Scarcity of reliable data on food allergy prevalence exists in Turkey. We aimed to assess reported and confirmed IgE-mediated food allergy prevalence, and define the spectrum of allergenic food.
MethodsWe prospectively evaluated the ISAAC Phase II study population for food allergy. Participants that reported experiencing food allergy symptom in the last year and/or were skin prick test positive for a predefined list of food allergens, were interviewed via telephone, and those considered as having food allergy were invited to undergo clinical investigation, including challenge tests.
ResultsA total of 6963 questionnaires were available. Parental reported food allergy prevalence and skin prick sensitisation rate were 20.2±0.9% and 5.9±0.6%. According to the above-defined criteria, 1162 children (symptom positive n=909, skin prick test positive n=301, both positive n=48) were selected and 813 (70.0%) were interviewed via telephone. Out of 152 adolescents reporting a current complaint, 87 accepted clinical investigation. There were 12 food allergies diagnosed in nine adolescents, with food allergy prevalence of 0.16±0.11%. The most common foods involved in allergic reactions were walnut (n=3) and beef meat (n=2), followed by hen's egg (n=1), peanut (n=1), spinach (n=1), kiwi (n=1), cheese (n=1), hazelnut (n=1) and peach (n=1).
ConclusionsWhile parental reported food allergy prevalence was within the range reported previously, confirmed IgE-mediated food allergy prevalence among adolescents was at least 0.16%, and the spectrum of foods involved in allergy differed from Western countries, implying environmental factors may play a role.
The term food allergy refers to immunologically mediated hypersensitivity reaction to any food, which is mostly IgE-mediated, but cellular mechanisms may also underlie in a small portion of patients. Other reactions to food that need to be distinguished from food allergies include food intolerance, pharmacological reactions, toxic reactions and food aversions,1 which are collectively regarded as non-allergic food hypersensitivity.2
Even though food allergies are a very common childhood health problem, there are little data about the true occurrence of the disease in a population setting. The reason for this is the high rate of perception of food allergy as causal of untoward reactions to food, diagnostic concerns and the time, resource and effort-consuming nature of population-based studies. In addition, there is significant variation in allergenic food profile among different geographical regions.3–15 Thus, there is universal scarcity of data on epidemiology of food allergy in population; this is also true for Turkey. Population-based studies typically employ questionnaires, skin prick tests (SPT), specific IgE (spIgE) measurements and food challenge tests. Only few studies employ double-blind placebo-controlled challenge tests (DBPCCT), a gold standard diagnostic procedure, because they require substantial effort from the study team and patient compliance is also an important concern. Self-reported food allergy prevalence reported in studies conducted up to date is quite variable, usually within the range of 5–35%.3,4,10–14,16,17 Confirmed prevalence implementing oral challenge tests is much lower, generally <1%, as documented in studies by different investigators.3,4,6,11–13,16 Knowledge of the epidemiological impact of the problem is crucial for the effective implementation of health policies.
In this study, we aimed to estimate food allergy prevalence and define allergenic food spectrum for urban adolescents from different geographical regions of Turkey.
Materials and methodsThe current study is a detailed evaluation for the presence of food allergy of patients from multicentre ISAAC Phase II study conducted in 2005–2006 and which aimed to investigate epidemiology of asthma and allergic disease in childhood. ISAAC Phase II option B protocol18 was implemented in 2005–2006 in five different cities (Ankara, Antalya, Manisa, Trabzon, Van) of Turkey under the support of Prime Ministry State Planning Organization and headed by Hacettepe University, Faculty of Medicine, Pediatric Allergy and Asthma Unit.
ISAAC Phase II StudyISAAC Phase II Study was a cross-sectional study that recruited 10–11-year-old primary schoolchildren. Sample size calculation and sampling process involved a two-step layered systematic cluster sampling method. In accordance with the ISAAC II methodology (option B), the study aimed to enrol at least 1000 students from each city, to be recruited from at least 10 schools, with the aim of recruiting at least 100 children who had reported wheezing over the last 12 months.18 City-specific minimum sample sizes were calculated for α=0.05, 1−β=0.80, δ=6.9% (percentage of physician-diagnosed asthma cases recorded in the previous ISAAC study conducted in Turkey19), and σ=1.2. Detailed information on the study population and sampling process is presented elsewhere.20–23 Apart from standard workup, food allergy module (Refer to Appendix A) was integrated, and skin prick testing was performed with standard food allergens (egg white, cow's milk, walnut, peanut and hazelnut) to all consented participants. Food allergy module consisted of six questions regarding ever and current experiencing any complaint following food intake, spectrum of complaints, presence of complaint following intake of commonly encountered food items, physician aid seeking and history of physician diagnosed food allergy. The questionnaire was validated on a sample of 40 parents/children through in-depth interviews.
Definition of ‘suspicious for food allergy’ patientsAny questionnaire holder who answered the question ‘Did your child have any allergic complaint after any food intake within last year?’ (Appendix A, Question 2) as ‘Yes’ and/or was sensitised to any of tested food allergens was defined as ‘suspicious for food allergy’, and thus was chosen for telephone interview.
