Determination of efficacy of allergic vaccines, in the context of clinical trial, is mainly based on the objective quantification of medication administered and symptoms evolution with little relevance of variables perceived by patients. IgE-mediated respiratory diseases have an important social and psychological repercussion for patients as well as economic consequences. In this sense, it seems important to record and analyse Patient-Reported Outcomes (PROs) which refers to any report coming from patients about a health condition and its treatment.1 PROs are gaining importance in clinical research because of their relevance in the overall treatment efficacy assessment.1
Among PROs, Health Related Quality of Life (HRQoL) has been extensively evaluated in rhinitis and asthma.1 Several validated tools have been developed to assess variations in HRQoL in rhinitis and asthma, including both the Rhinoconjunctivitis2 and Asthma3 Quality of Life Questionnaires (RQLQ, AQLQ) developed by Junniper, highlighted by their widespread use.
High-dose allergoid immunotherapy (Acaroid®) has demonstrated its clinical effectiveness and safety in several clinical trials,4,5 for treatment of allergic disease mediated by IgE aetiologically related to house dust mites. The aim of this study was to assess improvements in HRQoL of patients after the first two months of treatment with Acaroid®.
The tested product Acaroid® (Allergopharma KG, Reinbek, Germany) is an aluminium hydroxide-adsorbed depot allergoid preparation of standardised (in therapeutic units, TU) high concentrations of powdered diafiltered dust mite allergens modified with formaldehyde and glutaraldehyde. There are two different concentrations, A strength (1000TU/mL), and B strength (10,000TU/mL). Allergens quantified in the last step prior to allergoidisation are 11.66μg/mL Der p 1, and 10μg/mL Der p 2 in the 100% Dermatophagoides pteronyssinus formulation, and 20μg/mL Der f 1 and 15μg/mL Der f 2 in the 100% Dermatophagoides farinae formulation.
A retrospective observational study including patients with allergic rhinitis and/or bronchial asthma was performed at the Allergy Service of Doctor Negrin Universitary Hospital (Canary Islands, Spain) between October 2009 and August 2011. Both questionnaires were administered before the beginning of the immunotherapy and eight weeks later. The inclusion criteria were: adult patients between 18 and 65 years old, diagnoses with rhinitis and/or bronchial asthma IgE-mediated by house dust mites and were likely to receive immunotherapy with Acaroid®. All patients accepted participation in the study by signing the informed consent form.
The sRQLQ has 28 questions distributed in seven different domains: activity limitation, sleep problems, nose symptoms, eye symptoms, non-nose/eye symptoms, practical problems and emotional function. Patients respond to each question on a 7-point scale (0=not impaired at all–6=severely impaired). The sAQLQ includes 32 questions in four domains: symptoms, activity limitation, emotional function and environmental stimuli. Patients are asked to respond to all questions on a 7-point scale (7=not impaired at all–1=severely impaired). HRQoL was assessed at the beginning of the immunotherapy and eight weeks later. The Spanish validated versions for RQLQ and AQLQ were used in the study.6 Patients with allergic rhinitis and bronchial asthma fulfilled both questionnaires.
For both questionnaires, the overall score is the mean of all 28 or 32 responses (sRQLQ and sAQLQ, respectively), and the individual domain scores are the means of the items in those domains. The statistical significance of the differences between HRQoL scores at weeks 0 and 8 was determined using Student's t-test (paired samples, two tails, P<0.05). Previously, the Kolmogorov–Smirnov test and the Bartlett test were used to check both the normality and homoscedasticity of the samples.
