INTRODUCTION
Atopic dermatitis (AD) is a chronic inflammatory skin disorder that affects between 10 % and 12 % of the pediatric population. There is an increasing body of clinical and laboratory evidence suggesting that food hypersensitivity plays a pathogenic role in AD in a subset of patients, primarily infants and children 1.
In the early part of this century, Schloss reported several cases of patients who had improvement in their eczematous skin lesions after avoiding specific foods. That report was followed by many others with conflicting findings and led to controversy about the role of specific food allergens in AD 2. Some authors demonstrated by double-blind placebo-controlled food challenge (DBPCFC) an acute-onset clinical reactions consisting of urticaria, pruritus, and erythema in a subset of patients with AD, whereas others had delayed-onset eczematous reactions. No relationship has been established between reactivity in skin prick tests (SPT) and delayed-onset clinical reactions 3-5.
The prevalence of clinically relevant food hypersensitivity among children with AD remains an unanswered question. Approximately 40 % of infants and small children with moderate/severe AD have food allergy with a positive DBPCFC 6-8. Because the real prevalence of food allergy in AD remains unknown, the aim of this study is to determine the prevalence of clinically relevant food hypersensitivity in patients who were referred to a Service of Dermatology. These patients were referred to the dermatologist without selection for any adverse reaction to foods.
METHODS
Subjects
We studied 44 infants of both sexes, 27 males (61 %) and 17 females (39 %), less than 12 months old (range from 3 to 12 months, mean 7.5 months and mode 6 months), referred from the Service of Dermatology with the diagnosis of atopic dermatitis (AD) fulfilling the criteria of Hanifin-Rajka 9 and were not selected on the basis of suspected allergy to foods. They were recruited according to consecutive non-probabilistic sampling. The dermatologist evaluated patients at the initial visit, and an AD symptom score was assigned using the SCORAD index 10.
Full past medical history was recorded and a complete physical examination performed. All patients were asked about foods introduced into the diet and their tolerance.
Procedures
Skin test technique: Prick testing was done with a commercially available allergens (LETI Laboratory, Madrid, Spain). A 1 mm-one-peak lancet with shoulder to prevent deeper penetration was used. Histamine dihydrochloride (10 mg/ml) was used as a positive control, and saline solution was used as a negative control. SPT were carried out on the front surface of the forearm with reading after 15 minutes. A wheal diameter 3 mm larger than that produced by the negative control was considered positive. SPT were performed in all patients with whole cow's milk extract (5 mg/ml), with isolated cow's milk proteins (CMP): α -lactalbumin (5 mg/ml), β -lactoglobulin (5 mg/ml), and casein (10 mg/ml) and with other foods: egg-white, egg-albumin, ovomucoid and those introduced into diet.
In vitro test: A venous blood sample was obtained from the infant. The patients were screened for food-specific serum IgE to milk, α -Lactalbumin, β -Lactoglobulin and those foods with positive SPT. The levels of total and specific serum IgE were determined using CAP System fluorescein-enzyme immunoassay (CAP) (limit of the assay,0.35 KUA/L) (Pharmacia Diagnostics, Uppsala Sweden). The test was considered positive when a result of 0.35 KUA/L was obtained.
Challenge test: Before the challenge, foods were eliminated from the infants' diet for at least 2weeks. Open controlled food challenges (OCFC) with cow's milk were performed on all patients after one month of elimination from the diet, and the same test were carried out with the foods with positive allergy evaluation (sIgE and/or SPT) after two weeks of elimination from the diet. Elimination diet with cow's milk was extended to one month following the protocol of the study of Isolauri 3. During this month patients were reviewed in the hospital at the time of any exacerbation of AD.
All of the challenges were performed in the Unit of Allergy at the Hospital, where appropriate medication and resuscitation equipment was directly available. Informed consent was previously obtained from the parents. Each patient remained for 2 hours under observation after the last food dose intake before going back home. Before OCFC infants must fulfil the following conditions: controlled atopic dermatitis, absence of acute rash and not being treated with anti-histamines in the previous 7 days, topical corticoids (48 hours), systemic corticoids (one month) and oral and inhaled beta-adrenergics (12 hours).
