Familial Mediterranean fever (FMF) is the most common auto-inflammatory disease and is characterized by self-limiting episodes of fever and polyserositis. The aim of this study was to determine the atopic clinical findings associated with the MEFV gene.
MethodsA retrospective chart review was conducted of pediatric patients who had received a diagnosis of familial Mediterranean fever between August 2015 and November 2018.
ResultsA total of 454 patients with familial Mediterranean fever were evaluated. The median age of diagnosis was 60 months (min–max: 6–228) and the percentage of patients who were male was 57.5%. A MEFV gene mutation was determined in 310 (68.3%) children. The most frequent genetic mutation was a R202Q heterozygote mutation, which was found in 95 patients (20.9%). When compared with MEFV-negative patients, elevation of serum amyloid A and fibrinogen levels during an episode of FMF was found to occur more frequently in MEFV-positive patients (p=0.019 and 0.027, respectively). Male gender, cigarette exposure, and a younger diagnosis age were seen more frequently in patients who had episodes with fever (p=0.039, 0.022, and 0.001, respectively). Chronic cough with sputum and persistent purulent rhinitis were more frequent in the group which did not experience fever episodes (p=0.003 and 0.002, respectively).
ConclusionsWhile being a periodic fever syndrome, familial Mediterranean fever also presents as a multisystemic disease with heterogeneous clinical symptoms. Severe atopic diseases and recurrent respiratory tract infections are characteristic features of this disease.
Familial Mediterranean fever (FMF) is an autosomal recessive auto-inflammatory disease that occurs worldwide and predominantly affects populations of Mediterranean origin.1 The highest prevalence of the disease is 1/400–1/1000, in the Turkish population.2 Familial Mediterranean fever is caused by mutations in the MEFV gene coding for pyrin, which is a component of inflammasome and has a role in the inflammatory response and production of interleukin-1β. Self-limiting inflammatory attacks of fever and polyserositis, along with a high acute-phase response, is the typical phenotype expected in FMF. The most significant complication of FMF is amyloidosis, which requires long-term treatment with colchicine.3
The diagnosis of FMF relies mainly on clinical findings; a molecular analysis of the MEFV gene provides genetic confirmation.4
Periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome is considered the most common auto-inflammatory disease in childhood. Episodes usually last 3–7 days and recur at intervals of 2–8 weeks, with results being highly predictable in most pediatric cases. Initially, it was believed that PFAPA syndrome was a disorder limited to childhood, but an increasing number of reports has clearly proven that it can also affect non-pediatric patients at different ages.5 According to Federici et al. corticosteroids can reliably abort a PFAPA flare to the point that a failure to respond within a few hours should bring doubt to the diagnosis of a PFAPA syndrome.6,7
The aim of this study was to determine the atopic clinical findings associated with the MEFV gene.
Materials and methodsStudy populationThis retrospective study was conducted at the Gaziosmanpasa Taksim Education and Research Hospital's (Istanbul, Turkey) Pediatric Allergy and Immunology Department between August 2015 and November 2018. Patients admitted to the pediatric allergy and immunology polyclinic with classic symptoms of FMF (e.g., recurrent fever, abdominal pain) were included in the study. Almost all of the patients had classic symptoms of FMF. Serum amyloid (SAA) level, serum fibrinogen level, erythrocyte sedimentation rates, C-reactive protein, leukocyte counts, total lymphocyte counts, total neutrophil counts, and serum immunoglobulin level were evaluated during the FMF episodes. In patients who did not have classical symptoms, SAA and fibrinogen levels were examined in the symptomatic period (e.g., severe atopic disease, and recurrent respiratory tract infections). MEFV gene analysis was conducted on patients whose aforementioned values were high. Some diagnostic criteria were determined for these patients who showed clinical variety heterogeneously. After the patients were diagnosed with familial Mediterranean fever with these diagnostic criteria, they were followed with colchicine therapy clinically. The files of these patients were then examined retrospectively.
DefinitionsFever: Fever was described as an axillary temperature higher than 38°C.
Subfebrile fever: Subfebrile fever was described as an axillary temperature between 37.5°C and 38°C.
Complete PFAPA syndrome: Presence of fever, cervical adenitis, and aphthous stomatitis with tonsillitis, pharyngitis, or tonsillopharyngitis.
Incomplete PFAPA syndrome: Presence of fever with tonsillitis, pharyngitis, or tonsillopharyngitis with at least one of the symptoms of either cervical adenitis or aphthous stomatitis.
Recurrent tonsillitis, pharyngitis, or tonsillopharyngitis: Patients who had more than 10 episodes of tonsillitis, pharyngitis, or tonsillopharyngitis per year. Patients who were otherwise healthy and did not have cervical adenitis or aphthous stomatitis with an episode of tonsillopharyngitis.
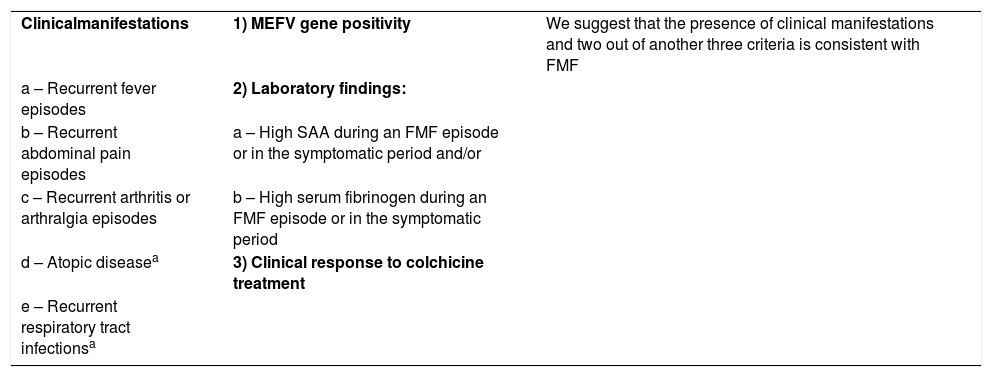
Diagnostic criteria for FMF:
Current criteria set for the diagnosis of familial Mediterranean fever in childhood.
Criteria description:
Fever axillary temperature of >38°C (6–72h of duration, ≥3 attacks)
Abdominal pain (6–72h of duration, ≥3 attacks)
Chest pain (6–72h of duration, ≥3 attacks)
Arthritis (6–72h of duration, ≥3 attacks, oligoarthritis)
Family history of FMF (7)
Based on these criteria, the following criteria were used in the present study:.
| Clinicalmanifestations | 1) MEFV gene positivity | We suggest that the presence of clinical manifestations and two out of another three criteria is consistent with FMF |
| a – Recurrent fever episodes | 2) Laboratory findings: | |
| b – Recurrent abdominal pain episodes | a – High SAA during an FMF episode or in the symptomatic period and/or | |
| c – Recurrent arthritis or arthralgia episodes | b – High serum fibrinogen during an FMF episode or in the symptomatic period | |
| d – Atopic diseasea | 3) Clinical response to colchicine treatment | |
| e – Recurrent respiratory tract infectionsa |
a These symptoms alone have no diagnostic value. They should be evaluated together with other symptoms.
Each sample was screened by direct sequencing for mutations located across the entire MEFV gene (50.0% of patients) or in exons 2, 3, 5, and 10 of the MEFV gene (50.0% of patients). Genomic DNA was extracted from ethylenediamine-tetraacetic acid-anticoagulated whole-blood samples using a Genomic DNA Purification Kit (Invitrogen, Carlsbad, CA, USA). Following selective amplification of whole exons or exons 2, 3, 5, and 10 with specific primers (primer sequences available upon request), polymerase chain reaction products were run in a 2% agarose gel containing ethidium bromide and visualized under UV light by an imaging system (Syngene InGenius, Cambridge, UK). Sequencing was then performed using the BigDye Terminator v3.1 Cycle Sequencing Kit and an ABI 3500 XL Genetic Analyzer (Applied-Biosystems, Foster City, CA, USA).