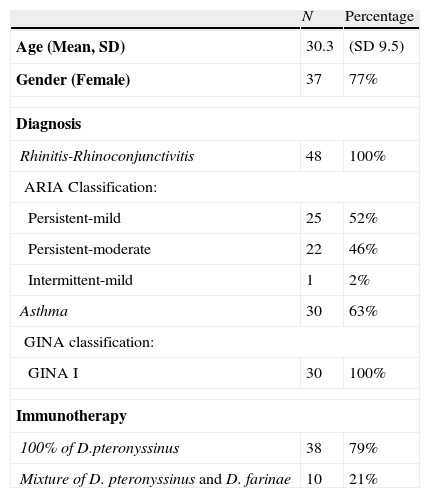
A total of 48 patients were requested to participate in this study, acceptance percentage was 100%. Baseline characteristics, degree of disease severity according to ARIA (for rhinoconjunctivitis) and GINA (for asthma) and immunotherapy administered are described in Table 1. All patients were sensitised to house dust mites, 92% of rhinoconjunctivitis patients and 93% of bronchial asthma patients were monosensitised, respectively. The rest of patients were poli-sensitised, however this sensitisation may not impact on clinical symptoms. 44 (92%) of 48 patients with rhinitis and 19 (63%) of 30 asthmatic patients fulfilled the questionnaires at weeks 0 and 8, respectively. Results reported below refer to this number of patients.
Baseline and clinical characteristics of patients included in the study.
| N | Percentage | |
| Age (Mean, SD) | 30.3 | (SD 9.5) |
| Gender (Female) | 37 | 77% |
| Diagnosis | ||
| Rhinitis-Rhinoconjunctivitis | 48 | 100% |
| ARIA Classification: | ||
| Persistent-mild | 25 | 52% |
| Persistent-moderate | 22 | 46% |
| Intermittent-mild | 1 | 2% |
| Asthma | 30 | 63% |
| GINA classification: | ||
| GINA I | 30 | 100% |
| Immunotherapy | ||
| 100% of D.pteronyssinus | 38 | 79% |
| Mixture of D. pteronyssinus and D. farinae | 10 | 21% |
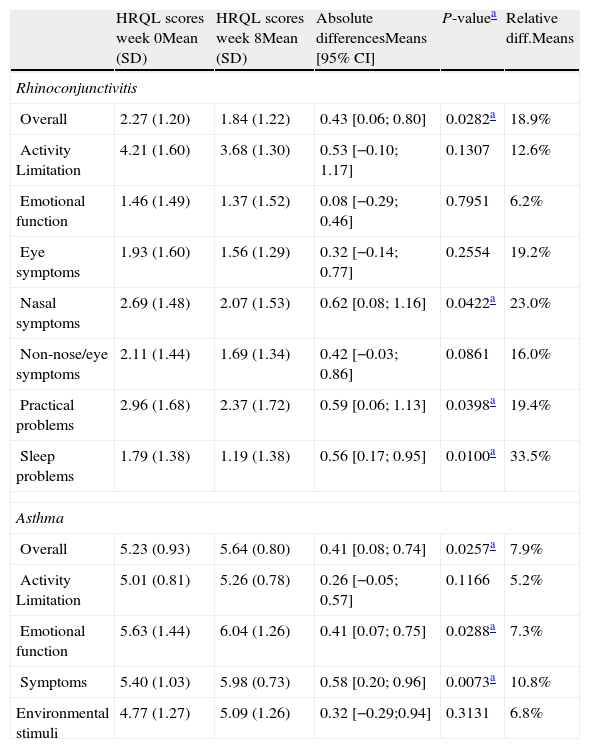
We observed statistically significant differences in the sRQLQ and sAQLQ overall scores after eight weeks of immunotherapy with Acaroid®. A decrease in sRQLQ overall score of 0.45 points was observed, which represents an improvement of HRQL of 19% (P-value <0.05). When the analysis is performed by domains, these differences were also significant for three of the seven domains assessed: sleep problems, practical problems and nose symptoms.
For asthmatic patients, an increase of 0.41 sAQLQ overall score was observed, representing an improvement in HRQL of 8% (P-value <0.05). The improved quality of life was also observed in all domains in the sAQLQ with statistical significance for symptoms and emotional function domains. All these results are summarised in Table 1.
Treatment of rhinoconjunctivitis/bronchial asthma IgE-mediated by house dust-mites with Acaroid® shows an important and statistically significant improvement in patients’ HRQoL after eight weeks of immunotherapy (Table 2).