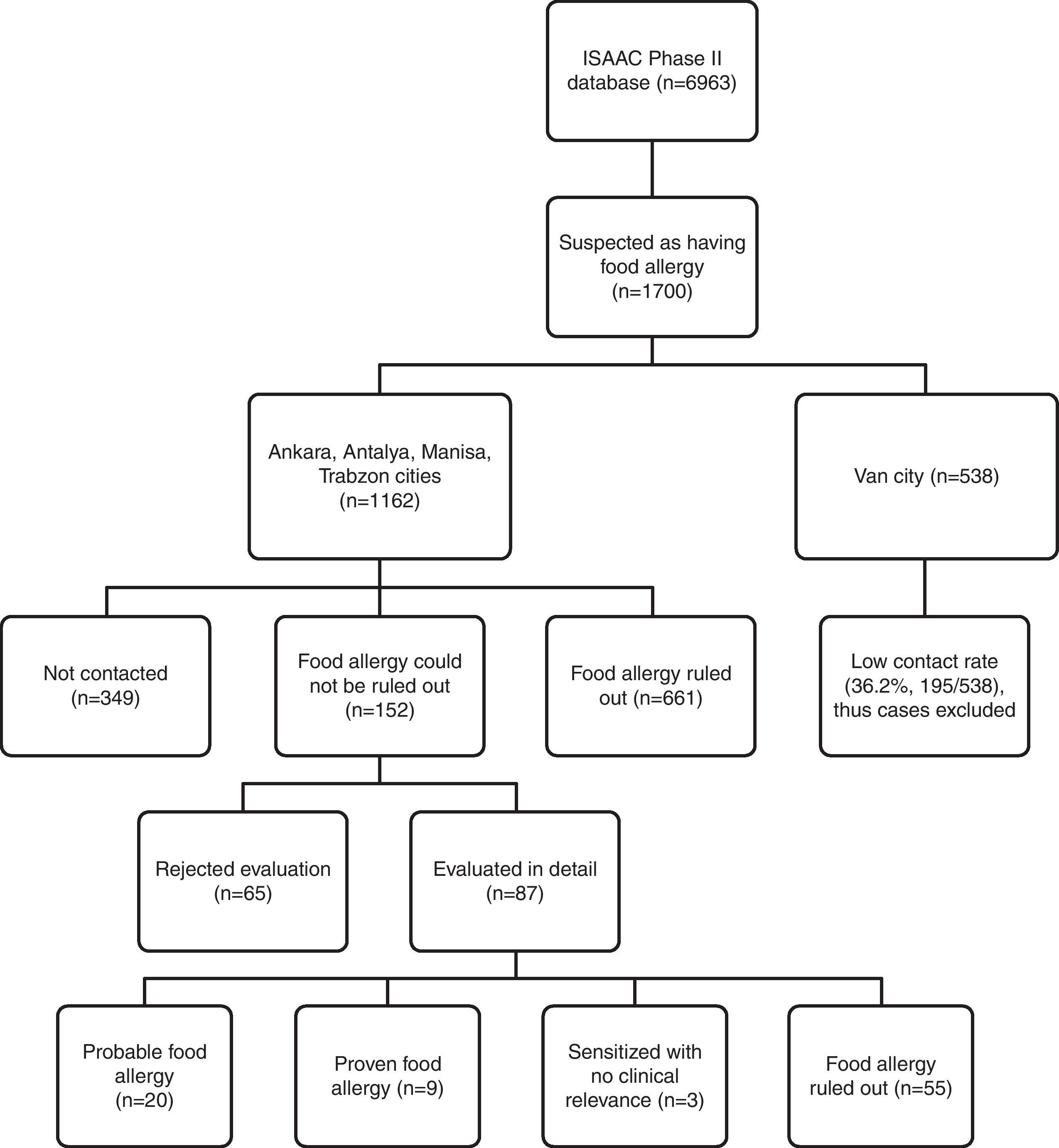
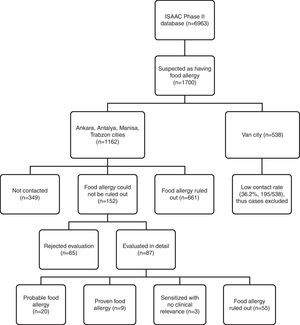
Telephone interview of patients ‘suspicious for food allergy’Questionnaire holders were contacted via telephone over the period of January 2010–June 2010 by a single paediatrician trained in food allergy. Due to low contact rates and absence of appropriate paediatric allergic setting for referral in Van province, cases from this centre were not included in estimation of confirmed food allergy prevalence because of potential bias. The flowchart of evaluation of ISAAC Phase II study population for food allergy is shown in Fig. 1.
The detailed telephone interview was conducted with aim to exclude non-allergic food hypersensitivity and non-IgE-mediated food allergy. Adolescents in whom suspicion for IgE-mediated food allergy persisted after obtaining clinical history were chosen for evaluation at allergy departments.
We preferred to interview patients themselves or their mothers/caregivers. The reason for this is that culturally Turkish mothers care for their children primarily and therefore are more likely to give reliable information on their children. The interview implemented a standard questionnaire regarding ever and current complaints following food ingestion or complaints linked to any food. Respondents were asked whether they have ever experienced pruritus, erythema, wheals, urticaria, nausea and vomiting, abdominal pain, diarrhoea, cough, dyspnoea, wheezing, lip swelling, itching or sense of swelling in the oropharynx, rhinitis or conjunctivitis symptoms, palpitations or syncope after food consumption. Then, the interviewer reviewed a list of foods commonly involved in food allergy and symptoms related to ingestion of these food items. Cases that reported consistent or suspicious symptoms after food consumption within 12 months, or those that reported ever consistent/suspicious symptoms but had not consumed the food in question within 12 months for any reason were defined as patients who might be food allergic. They were invited for evaluation at allergy departments in the corresponding city. Questionnaire holders were attempted to contact on at least three occasions before deciding they were unavailable.