Four hundred and fifty-four patients with FMF were included in the study. Demographic data such as the age of onset of FMF, diagnosis age, regular breast-milk intake (>6 months), family history of atopy, family history of periodic fever, consanguinity, tobacco exposure, interval between episodes, episode duration, atopic components and severity of atopic disease, and other clinical symptoms were obtained from the electronic files of the patients. Laboratory-result data were obtained from patient files.
Statistical analysisAll statistical analyses were performed using the Statistical Package for Social Sciences for Windows 15.0 (SPSS, version 15). All data are expressed as median, mean±standard deviation, or percentage. A Chi-squared test, Mann–Whitney U test, Kruskal–Wallis test, and ANOVA test were used as required, and values of p<0.05 were accepted as statistically significant.
Ethical considerationsEthical approval was granted in decision no. 2018/115 (dated 10.01.2018) by the Gaziosmanpasa Taksim Education and Research Hospital's Ethics Committee of Medical Research.
ResultsDemographic dataFour hundred and fifty-four patients (261 boys and 193 girls) diagnosed with FMF were followed-up in our clinic. While the median age of the patients at diagnosis was 60 months (min–max: 6–228 months), the median age of symptom onset was 12 months (min–max, 0–156 months). A total of 95.3% of the patients had at least one atopic component, while 89.6% had at least one infectious/auto-inflammatory component. Demographic data of the patients are summarized in Table 1.
Demographics of children with familial Mediterranean fever.
| n (%) | |
|---|---|
| Male | 261 (57.5) |
| Diagnosis age, median, (min-max), month | 60 (6–228) |
| Disease onset age, median, (min-max) month | 12 (0–156) |
| Breast-feeding, regular (>6 months) | 371 (81.7) |
| Tobacco exposure | 241 (53.1) |
| History of atopic disease, in family | 277 (61.0) |
| Consanguinity | 117 (25.8) |
| MEFV variant | 310 (68.3) |
| Fever (>38°C) | 322 (70.9) |
| Interval between episodes, weeks | |
| 2w | 172 (37.9) |
| 4w | 157 (34.6) |
| Irregular | 49 (10.7) |
| 3w | 40 (8.8) |
| 6–8w | 31 (6.8) |
| 1w | 5 (1.1) |
| Episode duration, days | |
| 1–3d | 182 (40.1) |
| 4–7d | 136 (30.0) |
| 8–14d | 100 (22.0) |
| Irregular | 36 (7.9) |
| Atopic component | 435 (95.8) |
| Number of asthma/wheezing attacks, year | |
| >12 | 172/362 (47.5) |
| 4–12 | 138/362 (38.1) |
| 1–3 | 52/362 (14.4) |
| Severity of allergic rhinitis | |
| Severe | 187/342 (54.7) |
| Moderate | 123/342 (36.0) |
| Mild | 32/342 (9.4) |
| Severity of atopic dermatitis | |
| Mild | 49/103 (47.5) |
| Moderate-severe | 54/103 (52.5) |
| Skin prick test positivity | 226/312 (72.4) |
| Acarus | 106/312 (34.0) |
| Pollen | 20/312 (6.4) |
| Multiple inhalant | 100/312 (32.1) |
| Immunologic components | 407 (89.6) |
| Respiratory components | 381 (83.9) |
| Gastrointestinal components | 312 (68.7) |
| Musculoskeletal components | 228 (50.2) |
| Skin components | 151 (33.3) |
| Hematological components | 112 (24.7) |
| Serum IgE elevation, (0–100) IU/ml | 184/418 (44.0) |
| Serum IgE level, median (min-max), IU/ml | 73 (1–2728) |
| Eosinophilia, (0–5) % | 93/445 (20.4) |
| Eosinophil level, median (min-max), % | 2.0 (0–23.0) |
| Serum amyloid A elevation during episode, (<0.5) mg/dL | 331/392 (84.4) |
| Serum amyloid A level, median (min-max), mg/dL | 4.6 (0.7–213) |
| Serum fibrinogen elevation during episode, (180–350) mg/dL | 129/425 (30.4) |
| Serum fibrinogen level, median (min-max), mg/dL | 324 (5–980) |
| C-reactive protein elevation during episode, (0–5) mg/L | 274/391 (70.1) |
| C-reactive protein level, median (min–max), mg/L | 10.0 (0–264) |
| Leukocytosis during episode, (4.1–11.0) 103/μL | 132/447 (29.5) |
| Leukocyte count, median (min–max), 103/μL | 9.6 (1.1–51.9) |
| Erythrocyte sedimentation rate elevation during episode, (0–20) mm/h | 193/347 (55.6) |
| Erythrocyte sedimentation rate level, median (min-max), mm/h | 22 (1–107) |
| Response to prednisolone during episode | 58/80 (72.5) |
| Response to colchicine | 221/232 (95.3) |
| Hypogammaglobulinemia | 68/419 (16.2) |
| Total | 454 (100.0) |
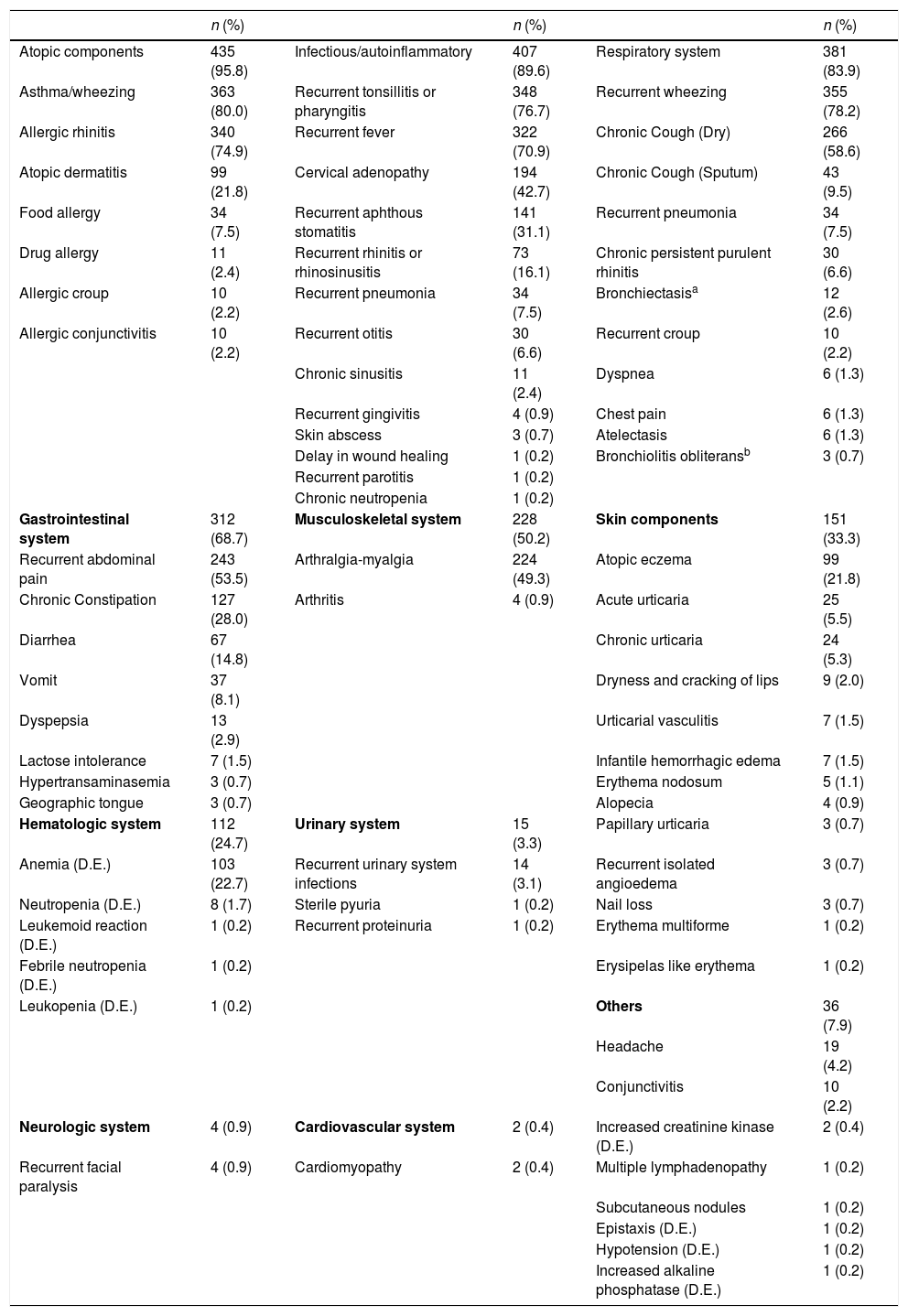
The most frequently seen clinical findings were atopic (95.3%) and immunological (89.6%). The most frequent atopic diseases were asthma/wheezing in 363 (80.0%) patients and allergic rhinitis in 340 (74.9%) patients. The most frequent immunological component was recurrent tonsillopharyngitis, which was found in 348 (76.7%) patients. Three hundred and eighty-one (83.9%) patients were found to have respiratory system involvement. Three hundred and fifty-five (78.2%) patients were found to have wheezing, while 266 (58.6%) had a dry cough due to asthma, 43 (9.5%) had chronic cough with sputum, 34 (7.5%) had recurrent pneumonia, 30 (6.6%) had persistent purulent rhinitis, 12 (2.6%) had bronchiectasis, and three (0.7%) had bronchiolitis obliterans. Three hundred and twelve (68.7%) patients were found to have gastrointestinal involvement. The most frequent gastrointestinal symptoms were abdominal pain (found in 243 (53.5%) patients) and chronic constipation (127 (28.0%) patients). Clinical data for these patients are summarized in Table 2.