The OCFC were made with a ready-to-use infant formula of cow's milk. In the challenge with cow's milk, rising doses (5, 10, 25, 50 and 100 ml) were given at 30-minute intervals until milk intake appropriate for the age was reached. In the case of the other foods, they were prepared under normal eating conditions, the dose was equivalent to a normal intake, and increasing doses with 30-minute intervals were given: 1/8 of the total dose, 1/4 of the total dose and later the rest of the total dose. If there was history of an IgE-mediated type immediate reaction it was started with 1/16. The challenge was discontinued when a clinical reaction was noticed. After two hours, the patient was sent home with instructions to the parents to record the symptoms of the infant for 48 hours. Patients were reviewed in the hospital at the time of any adverse reaction. If the response was positive with clear and objective signs of urticaria and/or angio-oedema, whether or not combined with other clinical symptoms, elimination of the food from the diet was indicated.
Follow up was carried out for one month after the challenge test. During this month patients were reviewed in the hospital if any exacerbation of AD occurred. AD was evaluated using the SCORAD index
Informed consent was previously obtained from the parents of each patient.
RESULTS
AD starts between the 2nd and 8th month of life, 80 % being concentrated between the 2nd and 4th month. 68 % received breast-feeding for 1 to 8 months, with a mean of 5 months. Almost half (45 %) of the infants from the sample studied started with AD when exclusively on breast feeding (without receiving bottles during their stay in the Maternity Ward after birth), and before having been introduced to any other food in their diet. As regards SCORAD score of AD, it was evaluated as mild in 14 children (32 %), moderate in 28 (64 %) and severe in 2 (4 %).
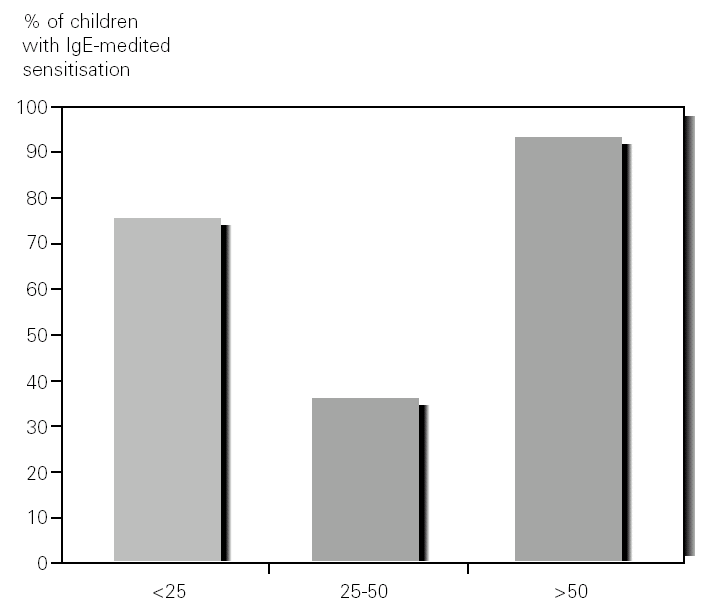
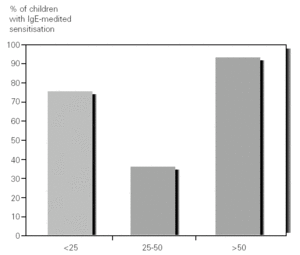
Food sensitization (positive prick test and/or specific IgE > 0.35 kU/l) was seen in 27 out of the 44 (61 %) patients studied. Among the patients with AD there was food sensitization in 43 % (6/14) of those who had mild AD, 68 % (19/28) of those with moderate AD and 100 % (2/2) of those who had severe AD (fig. 1).
Figure 1.--Percentage of children with food sensitisation, in the atopic dermatitis groups, mild (SCORAD: < 25), moderate (SCORAD: 25-50) and severe (SCORAD: > 50).
Sensitization to egg was present in 61 % (27/44) of the total infants with AD and a higher percentage was seen, 70 % (21/30), among those who received mother's milk (30/44) and 75 % (15/20) of those started with AD exclusively on mother's milk (20/44).
In all the infants, after one month of elimination diet and whether there was sensitization or not to this food, OCFC with cow's milk was carried out. Only in one patient was positive (2 %), who was sensitised to this food, and presented with peribuccal erythema and generalised urticaria. The paediatrician had previously eliminated CMP from the diet due to having infant colic. The rest of the infants (98 %) had a negative CMP challenge test. No improvement in the progress of the AD was seen during the month of cow's milk elimination from diet, nor a worsening, after its subsequent re-introduction into the diet and patients did not need to be reviewed at hospital because no exacerbation of AD appeared.