Evaluation of patients at allergy departmentsAdolescents in whom IgE-mediated food allergy could not be ruled by a telephone interview were invited for investigation and evaluated at paediatric allergy clinics in Ankara, Antalya, Manisa or Trabzon between March 2010 and July 2010. Physicians at corresponding clinics were instructed to lead the investigation with intention to diagnose or rule out IgE-mediated food allergy.
Diagnostic tools were history, physical examination, SPT, spIgE and food challenge tests. History regarding complaints structurally similar to that asked in the first interview, past medical history and family history were obtained and physical examination performed; skin prick testing and food challenge tests with suspected allergens were applied to all consented patients. In case commercial extracts for prick testing were not available, patients underwent prick-to-prick testing. A wheal diameter of at least 3mm in respect to negative control was accepted as a positive result. SpIgE testing was performed with Pharmacia ImmunoCAP assay (Phadia AB, Uppsala, Sweden). Cases evaluated as positive on basis of history and/or had evidence of sensitisation to food allergens were applied open challenge tests (OCT). When the patient reported a severe reaction, she/he was not challenged and the diagnosis of food allergy was established according to history and sensitisation findings. In order to maintain a methodological uniformity of patient evaluation across study centres, patients with inconsistent history and laboratory data were also open challenged. The dose interval was 20min.
Food masking procedures for tomato and kiwi in three blinded challenge tests have been described elsewhere.12
Statistical evaluationData was statistically evaluated using SPSS v.15 (SPSS Inc., Chicago, Illinois, USA). Descriptive analysis was carried out for ISAAC Phase II study population. Results were expressed as mean and standard error of mean (SEM) or as percentages of responses to each question. Independent samples t-test was used for comparison of means. We used chi-square test to compare proportions in independent groups, and applied Yates correction and Fisher's exact test where required. Test of a single proportion was used to assess whether prevalence rates were different for the whole study population compared to a subset of subjects. A significance level of p<0.05 was chosen. 95% confidence interval (CI) was calculated for prevalence rates. Adjustments for loss to follow-up at each stage were applied in order to estimate for prevalence of food allergy and probable food allergy.