Clinical manifestations of children with familial Mediterranean fever.
| n (%) | n (%) | n (%) | |||
|---|---|---|---|---|---|
| Atopic components | 435 (95.8) | Infectious/autoinflammatory | 407 (89.6) | Respiratory system | 381 (83.9) |
| Asthma/wheezing | 363 (80.0) | Recurrent tonsillitis or pharyngitis | 348 (76.7) | Recurrent wheezing | 355 (78.2) |
| Allergic rhinitis | 340 (74.9) | Recurrent fever | 322 (70.9) | Chronic Cough (Dry) | 266 (58.6) |
| Atopic dermatitis | 99 (21.8) | Cervical adenopathy | 194 (42.7) | Chronic Cough (Sputum) | 43 (9.5) |
| Food allergy | 34 (7.5) | Recurrent aphthous stomatitis | 141 (31.1) | Recurrent pneumonia | 34 (7.5) |
| Drug allergy | 11 (2.4) | Recurrent rhinitis or rhinosinusitis | 73 (16.1) | Chronic persistent purulent rhinitis | 30 (6.6) |
| Allergic croup | 10 (2.2) | Recurrent pneumonia | 34 (7.5) | Bronchiectasisa | 12 (2.6) |
| Allergic conjunctivitis | 10 (2.2) | Recurrent otitis | 30 (6.6) | Recurrent croup | 10 (2.2) |
| Chronic sinusitis | 11 (2.4) | Dyspnea | 6 (1.3) | ||
| Recurrent gingivitis | 4 (0.9) | Chest pain | 6 (1.3) | ||
| Skin abscess | 3 (0.7) | Atelectasis | 6 (1.3) | ||
| Delay in wound healing | 1 (0.2) | Bronchiolitis obliteransb | 3 (0.7) | ||
| Recurrent parotitis | 1 (0.2) | ||||
| Chronic neutropenia | 1 (0.2) | ||||
| Gastrointestinal system | 312 (68.7) | Musculoskeletal system | 228 (50.2) | Skin components | 151 (33.3) |
| Recurrent abdominal pain | 243 (53.5) | Arthralgia-myalgia | 224 (49.3) | Atopic eczema | 99 (21.8) |
| Chronic Constipation | 127 (28.0) | Arthritis | 4 (0.9) | Acute urticaria | 25 (5.5) |
| Diarrhea | 67 (14.8) | Chronic urticaria | 24 (5.3) | ||
| Vomit | 37 (8.1) | Dryness and cracking of lips | 9 (2.0) | ||
| Dyspepsia | 13 (2.9) | Urticarial vasculitis | 7 (1.5) | ||
| Lactose intolerance | 7 (1.5) | Infantile hemorrhagic edema | 7 (1.5) | ||
| Hypertransaminasemia | 3 (0.7) | Erythema nodosum | 5 (1.1) | ||
| Geographic tongue | 3 (0.7) | Alopecia | 4 (0.9) | ||
| Hematologic system | 112 (24.7) | Urinary system | 15 (3.3) | Papillary urticaria | 3 (0.7) |
| Anemia (D.E.) | 103 (22.7) | Recurrent urinary system infections | 14 (3.1) | Recurrent isolated angioedema | 3 (0.7) |
| Neutropenia (D.E.) | 8 (1.7) | Sterile pyuria | 1 (0.2) | Nail loss | 3 (0.7) |
| Leukemoid reaction (D.E.) | 1 (0.2) | Recurrent proteinuria | 1 (0.2) | Erythema multiforme | 1 (0.2) |
| Febrile neutropenia (D.E.) | 1 (0.2) | Erysipelas like erythema | 1 (0.2) | ||
| Leukopenia (D.E.) | 1 (0.2) | Others | 36 (7.9) | ||
| Headache | 19 (4.2) | ||||
| Conjunctivitis | 10 (2.2) | ||||
| Neurologic system | 4 (0.9) | Cardiovascular system | 2 (0.4) | Increased creatinine kinase (D.E.) | 2 (0.4) |
| Recurrent facial paralysis | 4 (0.9) | Cardiomyopathy | 2 (0.4) | Multiple lymphadenopathy | 1 (0.2) |
| Subcutaneous nodules | 1 (0.2) | ||||
| Epistaxis (D.E.) | 1 (0.2) | ||||
| Hypotension (D.E.) | 1 (0.2) | ||||
| Increased alkaline phosphatase (D.E.) | 1 (0.2) |
D.A.: During episode.
a Sweat test normal in all patients and two patients also have primary immunodeficiency.
b Two patients have primary immunodeficiency.
A MEFV gene mutation was determined in 310 (68.3%) of the children. One hundred and ninety-three (42.5%) patients were found to have a heterozygote mutation, 81 (17.8%) were found to have a compound heterozygote mutation, and 36 (7.9%) were found to have a homozygote mutation. The most frequent genetic mutation was a R202Q heterozygote mutation, found in 95 patients (20.9%). No mutation of the MEFV gene was detected in 144 patients (31.7%). Variants of the MEFV gene observed in the patients are presented in Table 3.
