorylated γ chains then bind and activate Syk, which has an essential role for Fc¿RI-proximal signalling pathways.6,7 However, many more kinases including Fyn, PI3-kinase, PLC-γ, and MAPKs are important for mast cell activation and degranulation.3,4 Therefore, the inhibition of these kinases by natural compounds found in R. cutefracta might suppress the release of hypersensitivity-inducing mediators in mast cells, thereby leading to inhibition of the allergic response.
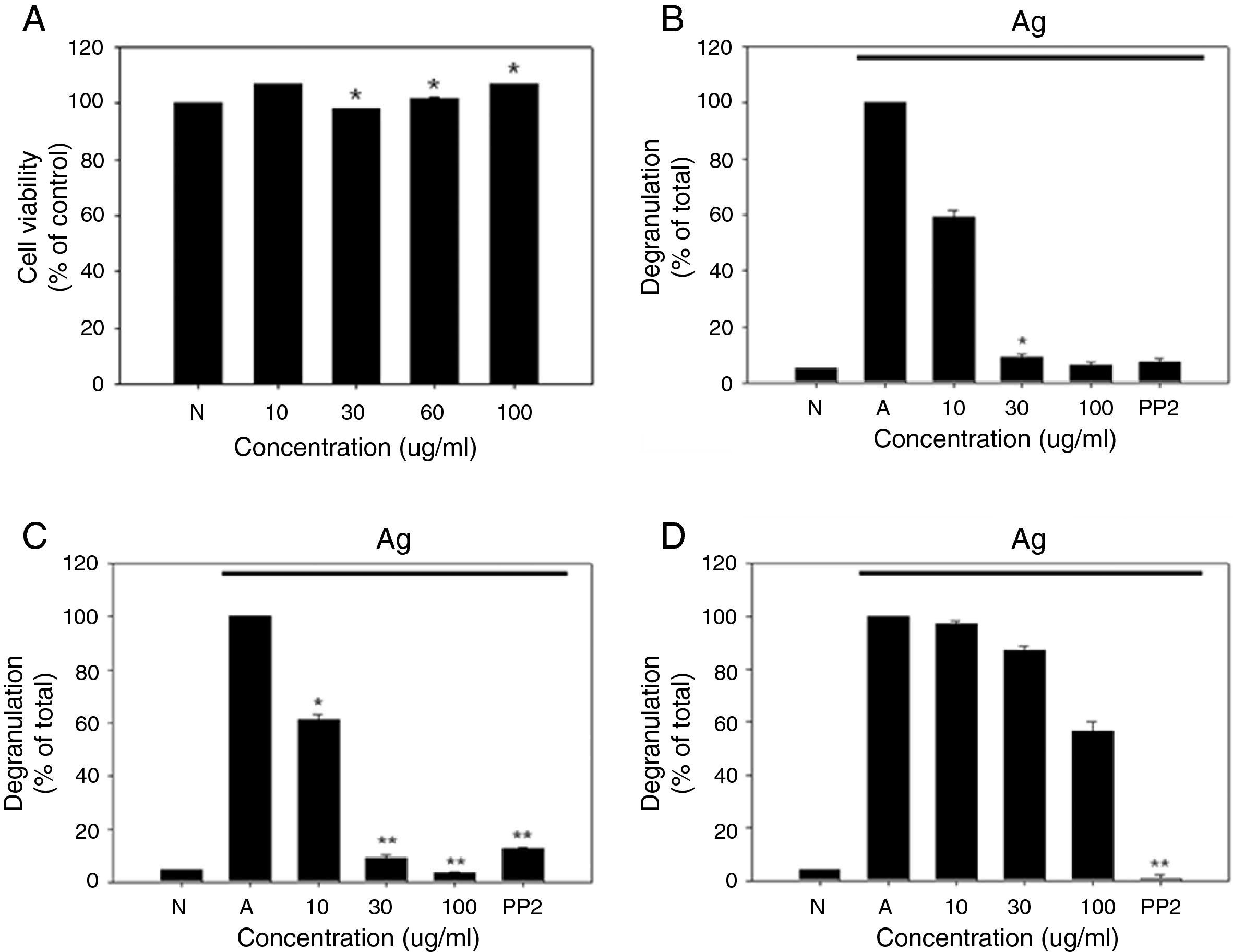
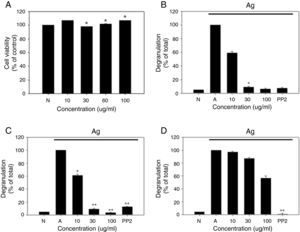
As a first step toward using R. cutefracta as a treatment for allergy, dried R. cutefracta (80g) was used to prepare R. cutefracta extract (RCE) by three extractions with ethanol. After filtration, excess water was removed with a rotary evaporator under vacuum at 50°C. Next, we determined whether RCE is toxic by assessing the viability of mast cells. RBL-2H3 cells were treated with various concentrations of RCE for 12h and cell viability was determined by MTT assay. Untreated cells were used as a negative control (Fig. 1A). We did not observe any increase in cell death due to RCE, although we tested a range of concentrations from 10μg/ml to 100μg/ml (Fig. 1A). Therefore, in the range of concentrations used in our study, RCE was not toxic to mast cells.