Role of the funding sourceISAAC Phase II Study was sponsored by a grant (03K 120 570-05-7) from the Prime Ministry State Planning Organization, Turkey. The Ministry of Health of Turkey (provision of nurses and allied health personnel) and Merck Sharp Dohme Pharmaceutical Co. Istanbul, Turkey [provision of Quantitest lancets (Panatrex Inc, Placentia, California, USA) for the skin prick tests] supported the study. The prospective evaluation phase that included telephone interview and detailed investigations were conducted with use of resources of ISAAC Phase II Study and of corresponding study centres.
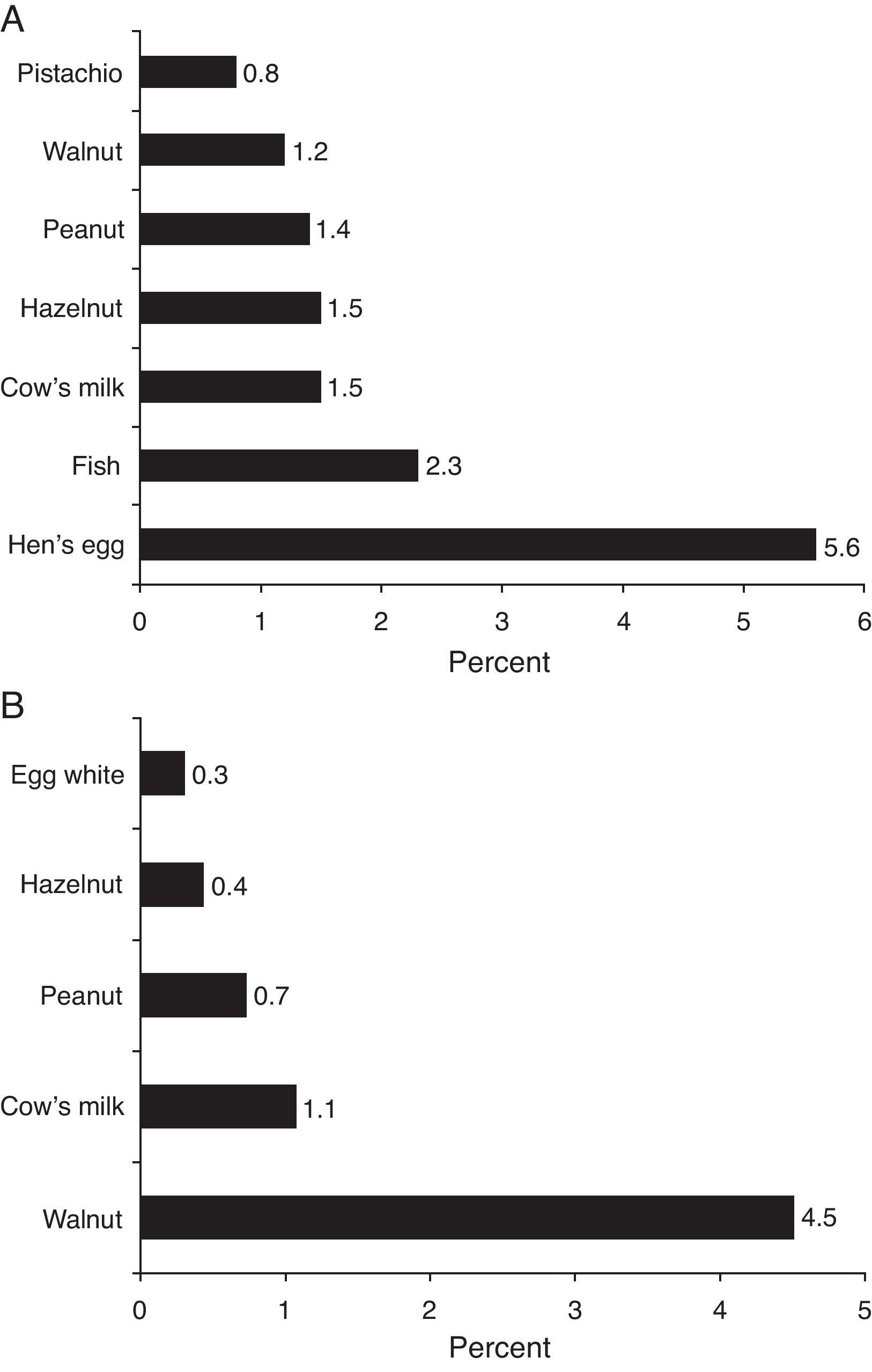
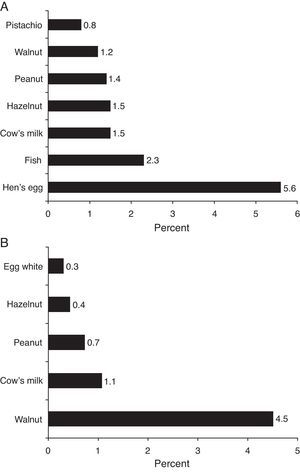
ResultsISAAC Phase II Study recruited 6963 children. Demographic and clinical features of the study population are given in Tables 1 and 2. Parental-reported food allergy prevalence was estimated as 20.22±0.94% (1408/6963, 95% CI) (Table 1). Sensitisation prevalence to common food allergens detected by skin prick testing was estimated as 5.9±0.6% (361/6134, 95% CI). Parental reported food allergy prevalence for specific food items and skin test sensitisation prevalence for common allergens are given in Fig. 2.
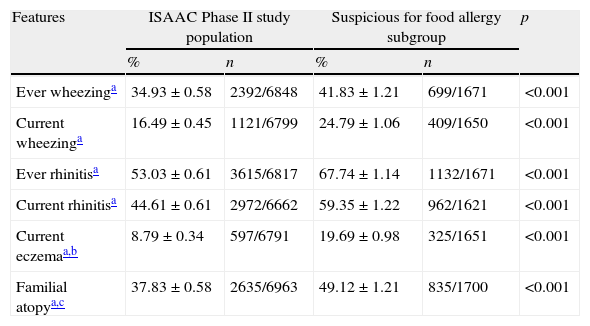
Clinical features of ISAAC Phase II study population and ‘suspicious for food allergy’ subgroup.
| Features | ISAAC Phase II study population | Suspicious for food allergy subgroup | p | ||
| % | n | % | n | ||
| Ever wheezinga | 34.93±0.58 | 2392/6848 | 41.83±1.21 | 699/1671 | <0.001 |
| Current wheezinga | 16.49±0.45 | 1121/6799 | 24.79±1.06 | 409/1650 | <0.001 |
| Ever rhinitisa | 53.03±0.61 | 3615/6817 | 67.74±1.14 | 1132/1671 | <0.001 |
| Current rhinitisa | 44.61±0.61 | 2972/6662 | 59.35±1.22 | 962/1621 | <0.001 |
| Current eczemaa,b | 8.79±0.34 | 597/6791 | 19.69±0.98 | 325/1651 | <0.001 |
| Familial atopya,c | 37.83±0.58 | 2635/6963 | 49.12±1.21 | 835/1700 | <0.001 |
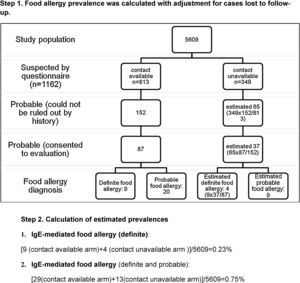
A total of 1700 questionnaire holders were defined as ‘suspicious for food allergy’ on basis of history and/or skin prick testing, and they were selected for telephone interview. Allergic co-morbidity prevalence was higher for this subgroup of children compared to the study population (Table 2). Contact rate for Van province was low (36%), and cases from this centre (1354 out of 6963 questionnaires) were not included in the estimation of confirmed food allergy prevalence because of potential bias. After exclusion of questionnaires from Van province, 1162 (out of 5609) questionnaires were labelled as ‘suspicious for food allergy’ (301 SPT positive, 909 symptom positive, 48 both positive), and for 813 of them contact was achieved, yielding an overall contact rate of 70%.