MEFV mutations in children with familial Mediterranean fever.
| MEFV variants | n |
|---|---|
| R202Q HETEROZYGOUS | 95 |
| E148Q HETEROZYGOUS | 41 |
| R202Q HETEROZYGOUS M694V HETEROZYGOUS | 24 |
| R202Q HOMOZYGOUS | 17 |
| V726A HETEROZYGOUS | 17 |
| R202Q HETEROZYGOUS E148Q HETEROZYGOUS | 13 |
| R202Q HETEROZYGOUS V726A HETEROZYGOUS | 7 |
| P369S HETEROZYGOUS R408Q HETEROZYGOUS | 6 |
| M680I HETEROZYGOUS | 5 |
| R202Q HOMOZYGOUS M694V HETEROZYGOUS | 5 |
| rs224205 HETEROZYGOUS | 5 |
| R202Q HETEROZYGOUS I591T HETEROZYGOUS | 4 |
| K695R HETEROZYGOUS | 4 |
| G304R HETEROZYGOUS | 3 |
| R202Q HETEROZYGOUS M680I HETEROZYGOUS | 3 |
| M680I HOMOZYGOUS | 2 |
| R202Q HETEROZYGOUS rs224205 HOMOZYGOUS | 2 |
| A744S HETEROZYGOUS | 2 |
| E148Q HOMOZYGOUS | 2 |
| R761H HETEROZYGOUS | 2 |
| R202Q HETEROZYGOUS E148Q HETEROZYGOUS M694V HETEROZYGOUS | 2 |
| R202Q HETEROZYGOUS M694V HETEROZYGOUS rs224205 HETEROZYGOUS | 2 |
| rs224205 HOMOZYGOUS | 2 |
| S141I HETEROZYGOUS | 2 |
| S619C HETEROZYGOUS | 2 |
| T267I HETEROZYGOUS | 2 |
| Q522H HETEROZYGOUS | 1 |
| R515S HETEROZYGOUS | 1 |
| E148Q HETEROZYGOUS R408Q HETEROZYGOUS P369S HETEROZYGOUS | 1 |
| E520V HETEROZYGOUS | 1 |
| D510N HETEROZYGOUS | 1 |
| R202Q HETEROZYGOUS R761H HETEROZYGOUS | 1 |
| R202Q HETEROZYGOUS rs224205 HETEROZYGOUS | 1 |
| L649V HETEROZYGOUS | 1 |
| E148Q HETEROZYGOUS R408Q HETEROZYGOUS P369S HETEROZYGOUS R744S HETEROZYGOUS | 1 |
| R202Q HETEROZYGOUS A448V HETEROZYGOUS | 1 |
| R202Q HETEROZYGOUS E148Q HETEROZYGOUS E520V HETEROZYGOUS | 1 |
| M694U HETEROZYGOUS | 1 |
| I591T HETEROZYGOUS | 1 |
| R202Q HETEROZYGOUS M680I HETEROZYGOUS M694 V HETEROZYGOUS | 1 |
| M694V HETEROZYGOUS | 1 |
| R202Q HETEROZYGOUS M694V HETEROZYGOUS V726A HETEROZYGOUS | 1 |
| R202Q HETEROZYGOUS P369S HETEROZYGOUS | 1 |
| R202Q HETEROZYGOUS V726A HETEROZYGOUS M694V HETEROZYGOUS | 1 |
| R202Q HETEROZYGOUS Z591T HETEROZYGOUS | 1 |
| M608I HETEROZYGOUS K965R HETEROZYGOUS | 1 |
| R202Q HOMOZYGOUS I591T HETEROZYGOUS | 1 |
| E148Q HETEROZYGOUS V726A HETEROZYGOUS | 1 |
| R202Q HOMOZYGOUS M694V HETEROZYGOUS rs224205 HETEROZYGOUS | 1 |
| R202Q HOMOZYGOUS P369S HETEROZYGOUS R408Q HETEROZYGOUS | 1 |
| R42R HETEROZYGOUS V726A HETEROZYGOUS | 1 |
| R5222405 HOMOZYGOUS | 1 |
| G304R HETEROZYGOUS A744S HETEROZYGOUS | 1 |
| E202Q HETEROZYGOUS | 1 |
| M694V HETEROZYGOUS rs224205 HETEROZYGOUS R202Q HOMOZYGOUS | 1 |
| rs224205 HOMOZYGOUS V726A HETEROZYGOUS | 1 |
| N99N HETEROZYGOUS | 1 |
| S288Y HETEROZYGOUS | 1 |
| S288Y HETEROZYGOUS M680I HETEROZYGOUS | 1 |
| E148Q HETEROZYGOUS rs224205 HETEROZYGOUS | 1 |
| G632S HETEROZYGOUS | 1 |
| TI77I HETEROZYGOUS | 1 |
| E148Q HETEROZYGOUS L110P HETEROZYGOUS | 1 |
| V726A HETEROZYGOUS rs224205 HETEROZYGOUS | 1 |
| E456D HETEROZYGOUS | 1 |
| NEGATIVE | 144 |
| TOTAL | 454 |
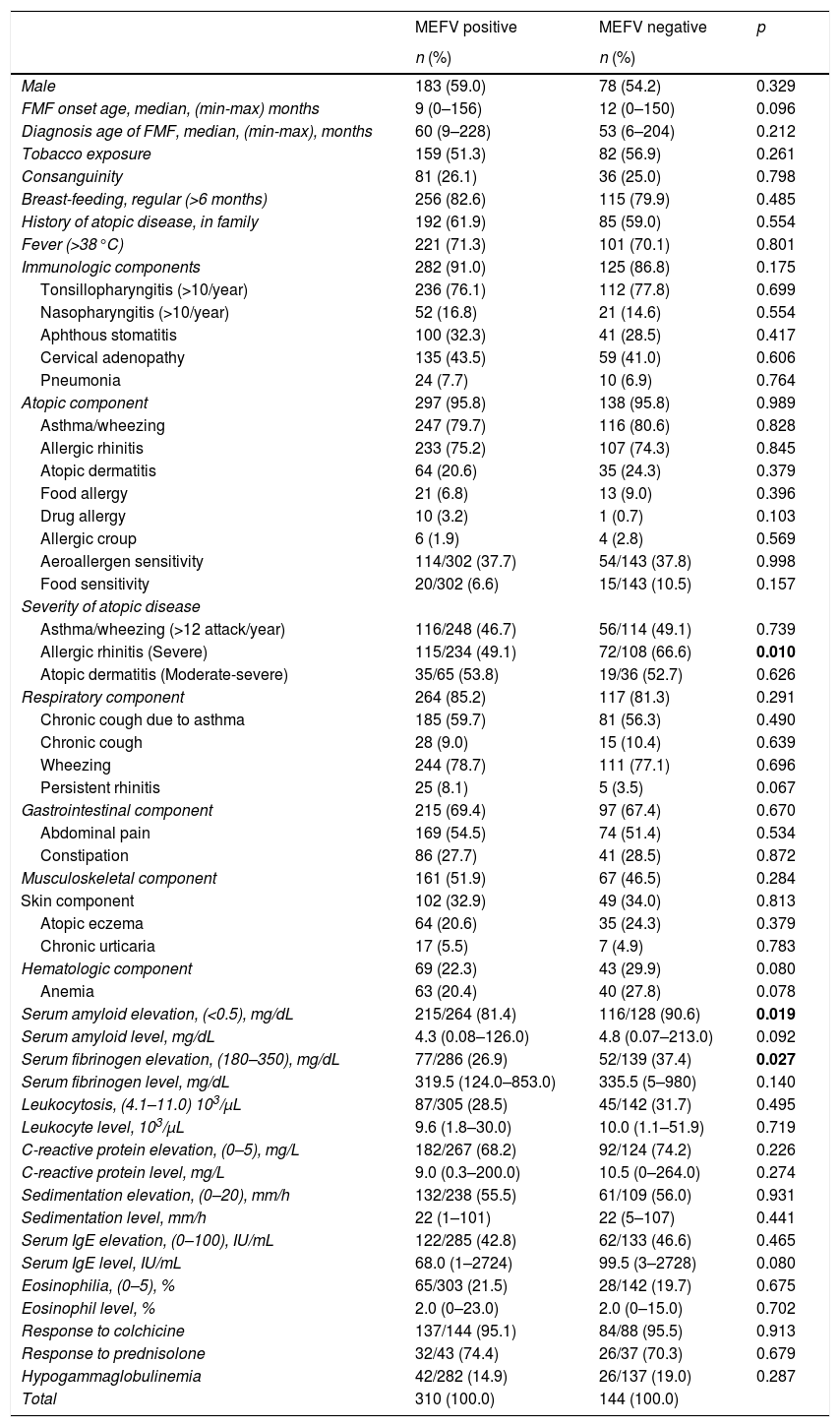
When compared with MEFV-negative patients, elevation of SAA and fibrinogen levels during an episode of FMF were more frequently observed among MEFV-positive patients (p=0.019 and 0.027, respectively). No statistically significant differences were found between MEFV-positive and MEFV-negative patients in terms of gender, onset age, diagnosis age, tobacco exposure, regularity of breast feeding, atopy in the family, consanguinity, fever, clinical symptoms, eosinophilia, serum IgE levels, and other laboratory parameters (p>0.05) (Table 4). No statistically significant differences were found between homozygote, heterozygote, compound heterozygote, and MEFV-negative patients in terms of clinical and laboratory parameters (p>0.05).