The effect of R. cutefracta on RBL-2H3 degranulation. (A) RBL-2H3 cells were treated by the indicated concentrations of RCE for 12h and cell viability was determined by MTT assay. (B) Degranulation of RBL-2H3 cells was measured by the release of β-hexosaminidase. (C and D) Thapsigargin and ionomycin-stimulated degranulation. Thapsigargin (300nM) and ionomycin (1M) were used to stimulate degranulation of RBL-2H3 cells. PP2 (10μM) is a general Src-family kinase inhibitor; N, no treatment; A, antigen stimulation only. The values are expressed as the mean±SEM from three independent experiments (*P<0.05 and **P<0.01).
Mast cell degranulation is a cellular process whereby secretory vesicles release hypersensitivity-inducing molecules including histamine, proteoglycans and proteases. β-Hexosaminidase is another molecule present within mast cell granules and this marker has been used widely to monitor degranulation.8 Mast cells were incubated with DNP-specific IgE (20ng/ml) overnight and various concentrations of RCE (10μg/ml, 20μg/ml, and 100μg/ml) for 30min, resulting in a dose-dependent decrease in degranulation, as measured by β-hexosaminidase release (Fig. 1B). In comparison, our control experiments with untreated cells or cells receiving antigen simulation only resulted in no degranulation and complete degranulation, respectively (Fig. 1B). Another control experiment using PP2 (10μM), a general Src-family kinase inhibitor, also inhibited degranulation and RCE at 30μg/ml and 100μg/ml were as potent as PP2 in inhibiting degranulation (Fig. 1B). We believe that this is the first demonstration of the anti-allergy properties of R. cutefracta.
An increase in cytosolic Ca2+ ionophore concentration ([Ca2+]i) is thought to be important for mast cell degranulation.9 Also, it is known that thapsigargin, a potent inhibitor of Ca2+-ATPase in the endoplasmic reticulum membrane and ionomycin, a Ca2+ ionophore, cause mast cell degranulation without Fc¿RI activation.10 To determine whether degranulation resulting from an increase in cytosolic [Ca2+] can be inhibited by RCE, RBL-2H3 cells were incubated with DNP-specific IgE (20ng/ml) overnight, treated with RCE at various concentrations (10μg/ml, 20μg/ml, and 100μg/ml) for 30min, and treated with thapsigargin (300nm) or ionomycin (1μM) for 10min. RCE treatment after thapsigargin pre-treatment resulted in a dose-dependent inhibition of mast cell degranulation, similar to that seen with DNP-IgE-induced degranulation (compare Fig. 1B and C). Ionomycin could also cause degranulation, but the inhibition of degranulation after subsequent treatment with RCE was weaker than that observed after thapsigargin treatment (compare Fig. 1C and D). Together, these data provide evidence that non-Fc¿RI-mediated mast cell degranulation could also be inhibited by R. cutefracta.
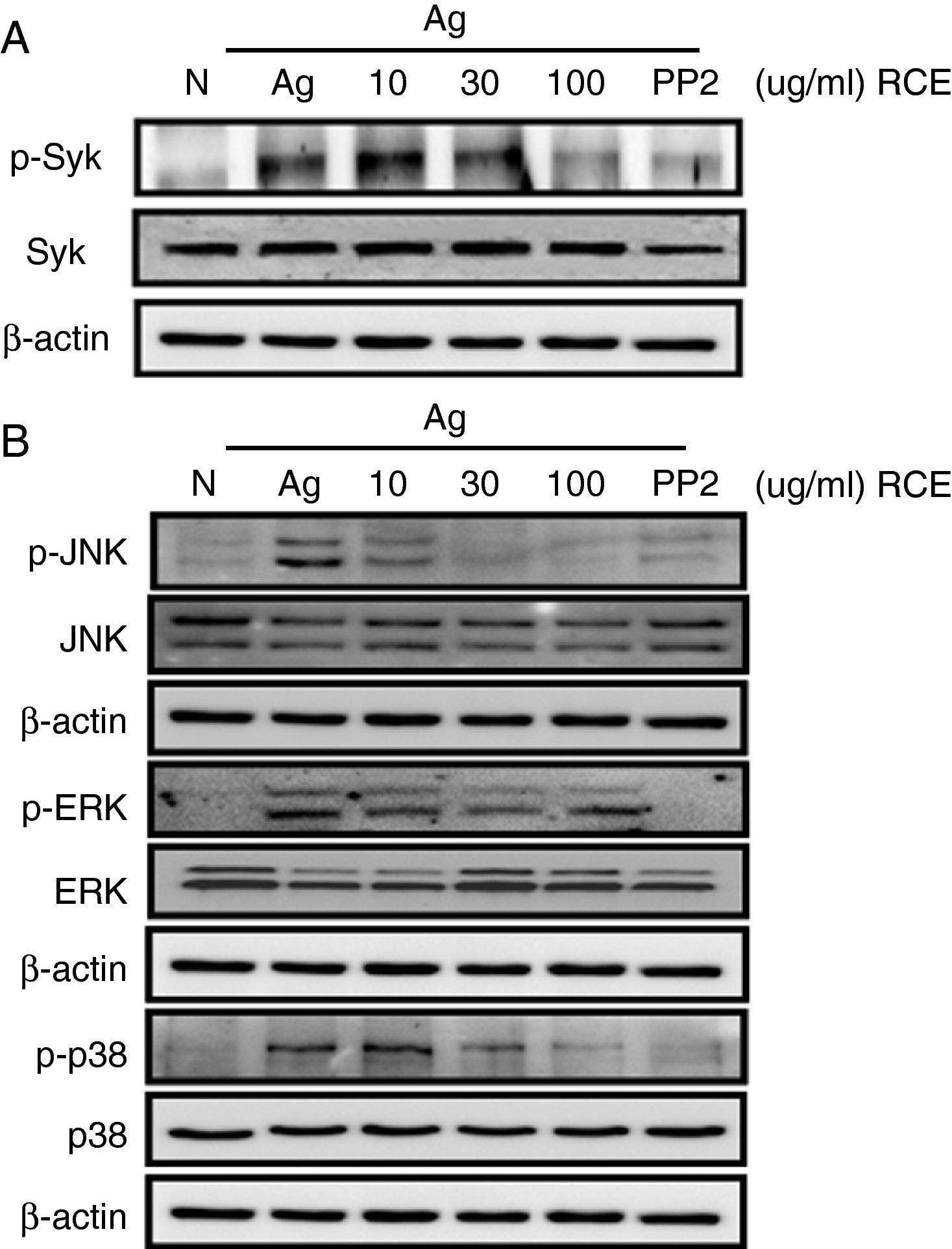