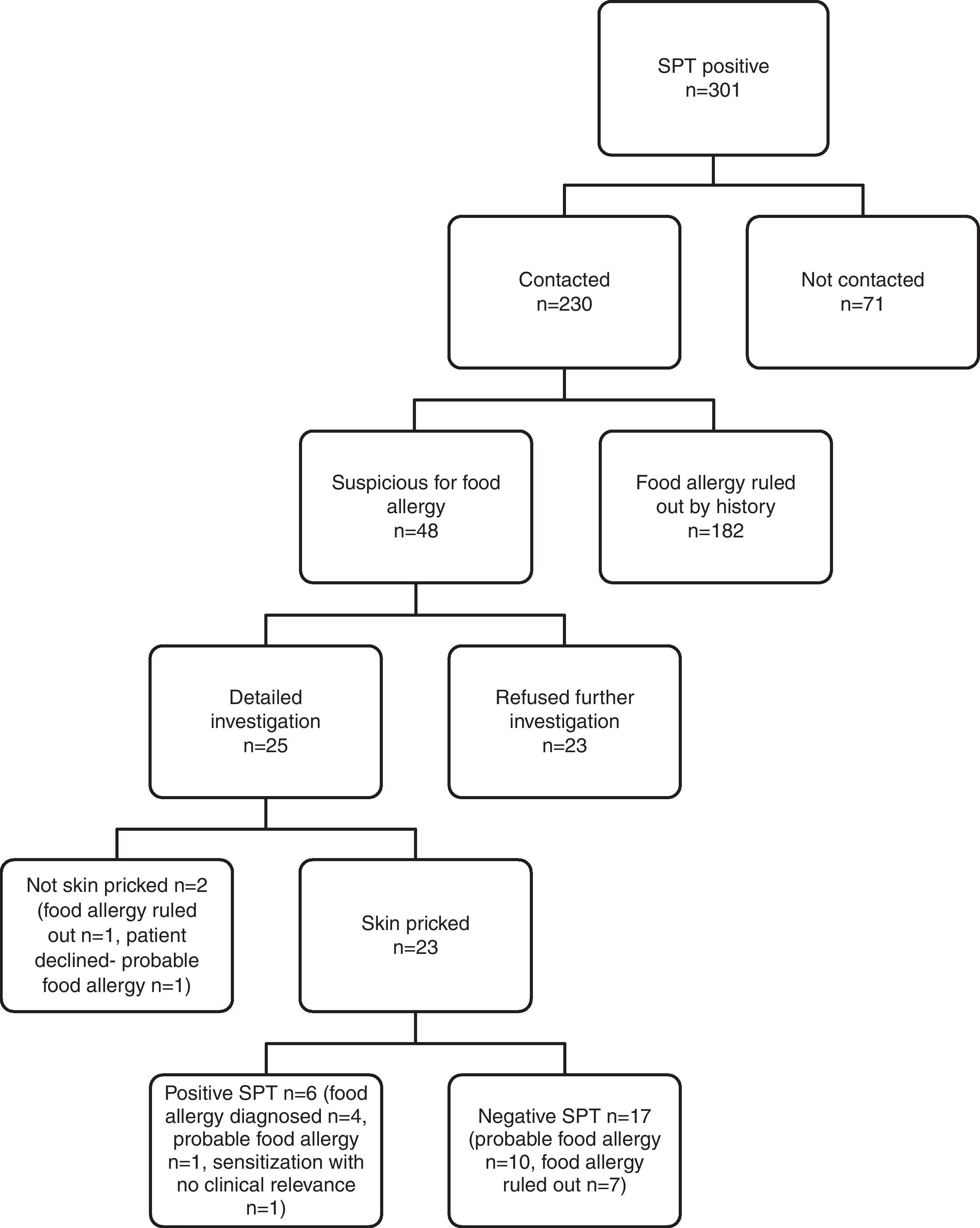
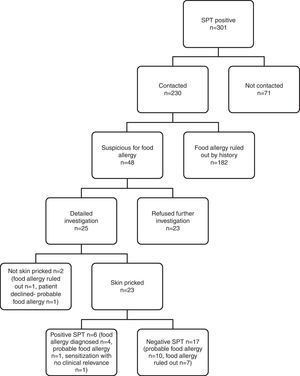
IgE-mediated food allergy could not be ruled out in 152 adolescents by history alone, and they were invited for further evaluation. Of them, 87 adolescents were admitted to allergy clinics for evaluation. Among admitted patients 79 consented to skin prick testing. The outcome of initially 301 SPT positive children from four study centres is shown in Fig. 3.
Subjects admitted to allergy clinics (n=87) reported 160 food allergies. There were 109 open food challenges planned and 82 performed. They resulted in 13 positive (in 12 patients) and 69 negative reactions. Among the 12 patients that reacted positively, eight patients were diagnosed food allergy and four patients offered DBPCCT. Two of them consented to DBPCCT and were negative for food allergy. The other two patients refused DBPCCT and thus formed a probable food allergy group. DBPCCT was available in three patients, all of which proved negative. Two had oropharyngeal reaction on open challenge with tomato, while the third patient reported oropharyngeal symptoms upon kiwi intake. We found in retrospect that she had been previously challenged with kiwi in blinded fashion, thus no further investigation was pursued.
Consequently, food allergy was diagnosed in nine patients, was ruled out in 55 patients, and three patients were sensitised without clinical relevance. There were 20 patients diagnosed as having ‘probable food allergy’ because they declined further investigation (Fig. 1).
Among nine patients diagnosed with food allergy, eight had been open challenged and one was not because of a potentially severe reaction. Ten open challenges in these patients resulted in cutaneous symptoms (n=6, 60%), gastrointestinal symptoms (n=4, 40%), oropharyngeal (n=2, 20%) and anaphylaxis (n=1, 10%). Reactions in challenge testing were mostly mild. Only one patient developed a potentially severe anaphylaxis with peanut, and refused further testing with other food items. She was administered i.m. adrenaline and hospitalised for follow up. Allergens found to cause food allergy in these patients were walnut (n=3), beef meat (n=2), hen's egg (n=1), peanut (n=1), hazelnut (n=1), peach (n=1), spinach (n=1), cheese (n=1) and kiwi (n=1).