Overall and by domains scores at weeks 0 and 8 for allergic rhinitis and bronchial asthma quality of life questionnaires.
| HRQL scores week 0Mean (SD) | HRQL scores week 8Mean (SD) | Absolute differencesMeans [95% CI] | P-valuea | Relative diff.Means | |
| Rhinoconjunctivitis | |||||
| Overall | 2.27 (1.20) | 1.84 (1.22) | 0.43 [0.06; 0.80] | 0.0282a | 18.9% |
| Activity Limitation | 4.21 (1.60) | 3.68 (1.30) | 0.53 [−0.10; 1.17] | 0.1307 | 12.6% |
| Emotional function | 1.46 (1.49) | 1.37 (1.52) | 0.08 [−0.29; 0.46] | 0.7951 | 6.2% |
| Eye symptoms | 1.93 (1.60) | 1.56 (1.29) | 0.32 [−0.14; 0.77] | 0.2554 | 19.2% |
| Nasal symptoms | 2.69 (1.48) | 2.07 (1.53) | 0.62 [0.08; 1.16] | 0.0422a | 23.0% |
| Non-nose/eye symptoms | 2.11 (1.44) | 1.69 (1.34) | 0.42 [−0.03; 0.86] | 0.0861 | 16.0% |
| Practical problems | 2.96 (1.68) | 2.37 (1.72) | 0.59 [0.06; 1.13] | 0.0398a | 19.4% |
| Sleep problems | 1.79 (1.38) | 1.19 (1.38) | 0.56 [0.17; 0.95] | 0.0100a | 33.5% |
| Asthma | |||||
| Overall | 5.23 (0.93) | 5.64 (0.80) | 0.41 [0.08; 0.74] | 0.0257a | 7.9% |
| Activity Limitation | 5.01 (0.81) | 5.26 (0.78) | 0.26 [−0.05; 0.57] | 0.1166 | 5.2% |
| Emotional function | 5.63 (1.44) | 6.04 (1.26) | 0.41 [0.07; 0.75] | 0.0288a | 7.3% |
| Symptoms | 5.40 (1.03) | 5.98 (0.73) | 0.58 [0.20; 0.96] | 0.0073a | 10.8% |
| Environmental stimuli | 4.77 (1.27) | 5.09 (1.26) | 0.32 [−0.29;0.94] | 0.3131 | 6.8% |
Powell et al.7 not only reported significant improvements in HRQoL of patients treated with a depot extract of phleum but also concluded that this improvement was higher in patients receiving higher doses of grass majority allergen (20μg phl p 5/doses). In this sense, Alvarez Cuesta et al.8 reported significant improvements using a modified therapeutic extract (allergoid).
Despite the small number of bronchial asthma patients included, we consider an important contribution of our study the improvements in HRQoL observed in these patients. In a previous study Ameal et al.9 reported improvements in HRQoL for asthma patients after seven weeks of immunotherapy, although this difference was not statistically significant until the end of the study (19 weeks).
Based on our findings and considering previous analyses published, we believe that the results reported in our study could be related with high concentrations of the major allergens of Dermatophagoides that are administered in each dose of Acaroid®.
One limitation could be the retrospective and observational design; nevertheless we consider that the assessment of the therapeutic product under real practice conditions could be clinically relevant. Other limitations of our study rely on the small sample size and the short period of follow-up. Nevertheless, it is necessary to highlight the clinical relevance of our findings. Patients perceived effects of the immunotherapy quickly and significantly. Patients’ satisfaction with their therapy is directly related with perceived effectiveness, which could also improve patients’ adherence to treatment. The significant improvement in HRQoL reported by asthmatic patients is also relevant despite their asthma level (100% GINA I).
In conclusion, this is the first study reporting significant improvements in HRQoL after a short period of immunotherapy, both the overall score and by domains score. Despite the short period of follow-up, the administration of Acaroid® in patients with IgE-mediated rhinoconjunctivitis and asthma has shown statistically significant improvements in patient's health-related quality of life. Confirmation of these results should be expected considering a higher sample size and a longer period of follow-up.
Ethical disclosuresProtection of human and animal subjectsThe authors declare that the procedures followed were in accordance with the regulations of the responsible Clinical Research Ethics Committee and in accordance with those of the World Medical Association and the Helsinki Declaration.
Confidentiality of dataThe authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentThe authors have obtained the informed consent of the patients and/or subjects mentioned in the article. The author for correspondence is in possession of this document.
This research was funded by the Allergy Department of the Hospital Universitario Dr. Negrín of Las Palmas de Gran Canaria, Spain.