Comparison of MEFV positive and MEFV negative children with familial Mediterranean fever.
| MEFV positive | MEFV negative | p | |
|---|---|---|---|
| n (%) | n (%) | ||
| Male | 183 (59.0) | 78 (54.2) | 0.329 |
| FMF onset age, median, (min-max) months | 9 (0–156) | 12 (0–150) | 0.096 |
| Diagnosis age of FMF, median, (min-max), months | 60 (9–228) | 53 (6–204) | 0.212 |
| Tobacco exposure | 159 (51.3) | 82 (56.9) | 0.261 |
| Consanguinity | 81 (26.1) | 36 (25.0) | 0.798 |
| Breast-feeding, regular (>6 months) | 256 (82.6) | 115 (79.9) | 0.485 |
| History of atopic disease, in family | 192 (61.9) | 85 (59.0) | 0.554 |
| Fever (>38°C) | 221 (71.3) | 101 (70.1) | 0.801 |
| Immunologic components | 282 (91.0) | 125 (86.8) | 0.175 |
| Tonsillopharyngitis (>10/year) | 236 (76.1) | 112 (77.8) | 0.699 |
| Nasopharyngitis (>10/year) | 52 (16.8) | 21 (14.6) | 0.554 |
| Aphthous stomatitis | 100 (32.3) | 41 (28.5) | 0.417 |
| Cervical adenopathy | 135 (43.5) | 59 (41.0) | 0.606 |
| Pneumonia | 24 (7.7) | 10 (6.9) | 0.764 |
| Atopic component | 297 (95.8) | 138 (95.8) | 0.989 |
| Asthma/wheezing | 247 (79.7) | 116 (80.6) | 0.828 |
| Allergic rhinitis | 233 (75.2) | 107 (74.3) | 0.845 |
| Atopic dermatitis | 64 (20.6) | 35 (24.3) | 0.379 |
| Food allergy | 21 (6.8) | 13 (9.0) | 0.396 |
| Drug allergy | 10 (3.2) | 1 (0.7) | 0.103 |
| Allergic croup | 6 (1.9) | 4 (2.8) | 0.569 |
| Aeroallergen sensitivity | 114/302 (37.7) | 54/143 (37.8) | 0.998 |
| Food sensitivity | 20/302 (6.6) | 15/143 (10.5) | 0.157 |
| Severity of atopic disease | |||
| Asthma/wheezing (>12 attack/year) | 116/248 (46.7) | 56/114 (49.1) | 0.739 |
| Allergic rhinitis (Severe) | 115/234 (49.1) | 72/108 (66.6) | 0.010 |
| Atopic dermatitis (Moderate-severe) | 35/65 (53.8) | 19/36 (52.7) | 0.626 |
| Respiratory component | 264 (85.2) | 117 (81.3) | 0.291 |
| Chronic cough due to asthma | 185 (59.7) | 81 (56.3) | 0.490 |
| Chronic cough | 28 (9.0) | 15 (10.4) | 0.639 |
| Wheezing | 244 (78.7) | 111 (77.1) | 0.696 |
| Persistent rhinitis | 25 (8.1) | 5 (3.5) | 0.067 |
| Gastrointestinal component | 215 (69.4) | 97 (67.4) | 0.670 |
| Abdominal pain | 169 (54.5) | 74 (51.4) | 0.534 |
| Constipation | 86 (27.7) | 41 (28.5) | 0.872 |
| Musculoskeletal component | 161 (51.9) | 67 (46.5) | 0.284 |
| Skin component | 102 (32.9) | 49 (34.0) | 0.813 |
| Atopic eczema | 64 (20.6) | 35 (24.3) | 0.379 |
| Chronic urticaria | 17 (5.5) | 7 (4.9) | 0.783 |
| Hematologic component | 69 (22.3) | 43 (29.9) | 0.080 |
| Anemia | 63 (20.4) | 40 (27.8) | 0.078 |
| Serum amyloid elevation, (<0.5), mg/dL | 215/264 (81.4) | 116/128 (90.6) | 0.019 |
| Serum amyloid level, mg/dL | 4.3 (0.08–126.0) | 4.8 (0.07–213.0) | 0.092 |
| Serum fibrinogen elevation, (180–350), mg/dL | 77/286 (26.9) | 52/139 (37.4) | 0.027 |
| Serum fibrinogen level, mg/dL | 319.5 (124.0–853.0) | 335.5 (5–980) | 0.140 |
| Leukocytosis, (4.1–11.0) 103/μL | 87/305 (28.5) | 45/142 (31.7) | 0.495 |
| Leukocyte level, 103/μL | 9.6 (1.8–30.0) | 10.0 (1.1–51.9) | 0.719 |
| C-reactive protein elevation, (0–5), mg/L | 182/267 (68.2) | 92/124 (74.2) | 0.226 |
| C-reactive protein level, mg/L | 9.0 (0.3–200.0) | 10.5 (0–264.0) | 0.274 |
| Sedimentation elevation, (0–20), mm/h | 132/238 (55.5) | 61/109 (56.0) | 0.931 |
| Sedimentation level, mm/h | 22 (1–101) | 22 (5–107) | 0.441 |
| Serum IgE elevation, (0–100), IU/mL | 122/285 (42.8) | 62/133 (46.6) | 0.465 |
| Serum IgE level, IU/mL | 68.0 (1–2724) | 99.5 (3–2728) | 0.080 |
| Eosinophilia, (0–5), % | 65/303 (21.5) | 28/142 (19.7) | 0.675 |
| Eosinophil level, % | 2.0 (0–23.0) | 2.0 (0–15.0) | 0.702 |
| Response to colchicine | 137/144 (95.1) | 84/88 (95.5) | 0.913 |
| Response to prednisolone | 32/43 (74.4) | 26/37 (70.3) | 0.679 |
| Hypogammaglobulinemia | 42/282 (14.9) | 26/137 (19.0) | 0.287 |
| Total | 310 (100.0) | 144 (100.0) | |
Chi square and Mann–Whitney U tests were applied. The values written in bold are statistically significant.
In patients with PFAPA syndrome and incomplete PFAPA syndrome, allergic rhinitis was more frequently observed compared to other patients (p=0.020). Fever was more frequent in patients with PFAPA syndrome and incomplete PFAPA syndrome compared to other patients (p=0.001). Aeroallergen sensitivity was more frequent in patients with complete PFAPA syndrome (p=0.025). Total IgE value was higher in patients with complete PFAPA syndrome compared to patients with recurrent tonsillitis (p=0.046). Eosinophil levels were higher in patients with complete PFAPA syndrome compared to patients with incomplete PFAPA syndrome and recurrent tonsillitis (p=0.022) (Table 5).