Patients diagnosed as having ‘probable food allergy’ reported symptoms with intake of hen's egg (n=5), walnut (n=5), chocolate (n=3), fish (n=2), hazelnut (n=2), peanut (n=2), almond (n=1), blackberry (n=1), tomato (n=1), meat and meat products (n=3), raspberry (n=1), black pepper (n=1), red pepper (n=1), and pickled cucumbers (n=1). In this patient group, 17 out of 20 were skin pricked. Oral challenge tests were available in two patients, one of which reported itching upon egg ingestion, and the other patient developed urticaria and itching six hours after chocolate intake. Both had negative skin prick testing and declined double-blind challenge. Out of 29 planned open challenges, 27 could not be performed.
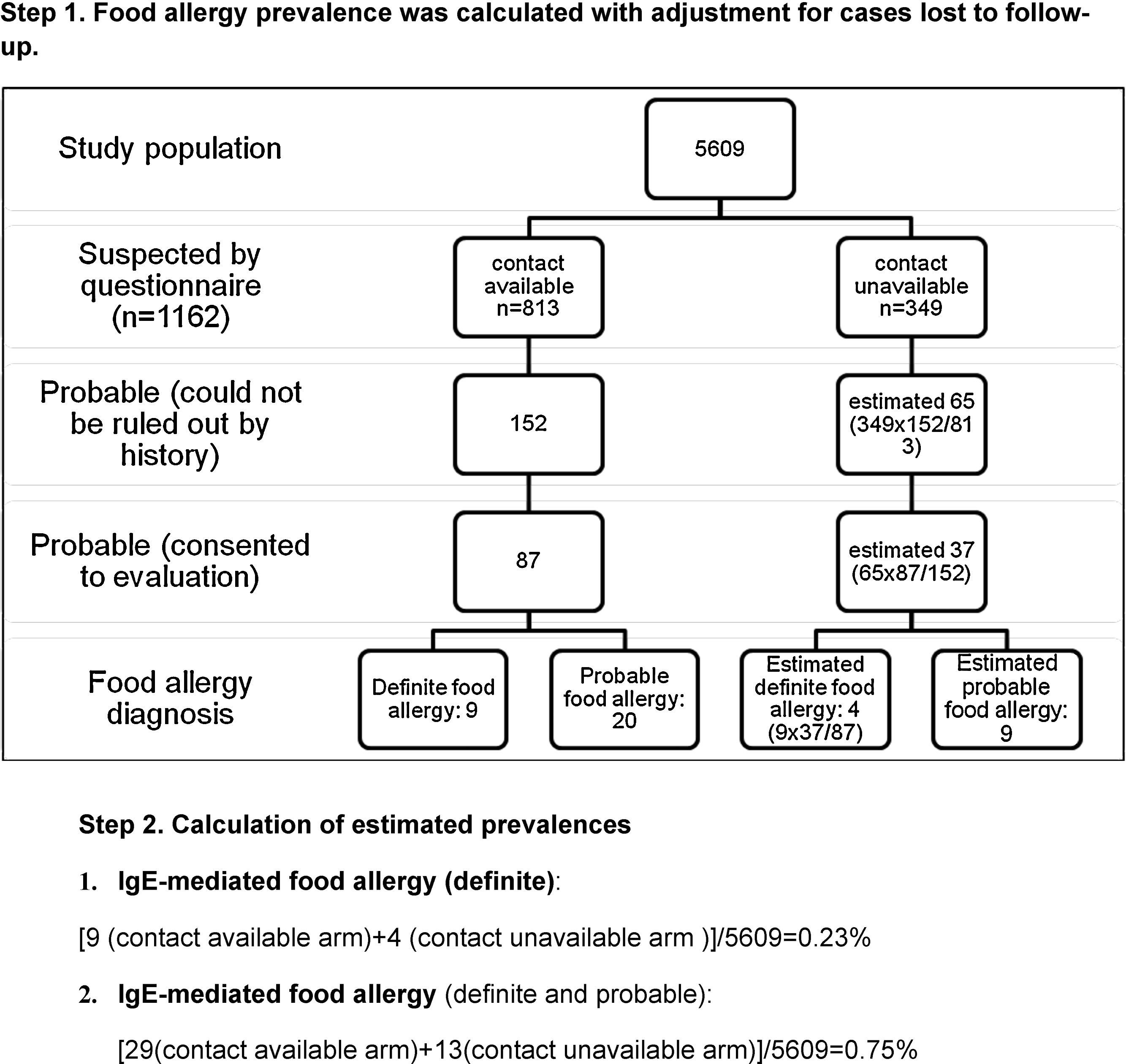
Further evaluation implementing food challenge testing proved IgE-mediated food allergy in 10% (9/87) of those diagnosed by history alone. Thus, IgE-mediated food allergy prevalence in ISAAC Phase II Study population was estimated to be at least 0.16±0.11% (9/5609, 95% CI).
DiscussionISAAC Phase II study was the first study to encompass such a large number of children in the region with the aim of assessing allergic disease burden. Parental reported food allergy prevalence was 20.2±0.94% (1408/6963, 95% CI), the sensitisation rate to food allergens was 5.9±0.6% (361/6963, 95% CI). IgE-mediated food allergy prevalence was estimated to be at least 0.16±0.11% (9/5609, 95% CI) in ISAAC Phase II study group. Allergenic foods in nine patients diagnosed as having food allergy were unique to this study [walnut (n=3), beef meat (n=2), hen's egg (n=1), peanut (n=1), hazelnut (n=1), peach (n=1), spinach (n=1), cheese (n=1) and kiwi (n=1)]. Thus, we conclude that while prevalence was within the previously reported range, there might be regional differences in allergenic food profile.