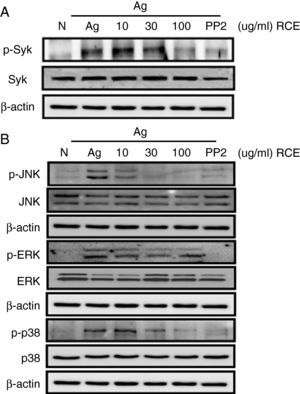
To understand how R. cutefracta suppresses mast cell activation and degranulation, we examined its effects on Fc¿RI-mediated signalling events by Western blot analyses, including phosphorylation of Syk and the MAP kinases (ERK1/2, JNK1/2 and p38). Syk is a key mediator of immunoreceptor signalling in mast cells; it is activated upon engagement of the Fc receptor and it is a critical link to many downstream events including calcium mobilisation.4 Treating the mast cells with increasing concentrations of RCE resulted in a gradual decrease in Syk phosphorylation (as detected by α-P-Syk Tyr525/526 antibody), thus verifying the dose-dependency of RCE and mast cell degranulation (Fig. 2A). Some secondary signalling events following KIT activation in mast cells, including phosphorylation of the mitogen-activated protein kinases (MAPKs), are important events in the transcriptional regulation of activated mast cells.4 When mast cells were treated with increasing concentrations of RCE, we observed significant decreases in JNK phosphorylation (as detected by α-P-JNK Thr183/Tyr185 antibody) and p38 phosphorylation (as detected by α-P-p38 Thr180/Tyr182 antibody) and a slight decrease in ERK1/2 phosphorylation (as detected by α-P-Erk1/2 Thr202/Tyr204 antibody) (Fig. 2B). Together, these results support our observation that RCE could inhibit mast cell degranulation by inhibiting cell signalling intermediaries. Furthermore, it is likely that RCE inhibits mast cell degranulation by preventing the phosphorylation of regulatory molecules downstream of Fc¿RI, such as Syk and the MAP kinases.
The effect of R. cutefracta on Syk and MAPK phosphorylation. RBL-2H3 cells were treated as described to initiate degranulation, with or without RCE treatment. The cell lysates were subjected to Western blot analysis to detect phosphorylated forms of (A) Syk and (B) MAP kinase. PP2 (10μM) is a general Src-family kinase inhibitor; N, no treatment; A, antigen stimulation only.
In conclusion, ours is the first study demonstrating that R. cutefracta inhibits degranulation in mast cells. Furthermore, only a few other mushroom species including Flammulina velutipes, Hypsizigus marmoreus, and Agaricus blazei are known to protect against hypersensitivity. Therefore, R. cutefracta merits further evaluation as a natural treatment for allergy.
This work was supported by the Regional Innovation Center (RIC) of the Ministry of Commerce, Industry and Energy through the Bio-Food and Drug Research at Konkuk University and also supported by the Ministry of Education, Science and Technology (MEST) and Korea Industrial Technology Foundation (KOTEF) through the Human Resource Training Project for Regional Innovation. This work was also supported by Korea Research Foundation Grant funded by the Korean government (KRF-2006-521-F00082).