Comparison of PFAPA variants and PFAPA negative children with familial Mediterranean fever.
| Recurrent tonsillitis | Incomplete PFAPA | Complete PFAPA | PFAPA negative | p | |
|---|---|---|---|---|---|
| n (%) | n (%) | n (%) | n (%) | ||
| Male | 79 (56.4) | 76 (59.4) | 49 (62.0) | 57 (53.3) | 0.638 |
| Diagnosis age of FMF, mean±SD, months | 64.3±4.8 | 61.9±4.0 | 59.5±5.4 | 69.5±6.0 | 0.638 |
| FMF onset age, mean±SD, months | 20.1±3.2 | 15.9±2.4 | 20.3±3.7 | 20.6±4.6 | 0.859 |
| Tobacco exposure | 75 (53.6) | 72 (56.3) | 43 (54.4) | 51 (47.7) | 0.604 |
| Consanguinity | 31 (22.1) | 36 (28.1) | 18 (28.8) | 32 (29.9) | 0.447 |
| Breast-feeding, regular (>6 months) | 111 (79.3) | 111 (86.7) | 63 (79.7) | 86 (80.4) | 0.387 |
| History of atopic disease, in family | 84 (60.0) | 85 (66.4) | 55 (65.8) | 56 (52.3) | 0.123 |
| Fever (>38.0°C) | 96 (68.6) | 109 (85.2) | 71 (89.9) | 46 (43.0) | 0.001 |
| MEFV gene positivity | 95 (67.9) | 84 (65.6) | 56 (70.9) | 75 (70.1) | 0.840 |
| Atopic component | 135 (96.4) | 124 (96.9) | 77 (97.5) | 99 (92.5) | 0.270 |
| Asthma/wheezing | 113 (80.7) | 105 (82.0) | 65 (82.3) | 80 (74.8) | 0.483 |
| Allergic rhinitis | 102 (72.9) | 105 (82.0) | 63 (79.7) | 70 (65.4) | 0.020 |
| Atopic dermatitis | 28 (20.0) | 27 (21.1) | 23 (29.1) | 21 (21.6) | 0.380 |
| Food allergy | 8 (5.7) | 11 (8.6) | 5 (6.3) | 10 (9.3) | 0.671 |
| Drug allergy | 2 (1.4) | 2 (1.6) | 4 (5.1) | 3 (2.8) | 0.336 |
| Allergic croup | 3 (2.1) | 2 (1.6) | 1 (1.3) | 4 (3.7) | 0.628 |
| Aeroallergen sensitivity | 42/138 (30.4) | 48/125 (38.4) | 40/78 (51.3) | 38/104 (36.5) | 0.025 |
| Food sensitivity | 10/137 (7.3) | 9 (7.1) | 6/78 (7.7) | 10/104 (9.6) | 0.898 |
| Respiratory component | 117 (83.6) | 109 (85.2) | 69 (87.3) | 86 (80.4) | 0.606 |
| Chronic cough due to asthma | 75 (53.6) | 82 (64.1) | 53 (67.1) | 56 (52.3) | 0.068 |
| Chronic cough | 12 (8.6) | 12 (9.4) | 3 (3.8) | 16 (15.0) | 0.077 |
| Wheezing | 113 (80.7) | 103 (80.5) | 63 (79.7) | 76 (71.0) | 0.236 |
| Persistent rhinitis | 9 (6.4) | 6 (4.7) | 6 (7.6) | 9 (8.4) | 0.513 |
| Nasopharyngitis (>10/year) | 22 (15.7) | 28 (21.9) | 11 (13.9) | 12 (11.2) | 0.148 |
| Pneumonia | 9 (6.4) | 10 (7.8) | 6 (7.6) | 9 (8.4) | 0.945 |
| Skin component | 44 (31.4) | 37 (28.9) | 29 (36.7) | 41 (38.3) | 0.398 |
| Atopic dermatitis | 28 (20.0) | 27 (21.1) | 23 (29.1) | 21 (19.6) | 0.380 |
| Chronic urticaria | 8 (5.7) | 3 (2.3) | 4 (5.1) | 9 (8.4) | 0.225 |
| Gastrointestinal component | 91 (65.0) | 102 (79.7) | 62 (78.5) | 57 (53.3) | 0.001 |
| Abdominal pain | 70 (50.0) | 88 (68.8) | 47 (59.5) | 38 (35.5) | 0.001 |
| Constipation | 37 (26.4) | 40 (31.3) | 30 (38.0) | 20 (18.7) | 0.025 |
| Musculoskeletal component | 61 (43.6) | 70 (54.7) | 51 (64.6) | 46 (43.0) | 0.007 |
| Hematologic component | 31 (22.1) | 36 (28.1) | 21 (26.6) | 24 (22.4) | 0.626 |
| Anemia | 29 (20.7) | 33 (25.8) | 21 (26.6) | 20 (18.7) | 0.446 |
| Serum amyloid elevation, (<0.5), mg/dL | 107/126 (84.9) | 89/111 (80.2) | 43/53 (81.1) | 92/102 (90.2) | 0.206 |
| Serum amyloid level, mean±SD, mg/dL | 17.6±2.6 | 11.5±2.3 | 26.9±5.3 | 14.1±3.1 | 0.319 |
| Serum fibrinogen elevation, (180–350), mg/dL | 44/133 (33.1) | 39/125 (31.2) | 23/65 (35.4) | 23/102 (22.5) | 0.238 |
| Serum fibrinogen level, mean±SD, mg/dL | 330.1±9.4 | 333.8±15.3 | 340.3±14.8 | 325.5±11.7 | 0.457 |
| Leukocytosis, (4.1–11.0) 103/μL | 43/138 (31.2) | 40/125 (32.0) | 16 (20.3) | 33/105 (31.4) | 0.262 |
| Leukocyte level, mean±SD, 103/μL | 11.6±0.7 | 10.8±0.5 | 11.0±0.6 | 10.1±0.6 | 0.300 |
| C-reactive protein elevation, (0–5), mg/L | 86/120 (71.7) | 79/117 (67.5) | 44/64 (68.8) | 65/90 (72.2) | 0.859 |
| C-reactive protein level, SD, mg/L | 29.9±5.4 | 20.4±3.2 | 28.5±6.1 | 23.4±5.6 | 0.638 |
| Sedimentation elevation, (0–20), mm/h | 69/107 (64.5) | 57/110 (51.8) | 28/56 (50.0) | 39/74 (52.7) | 0.170 |
| Sedimentation level, mean±SD, mm/h | 28.1±2.0 | 22.6±1.9 | 25.6±2.6 | 26.1±2.1 | 0.163 |
| Serum IgE elevation, (0–100), IU/mL | 53/130 (40.8) | 47/115 (40.9) | 40/74 (54.1) | 44/99 (44.4) | 0.256 |
| Serum IgE level, mean±SD, IU/mL | 146.5±38.8 | 198.4±36.4 | 209.1±41.9 | 219.3±68.6 | 0.046 |
| Eosinophilia, (0–5), % | 25/138 (18.1) | 20/124 (16.1) | 20/78 (25.6) | 28/105 (26.7) | 0.137 |
| Eosinophil level, mean±SD, % | 2.44±0.3 | 2.34±0.2 | 3.05±0.5 | 3.72±0.51 | 0.022 |
| Response to colchicine | 73/76 (96.1) | 69/72 (95.8) | 34/37 (91.9) | 45/47 (95.7) | 0.774 |
| Response to prednisolone | 22/29 (75.9) | 16/25 (64.0) | 17/21 (81.0) | 3/5 (60.0) | 0.529 |
| Hypogammaglobulinemia | 24/131 (18.3) | 18/117 (15.4) | 8/74 (10.8) | 18/97 (18.6) | 0.481 |
| Total | 140 (100.0) | 128 (100.0) | 79 (100.0) | 107 (100.0) |
Chi square, Kruskal–Wallis and one-way ANOVA tests were applied. The values written in bold are statistically significant.