The most common food involved was egg (100 % of the infants sensitised to foods) and the second was cow's milk (30 % of those sensitised to foods). None of them had egg introduced into the diet. We carried out the challenge test with egg in those sensitised (positive prick test and/or specific IgE > 0,35 kUA/l) when the children were 15 months old, before the triple virus vaccine. OCFC with egg was positive in 44 % (12/27) of the sensitised children. In those with negative OCFC, there was no exacerbation in DA next moth after reintroduction in the diet.
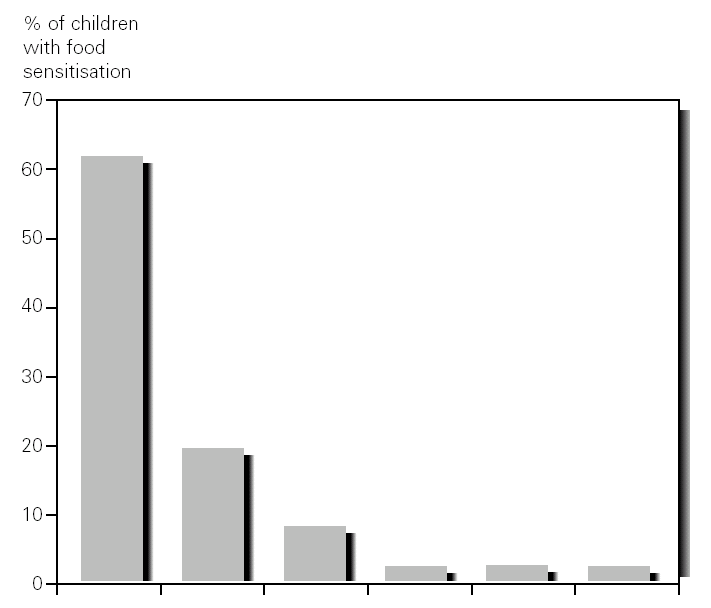
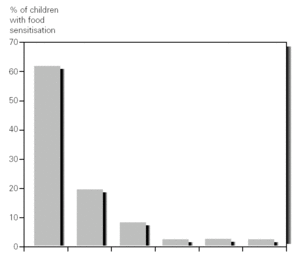
Other foods sensitization observed were chicken meat in 7 % of patients (3/44), hake in 2 % (1/44), tomato 2 % (1/44) and banana in 2 % (1/44) (fig. 2). We did not observed differences in the evolution of DA after two weeks of elimination diet and OCFC with these foods was negative in all cases.
Figure 2.--Percentages of sensitisation to food in infants less than 12 months old with atopic dermatitis.
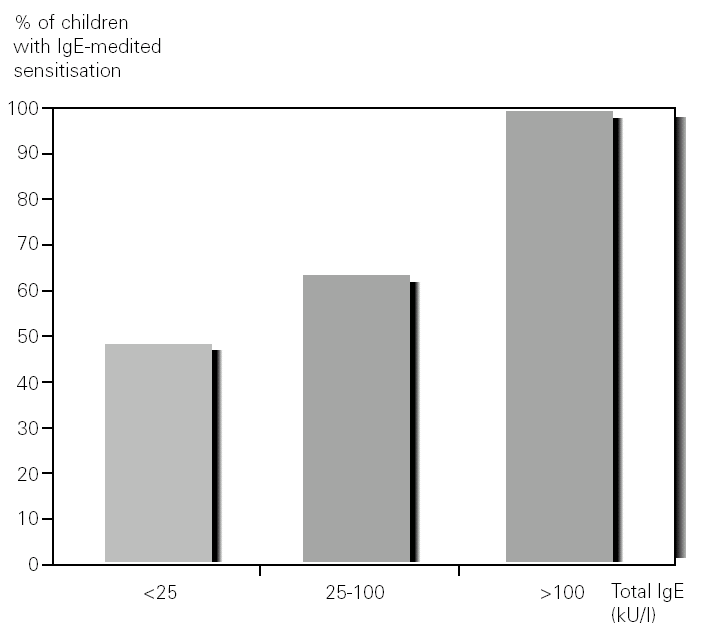
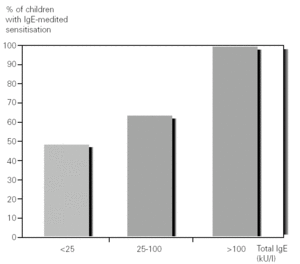
61 % of the patients (27/44) had a total IgE value between 0 and 25 KUA/L, of which 48 % (13/27) were sensitised; 25 % (11/44) between 25 and 100 KUA/L, with 64 % (7/11) sensitised and 14 % (6/44) above 100 KUA/L, with 100 % (6/6) sensitised (fig. 3).