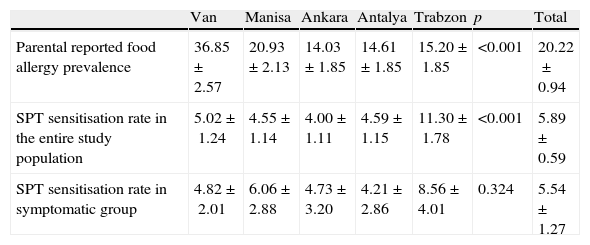
Zuberbier et al.11 addressed 13,300 Berlin inhabitants of adult age by questionnaire and reported lifetime self-reported food allergy prevalence as 34.9%. Telephone interview of 11,816 Istanbul (Turkey) inhabitants of adult age group yielded a lifetime self-reported food allergy prevalence of 9.5% in a recent study by Gelincik et al.13 Osterballe et al.5 investigated a cohort of 1272 young adults and calculated reported food hypersensitivity prevalence as 19.6%. Orhan et al.12 assessed 2739 school children (6–9-year olds) from the Eastern Black Sea region of Turkey and estimated parental reported food allergy prevalence as 5.7%. Parental reported food allergy prevalence in Trabzon (a central city in the Eastern Black Sea region, Turkey) in ISAAC Phase II population was 15% (Table 3 in Appendix B for findings by study centre), which is somewhat higher. This discrepancy might have resulted from different age groups assessed or different response rates to questionnaire. Systematic review by Rona et al.3 on the topic indicates similar reported food allergy prevalence in the range of 3–35% for any food.
ISAAC Phase II study demonstrated that parents most frequently blamed hen's egg for causing allergy (Fig. 2A). Parental reported food allergy prevalence for common food allergens was compatible with ranges stated in systematic reviews3,4 on the topic.
Prevalence of sensitisation to food allergens (5.9%) on skin prick test in ISAAC Phase II study population was consistent with previous studies. Obeng et al.24 performed skin prick testing with peanut and six fruits in 1714 Ghanaian children 5–16 years of age and reported the sensitisation rate as 5%. Venter et al.25 followed prospectively a birth cohort of 969 children on the Isle of Wight, and reported cumulative sensitisation prevalence to cow's milk, hen's egg, whey, sesame, and fish at ages 1–3 as 5.3%. Specific IgE measurements at ages 0, 1, 2, 3, 5 were available in 216 children from MAS birth cohort and sensitisation point prevalence was estimated as 10% during first 6 years of life.26 Sensitisation rates for ISAAC Phase II study population did not significantly differ from sensitisation rates for symptomatic subgroup of cases (cases that reported a symptom upon food ingestion). In contrast to this finding, ranges reported for sensitisation rates in symptomatic subgroup vs. general population in systematic review by Rona et al.3 were 2% to 5% vs. 7% to 17%. Orhan et al.12 reported a quite high sensitisation rate (33.1%) in symptomatic subset of patients compared to rates (8.6%) for the same region in ISAAC Phase II study (Table 3 in Appendix B). But since skin prick testing was not available in all patients in the study by Orhan et al.,12 sensitisation prevalence for the paediatric population in the Eastern Black Sea region (Turkey) could not be deduced from this study. Sensitisation rate for general population in Trabzon province was 11.3% in our study. Most common foods that caused a positive SPT reaction in ISAAC Phase II study population were walnut, cow's milk and peanut (Fig. 2B). While hen's egg, cocoa and cow's milk most commonly resulted in positive SPT in the study by Orhan et al.,12 hen's egg and tomato were most commonly encountered in adults in the study by Gelincik et al.13 Since inhalant antigen sensitisation rates increase with age,26 adults are more likely to have sensitisation to fruit and vegetable antigens, which cross-react with birch and grass pollens,27 and this is reflected by SPT and spIgE findings of a study conducted on adults.13
Despite high reported food allergy rate, proven food allergy prevalence estimated in ISAAC Phase II study population was somewhat lower than that reported previously for various populations.3–6,11,25 This might have resulted because of the loss to follow-up of potential food allergic cases, thus we decided to apply correction for this factor in order to estimate the range for IgE-mediated food allergy prevalence. Assuming that the outcome of questionnaire holders who could not be contacted via telephone is similar (thus four extra patients would have been diagnosed), IgE-mediated food allergy prevalence is estimated as 0.23±0.13% (13/5609, 95% CI) (Appendix C). Assuming that patients who declined complete investigation have food allergy (because the probability of food allergy is expected to be higher in these patients than in the general population), IgE-mediated food allergy prevalence (definite and probable) in ISAAC Phase II Study population could have been as high as 0.75%*±0.23% (42*/5609, 95% CI) (Appendix C). We found that these estimates were within the range reported for Turkish population.12,13 Gelincik et al.13 estimated IgE-mediated food allergy prevalence in adulthood as 0.1%, while it was 0.8% for primary schoolchildren in the study by Orhan et al.12 Osterballe et al.5 investigated a cohort of 1272 young adults with oral food challenge tests and reported food allergy prevalence as 1.7%. The same investigator estimated confirmed food allergy prevalence in the paediatric population as 2.3% for 3-year olds and 1% for children older than 3 years of age.16
In addition, spectrum of allergenic foods was different from previous studies, which may be related to geographic and cultural factors unique to this region. Recent reports underline the presence of region specific allergens beside common childhood allergens (egg, peanut, soy and wheat) and pollen associated group 2 allergens in adulthood. Gelincik et al.13 reported hen's egg, tomato, black pepper, red chilli, food additives and chocolate as the most common food items that caused positive reaction on oral challenge testing. This spectrum was somehow different for children in the study by Orhan et al.12 who reported chocolate, hen's egg, beef, and cow's milk as the most common foods accused of food allergy. A recent study from Australia defined raw eggs, peanut and sesame as the most common foods to which infants reacted on challenge.28 Kajosaari et al.14 defined citrus fruits, tomato, hen's egg, strawberry and fish as the most allergenic foods by history, provocation and elimination performed at home in 866 Finnish children 1–6 years of age. A group of investigators from Israel found cow's milk, egg, sesame and soy as the most allergenic.29 Some authors speculate that sesame allergy in Middle East region might be related to early introduction of sesame oil based product halvah into diet.9 Moreover, there is evidence that sesame allergy is an emerging global problem.30 Rance reported mustard as an important evolving allergen in French children.7,8 A large population-based questionnaire survey was conducted in two Asian countries, Singapore and Philippines, which included 23,425 respondents of paediatric age. Although investigators relied on history alone to diagnose IgE-mediated food allergy, they concluded that while shellfish allergy is more common among native children, compared to children born outside Asia, tree nut and peanut allergy are more common in the latter.15 Beef meat was persistently found among common allergic foods both in our study and in the study by Orhan et al.12 Although Anatolian region is well known for its cuisine with abundant meat, beef is also heavily consumed in central and western Europe, where beef allergy has been concluded to be rare.31 All these geographic differences require further investigation, which will shed light on evolvement of food allergy in respect to environment.