Male gender, cigarette exposure, and young diagnosis age were more frequent in patients who had episodes with fever (p=0.039, 0.022, and 0.001, respectively). Recurrent tonsillopharyngitis, aphthous stomatitis, and cervical adenitis were more frequent in patients who had episodes with fever (p=0.001, 0.001, and 0.001, respectively). Allergic rhinitis was more frequent in patients who had episodes with fever (p=0.002). In the patient group with subfebrile fever, chronic cough and persistent rhinitis due to FMF were more frequent (p=0.003 and 0.002, respectively). In the patient group that had episodes with fever, gastrointestinal and musculoskeletal system symptoms were more frequent (p=0.001 and 0.001, respectively). SAA, fibrinogen, and C-reactive protein levels were elevated in patients who had episodes with fever, as compared to patients who had episodes with subfebrile fever (p=0.024, 0.045, 0.042, and 0.016, respectively) (Table 6).
Comparison of children with familial Mediterranean fever according to fever and sub-febrile fever.
| Fever (>38°C) | Sub-febrile fever (<38°C) | p | |
|---|---|---|---|
| n (%) | n (%) | ||
| Male | 195 (60.6) | 66 (50.0) | 0.039 |
| FMF onset age, median, (min-max) months | 9.5 (0–156) | 12 (0–150) | 0.169 |
| Diagnosis age of FMF, median, (min-max), months | 60 (6–228) | 72 (9–204) | 0.001 |
| Tobacco exposure | 182 (56.5) | 59 (44.7) | 0.022 |
| Consanguinity | 85 (26.4) | 32 (24.2) | 0.634 |
| Breast-feeding, regular (>6 months) | 258 (80.1) | 113 (85.6) | 0.170 |
| History of atopic disease, in family | 200 (60.1) | 77 (58.3) | 0.453 |
| MEFV positivity | 221 (68.6) | 89 (67.4) | 0.801 |
| Immunologic components | 307 (95.3) | 100 (75.8) | 0.001 |
| Tonsillopharyngitis (>10/year) | 277 (86.0) | 71 (53.8) | 0.001 |
| Nasopharyngitis (>10/year) | 46 (14.3) | 27 (20.5) | 0.104 |
| Aphthous stomatitis | 120 (37.3) | 21 (15.9) | 0.001 |
| Cervical adenopathy | 163 (50.6) | 31 (23.5) | 0.001 |
| Pneumonia | 21 (6.5) | 13 (9.8) | 0.221 |
| Atopic component | 312 (96.9) | 123 (93.2) | 0.073 |
| Asthma/wheezing | 259 (80.4) | 104 (78.8) | 0.691 |
| Allergic rhinitis | 254 (78.9) | 86 (65.2) | 0.002 |
| Atopic dermatitis | 71 (22.0) | 28 (24.2) | 0.844 |
| Food allergy | 28 (8.7) | 6 (4.5) | 0.127 |
| Drug allergy | 9 (2.8) | 2 (1.5) | 0.421 |
| Allergic croup | 8 (2.5) | 2 (1.5) | 0.523 |
| Aeroallergen sensitivity | 114/316 (36.1) | 54/129 (41.9) | 0.253 |
| Food sensitivity | 28/315 (8.9) | 7/130 (5.2) | 0.212 |
| Severity of atopic disease | |||
| Asthma/wheezing (>12 attack/year) | 126/259 (48.6) | 46/103 (44.6) | 0.544 |
| Allergic rhinitis (Severe) | 134/245 (54.6) | 53/97 (54.6) | 0.892 |
| Atopic dermatitis (Moderate-severe) | 37/72 (51.3) | 17/29 (58.6) | 0.803 |
| Respiratory component | 268 (83.2) | 113 (85.6) | 0.531 |
| Chronic cough due to asthma | 185 (57.5) | 81 (61.4) | 0.442 |
| Chronic cough | 22 (6.8) | 21 (15.9) | 0.003 |
| Wheezing | 256 (79.5) | 99 (75.0) | 0.291 |
| Persistent rhinitis | 14 (4.3) | 16 (12.1) | 0.002 |
| Gastrointestinal component | 242 (75.2) | 70 (53.0) | 0.001 |
| Abdominal pain | 190 (59.0) | 53 (40.2) | 0.001 |
| Constipation | 107 (33.2) | 20 (15.2) | 0.001 |
| Musculoskeletal component | 183 (56.8) | 45 (34.1) | 0.001 |
| Skin component | 105 (32.6) | 46 (34.8) | 0.646 |
| Atopic eczema | 71 (22.0) | 28 (21.2) | 0.844 |
| Chronic urticaria | 13 (4.0) | 11 (8.3) | 0.063 |
| Hematologic component | 83 (25.8) | 29 (22.0) | 0.393 |
| Anemia | 78 (24.2) | 25 (18.5) | 0.222 |
| Serum amyloid A elevation, (<0.5), mg/dL | 227/269 (84.4) | 104/123 (84.6) | 0.966 |
| Serum amyloid A level, mg/dL | 5.5 (0.07–213.0) | 3.1 (0.07–104.0) | 0.024 |
| Serum fibrinogen elevation, (180–350), mg/dL | 102/298 (34.2) | 27/127 (23.3) | 0.008 |
| Serum fibrinogen level, mg/dL | 335 (5.0–980.0) | 310 (103.0–853.0) | 0.045 |
| Leukocytosis, (4.1–11.0) 103/μL | 97/317 (30.6) | 35/130 (26.9) | 0.439 |
| Leukocyte level, 103/μL | 10.0 (1.1–51.9) | 9.1 (1.8–28.0) | 0.042 |
| C-reactive protein elevation, (0–5), mg/L | 202/277 (72.9) | 72/114 (63.2) | 0.055 |
| C-reactive protein level, mg/L | 11.9 (0.5–264.0) | 7.25 (0–146.0) | 0.016 |
| Sedimentation elevation, (0–20), mm/h | 142/245 (58.0) | 51/102 (50.0) | 0.174 |
| Sedimentation level, mm/h | 22 (1–107) | 20.5 (3–60.0) | 0.114 |
| Serum IgE elevation, (0–100), IU/mL | 131/299 (43.8) | 53/119 (44.5) | 0.893 |
| Serum IgE level, IU/mL | 73.0 (1–2724) | 71.0 (2–2728) | 0.439 |
| Eosinophilia, (0–5), % | 63/316 (19.9) | 30/129 (23.3) | 0.435 |
| Eosinophil level, % | 2.0 (0–23.0) | 2.0 (0–22.0) | 0.331 |
| Response to colchicine | 162/170 (95.3) | 59/62 (95.2) | 0.966 |
| Response to prednisolone | 52/70 (74.3) | 52/252 (74.3) | 0.344 |
| Hypogammaglobulinemia | 47/300 (15.7) | 21/119 (17.6) | 0.620 |
| Total | 322 (100.0) | 122 (100.0) | |
Chi square and Mann–Whitney U tests were applied. The values written in bold are statistically significant.
Members of the consanguineous group were younger when diagnosed than members of the non-consanguineous group were (p=0.044). Members of the consanguineous group had less muscle-joint pain than members of the non-consanguineous group (p=0.036). No statistically significant differences were found between consanguineous group and non-consanguineous group patients in terms of gender, onset age, MEFV positivity, fever, atopic disease, abdominal pain, atopic disease, PFAPA syndrome, tobacco exposure, regularity of breast feeding, atopy in the family, clinical symptoms, eosinophilia, serum IgE levels, and other laboratory parameters (p>0.05).