Figure 3.--Percentage of children with IgE-mediated hypersensitivity in relation to total serum IgE levels.
DISCUSSION
The pathogenic role of food allergy in AD was first suggested by clinical observations and uncontrolled studies performed more than 80 years ago and has been supported further by the results from large controlled studies using DBPCFC performed in the past 20 years 1. Moreover, our study confirms that infants with AD frequently do have IgE-mediated food sensitization. However this is a sensitization that often does not lead to the appearance of symptoms of AD.
Of the 44 patients in the study, 69 % had a moderate/severe AD of which 40 % (12/30) had an immediate response in OCFC. These results are similar to those observed by other authors, where approximately 40 % of infants and young children with moderate-severe AD had a food allergy confirmed using DBPCFC 8,10. But in these studies they include patients with suspected adverse reactions to foods, which could explain the symptomatic sensitization results obtained in them.
The majority of existing studies in patients with AD have been carried out on patients sent to Allergy Clinics and in these, AD is normally accompanied by other allergic diseases, which are generally the main reason they are sent for an allergy evaluation. To prevent this bias that may have affected previous studies, the sample of infants of the present study was gathered in a Paediatric Dermatology Clinic where the infants were sent for diagnosis and treatment of AD without suspected food allergy. The same was done by Eigenmann et al, who also studied a sample of patients, but from six months to twenty years old, who were referred to a pediatric dermatologist with suspected AD, and detected food allergy (with positive DBPCFC) in 37 % of patients, higher than that obtained by us, which was 27 % (12/44), but similar to the percentage obtained by this same author, 39 %, in a sample of patients who attended his Allergy Clinic for AD or other allergic diseases 11. This is even more than that observed by Burks, in which the selection of the sample was criticised because two thirds of the patients came from an Allergy Clinic. But if we closely review the study, and looking at the characteristics of the patients studied by Eigenmann et al, we see that the majority of these patients had a history of adverse reactions to foods as well as AD.
Although virtually any food protein can cause a reaction, only a small number of foods account for more than 90 % of reactions in infants (cow's milk, eggs, peanut, soy) 4,12. In children with AD and food allergy, two thirds are reactive to eggs 13. In our study sample, the foods most commonly implicated were egg followed by cow's milk, but we did not observe sensitizations to peanut or soy maybe a cause of the age of the patients.
Some authors consider that egg and cow's milk could be aetiologically implicated in infant AD. This consideration, however, does not seem to be confirmed in our study. On evaluating the results obtained in the sample of 44 infants, firstly, we see that in 45 % of them the AD started while they were on breast-feeding, before introducing any other food into the diet, confirming that reported by other authors. As in the sample of 112 children less than 2 years old studied by Majamaa et al 14, where the symptoms had started in 78 % of them during exclusive breast-feeding. Secondly, the food that was most frequently implicated in sensitization, egg, as happened in our study, had not been introduced into the diet. Egg protein is secreted into breast milk after maternal dietary ingestion and, exposure to egg protein during breastfeeding is a route of hidden exposure that may result in sensitization of at-risk infants 15.
In the DBPCFC with foods, according to several authors, two types of positive responses can be seen: immediate and delayed. Double-blind challenge on patients with AD, generally show clinical reactions of the immediate type, which occur between 5 minutes and two hours, with mainly cutaneous symptoms (pruritus, erythema, morbilliform exanthem, bumps mainly in the areas of the eczema), secondly digestive system (nausea, vomiting, abdominal pain, diarrhoea), and can also have respiratory symptoms (wheezing, nasal congestion, sneezing, cough) but they do not mention reactions which reproduce the atopic eczema 13,16,17. Some patients, after the immediate response, experience a second episode of increasing pruritus with morbilliform or transitory erythematous exanthem which starts 4 to 10 hours after the initial positive response 3, and in a small proportion of cases the exacerbation of the symptoms occur from 2 to 5 days after the normal ingestion of the food 18. In the years following, after an avoidance diet, in those who remain sensitised and the eczema is reduced, the symptoms with the DBPCFC is generally urticaria 19.