The major limitation of our study is suboptimal participation rate and a relatively long time lapse between ISAAC Phase II study and the telephone interview phase. If it were shorter, contact rates, participation rates and compliance to challenge tests would be higher. Moreover, during this period, some subjects might have outgrown their allergies, and some developed new allergies. Although every effort was shown to prevent loss to follow-up, this remains an important issue in population-based studies,11,13 thus authors agree that only crude estimates can be drawn from these results. Time and effort consuming nature of food challenges is also an important reason for discrepancy between planned and performed procedures. Another important drawback of our study is that DBPCCT was not available for all the suspected patients.
Turkey is a developing country and has a very heterogeneous population in terms of socioeconomic and educational status across its length from east to west. This renders conduction of population-based studies difficult, especially if the study is to involve a city from an underdeveloped part of the country. Limitations of the current study are predictable under these circumstances. Because data on epidemiology of food allergy for paediatric age group in Turkey is limited to a regional study by Orhan et al.,12 despite its limitations, this is the first multicentre study recruiting a large population of children from different geographic regions of Turkey. The results of this study are valuable in this aspect, and enable us to draw conclusions on reported food allergy prevalence, food allergen sensitisation rate and proven IgE-mediated food allergy prevalence. Finding of high reported food allergy rate and low proven food allergy prevalence was in line with results of previous studies. Furthermore, this study shows us that the allergenic food profile might differ for different geographic regions. Further investigation into the field with well-planned studies implementing double-blind placebo-controlled challenge tests is essential.
Ethical disclosuresPatients’ data protectionConfidentiality of data. The authors declare that they have followed the protocols of their work centre on the publication of patient data and that all the patients included in the study have received sufficient information and have given their informed consent in writing to participate in that study.
Right to privacy and informed consentRight to privacy and informed consent. The authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchProtection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this investigation.
Conflict of interestThe authors have no conflict of interests to declare.
We are indebted to Dr. İlke Kılıç (Karadeniz Technical University, Faculty of Medicine, Pediatric Allergy Division, Trabzon, Turkey), Dr. Ahmet Türkeli (Celal Bayar University, Faculty of Medicine, Pediatric Pulmonology and Allergy Unit, Manisa, Turkey), Dr. Betül Büyüktiryaki (Hacettepe University, Faculty of Medicine, Pediatric Allergy and Asthma Unit, Ankara, Turkey), Dr. Serkan Filiz (Akdeniz University, Faculty of Medicine, Pediatric Immunology and Allergy Unit, Antalya, Turkey), Dr. Recep Sancak (Ondokuz Mayıs University, Faculty of Medicine, Pediatric Allergy Unit, Samsun, Turkey) and Dr. Yakup Canıtez (Uludağ University, Faculty of Medicine, Pediatric Allergy Unit, Bursa, Turkey) who helped us to evaluate patients at the allergy departments. The processing of data would have been impossible without the supporting attitude of Dr. Banu Çakır (Hacettepe University, Faculty of Medicine, Public Health Department, Ankara, Turkey) and Dr. Pınar Özdemir (Hacettepe University, Faculty of Medicine, Biostatistics Department, Ankara, Turkey). We are also very grateful to nurses and technicians at allergy departments who helped us with diagnostic and laboratory procedures.
See Table 3.
| Van | Manisa | Ankara | Antalya | Trabzon | p | Total | |
| Parental reported food allergy prevalence | 36.85±2.57 | 20.93±2.13 | 14.03±1.85 | 14.61±1.85 | 15.20±1.85 | <0.001 | 20.22±0.94 |
| SPT sensitisation rate in the entire study population | 5.02±1.24 | 4.55±1.14 | 4.00±1.11 | 4.59± 1.15 | 11.30±1.78 | <0.001 | 5.89± 0.59 |
| SPT sensitisation rate in symptomatic group | 4.82±2.01 | 6.06±2.88 | 4.73±3.20 | 4.21±2.86 | 8.56±4.01 | 0.324 | 5.54±1.27 |