DiscussionThe MEFV gene mutation is located on the short arm of chromosome 16 and consists of 10 exons. The most common genetic mutations are encoded on exons 10 and 2, which are responsible for more than 85% of FMF cases among populations in the Mediterranean basin.2 The incidence of mutations in the MEFV gene may differ between ethnic groups. In the Turkish population, the most commonly seen mutation is M694V, M680I, and V726A.8 In 2012, a group of clinical and molecular experts reached a consensus to test for a total of 14 MEFV variants. This included nine clearly pathogenic variants (M694V, M694I, M680I, V726A, R761H, A744S, I692del, E167D, and T267I) and five variants of unknown significance (E148Q, K695R, P369S, F479L, and I591T).9 They concluded that in some cases, disease in heterozygotes could not be distinguished from that of homozygous patients, and FMF could therefore be viewed as a dominant condition with low penetrance.3 There have been studies that have investigated the MEFV gene mutation carrier state of healthy individuals. In Turkey, the frequency of carriers is reported to be 20.0%.10 In the present study, SAA and fibrinogen levels during an episode of FMF were found to be higher in MEFV-positive patients as compared to MEFV-negative patients. Otherwise, this study found no differences between MEFV-positive and MEFV-negative patients in terms of clinical and laboratory findings. Also, no statistically significant differences were found between homozygote, heterozygote, compound heterozygote, and MEFV-negative patients in terms of clinical and laboratory parameters. Recently, we have reported that R202Q and E148Q mutations also give clinical findings and that these patients responded to colchicine treatment.11 In the present study, the most frequently observed mutations were R202Q heterozygote and E148Q heterozygote mutations. Clinical findings and levels of acute phase reactants (leukocyte count, C-reactive protein, and erythrocyte sedimentation rate) did not differ significantly between MEFV-positive and negative patients. MEFV-negative patients are diagnostically challenging cases and the presence of recurrent symptoms such as fever, abdominal pain, elevation in SAA and/or fibrinogen levels during the episodes, and response to colchicine treatment should all be taken into consideration for diagnosis. SAA and fibrinogen levels may be elevated during an attack in MEFV-positive and MEFV-negative patients. Rarely, SAA and fibrinogen levels are within the normal range between the episodes. MEFV-positivity, the presence of the classical symptoms, and response to colchicine treatment should be taken into consideration during diagnostic work-up in this patient group. This would suggest that mutations which in the past were thought to be unrelated to the clinical/symptomatic presentation of FMF are in fact symptomatic, and that it is wrong to classify these patients according to the MEFV gene.
The heterogeneity of our study's clinical findings may cause confusion, since most of these symptoms are also seen in healthy children. But none of these symptoms have diagnostic value for FMF. Classic symptoms such as recurrent fever and abdominal pain are the symptoms that should be considered relevant for diagnosis. Although examining every patient with atopic disease and recurrent respiratory infection who also has the MEFV gene may be unnecessary and may lead to misdiagnosis, the presence of atopic disease and recurrent respiratory tract infections may nevertheless provide a diagnostic clue for the clinician. The presence of classic symptoms (e.g., recurrent fever, abdominal pain) associated with atopic disease and recurrent respiratory infections will also guide the diagnosis. In these patients, the diagnosis should be made by evaluating such parameters as MEFV gene positivity, amyloidal and fibrinogen elevation during episode, and response to colchicine, in addition to clinical findings.
PFAPA syndrome (periodic fever, aphthous stomatitis, pharyngitis and cervical adenitis) is the most common cause of periodic fever in childhood. Corticosteroids are effective in aborting PFAPA flares, but do not prevent recurrence.12,13 We classified the patients with PFAPA syndrome into three groups: patients with recurrent tonsillopharyngitis; patients with incomplete PFAPA (recurrent tonsillopharyngitis and aphtous stomatitis or cervical adenopathy); and patients with complete PFAPA (i.e., patients fulfilling all diagnostic criteria). No significant difference was found among these three groups regarding the elevation levels of acute phase reactants, MEFV positivity, the presence of accompanying classical clinical symptoms, response to steroid treatment during the attack, and response to colchicine treatment. In most children, the disease has a spontaneous resolution that occurs within a few years (in most cases before puberty). However, in 20% of cases, the initial recovery is followed by a new occurrence of febrile flares in adulthood.14
The utility of tonsillectomy in the treatment of PFAPA syndrome is still disputed. It is reported in the literature that 65% of PFAPA patients benefit from tonsillectomy, while the rest do not.15 In our study, we showed that fever was higher in patients with tonsillopharyngitis whether they had PFAPA syndrome or not. Forty-three (9.5%) of our patients received tonsillectomy and 22 (4.8%) of these responded to tonsillectomy. However, it was found that only episodes of fever regressed in patients who responded to such treatment. In the follow-up for most of our patients, severe atopy, abdominal pain, and muscle-joint pain were found to continue. This suggests that tonsillectomy caused a pseudo-recovery in these patients and that both families and doctors were misled by the apparent recovery.
In studies conducted recently, the frequency of atopic disease was not found to be higher in FMF patients when compared with healthy children. Some studies even suggested the hypothesis that FMF is protective against atopic diseases.16–18 However, it should be considered that the number of patients is limited in these studies and the average age was higher than the average age in our study. It is also known that diseases such as food allergies and atopic dermatitis also decrease with age.19 It has been shown that TH17 cells are associated with pyrin dysfunction and fever stimulation in FMF patients.20 It was also previously reported that H17 cells played a defensive role against allergic diseases, auto-immunity, and various pathogens. In our study, 95% of the patients were found to have at least one allergic disease and the atrophies of these diseases generally had a severe course. In our study, the number of attacks in a year was more than 12 in patients with asthma/wheezing. Symptoms were found to be persistent in 54.7% of the patients with allergic rhinitis, while the SCORAD index was over 25 in 52.5% of the patients with atopic dermatitis. Another feature of our study was the finding that patients with PFAPA syndrome had allergic rhinitis incidence, aeroallergen sensitivity, and a high total IgE and eosinophils. The significance of this study is reporting that atopy is a component of FMF, rather than reporting that atopic diseases are frequently seen in FMF patients. Atopic symptoms of these patients increase in the active periods of the disease.
The immune response generated by TH17 cells has been shown to be involved in the defence against extracellular bacteria and fungi (e.g., Klebsiella pneumoniae and Candida albicans). Deficiencies in IL-17 or IL-17R lead to increased susceptibility to opportunistic pathogens such as Staphylococcus aureus and Candida albicans. In addition, it is believed that TH17 cells have a role in various viral infections, primarily influenza and herpes virus infections.21 In the present study, 89.6% of our patients were found to have immunological symptoms, including recurrent infections. Recurrent respiratory tract infections were one of the most obvious characteristics of this disease. In our clinical observations, we found that while the complaints of some patients began at birth, the complaints of others started while at school or in kindergarten. In addition, the episodes experienced by a great number of the patients were found to increase in the winter months. This suggests that these patients may be sensitive to pathogens of the respiratory tract.
Recently, Butbul Aviel et al. reported that PFAPA and FMF are strongly associated and may clinically overlap, although they are distinct entities.22 The significance of the current study was the demonstration of the association of the MEFV gene with severe atopic diseases and recurrent respiratory infections. Patients with FMF and PFAPA have been reported to be treated with colchicine.4,11 We found that recurrent respiratory infections (rhinosinusitis, tonsillopharyngitis, croup, and pneumonia) improved with colchicine treatment. On the other hand, we observed a variable response to colchicine treatment in atopic diseases such as asthma, allergic rhinitis, and eczema. Treatment-focused prospective studies are needed to explore the role of colchicine in the treatment of atopic diseases, considering that these patients usually take allergy medication (antihistamines, steroids, etc.) as well.
In the present study, we endeavoured to show that rather than being just a periodic fever syndrome, FMF is a multisystemic disease accompanied by severe atopy and recurrent respiratory infections. Patients with severe atopy and recurrent respiratory infections should be questioned for FMF symptoms and should be examined for FMF.
Ethical disclosuresConfidentiality of dataThe authors declare that no patient data appears in this article.
Right to privacy and informed consentThe authors declare that no patient data appears in this article.
Protection of human subjects and animals in researchThe authors declare that no experiments were performed on humans or animals for this investigation.
FundingNone.
Conflict of interestThe authors have no conflict of interest to declare.