Some authors believe that the responses to the food provocation test can also appear in a delayed form, much later than the first three hours, generally in the following 48 hours or even in the days after, specifying that for the appearance of eczema the patient should take the food for several days. Delayed symptoms are difficult to diagnose and attribute to a particular food, or, as reported by Werfel and Kaap, it may be impossible 20. Vanto et al. studied 305 infants, the majority (74 %) of them with AD, suspected of having hypersensitivity to CMP and concluded that it is very difficult to asses the results of a positive delayed response since the improvement in the AD during the elimination period was not total, not being completely free of eczema, and to evaluate the difference between the initial skin disorder and the additional ones during the days of provocation was, in many cases, very difficult 21. They suggested that in an orthodox clinical trial, such as indicated by Metcalfe and Sampson, it should include confirmation by carrying out three double-blind tests, which would surely be difficult for the parents to accept 22.
Burks et al found that in 266 DBPCFC carried out on 165 patients with AD, the symptoms began in the first two hours and did not have any significant delayed responses 13. In the studies by Sampson only some patients developed diffuse pruritus and rarely maculoerythematous exanthem at 4-8 hours after a positive DBPCFC 12,23,24.
In the patients in our study we found that 12 (27 %) had an immediate response in the provocation test and no delayed responses were seen.
However, there is still controversy over whether food allergy in patients affected by AD is implicated in the etiopathogenesis of the disease or is only an expression of the atopic constitution of the patient.
Therefore, once there is diagnosed a food allergy, its influence on the evolution of AD will need to be evaluated. Many authors report that with a suitable elimination diet, food allergic patients could experience a noticeable improvement, but they do not document this assertion. There are few prospective studies that have studied the efficacy of the elimination diet in AD. In a study by Sampson and McCaskill 13, in which they followed up 113 children with AD for 1 to 2 years, who had a suitable elimination diet based on the results of the challenge test, there was a significant improvement in clinical symptoms compared to those who did not demonstrate food allergy. In a later prospective study by Sampson and Scanlon, in which a small sample of children with AD were followed up for 3 to 4 years, only 17 children, who were allergic to foods and had an elimination diet, significantly improved in the 3-4 year of follow up 23. But, Novembre et al, in a recent study on the natural progress of AD over 9 years in children from 2 to 11 years old, observed that it is better in those who have allergic sensitization, although do not have an elimination diet 9.
Resano et al, in their study followed up 74 children and adults with AD and during the first 18 months of follow-up they did not see any differences in the outcome between those allergic to foods with elimination diet and those not allergic to foods. Only at three years was a significant improvement seen in those who followed an elimination diet 25. As Eigenmann et al pointed out recently, there is a lack of double-blind prospective studies to test the efficacy of the elimination diet in AD 11. On the other hand, as commented by this author, at least one third of patients can tolerate the food after one or two years of elimination diet but, however, continue having AD and in patients with good progress of AD where food allergy persists, on carrying out challenge test with the food, a response of skin urticaria is obtained.
CONCLUSIONS
A high prevalence of sensitization to foods is seen in infants with AD. This sensitization was proportionally higher in infants with moderate/severe AD, and with increased levels of serum total IgE. This could be explained by the atopic condition of our patients. The most frequent sensitization was to egg, although with little clinical relevance since it had not been introduced into the diet. The clinical relevance of the food sensitizations observed has not been verified with regard to AD. Allergy and sensitization to foods in our patients appear associated as one more expression of the atopic constitution of these children but they are not the cause of AD. More studies are needed to confirm these results.
Correspondence:
Dr. Antonio Martorell
Consorci Hospital General Universitari Valencia. Unit of Allergology
Avda. Tres Cruces, s/n
46014 Valencia
Tel.: 96-197 20 00
Fax: 96-197 22 10
E-mail: martorell_ant@gva.es