In Africa, few studies of sensitisation profiles have been performed in children or adolescents and, in Angola, there are none. The objectives of the present study were to assess the sensitisation profile of Angolan schoolchildren and to determine the relationship between that pattern, sociodemographic factors, asthma and other allergic diseases.
Materials and MethodsCross-sectional, observational study in 5-14-year-old children, performed between September and November 2017, in the Province of Bengo, Angola. Five schools (15%) were randomly selected in the geographical area of the study: three from an urban area, and two from a rural area. Data were collected using the Portuguese versions of the ISAAC questionnaires for children and adolescents, regarding asthma, rhinitis and eczema. Skin prick tests (SPT) were performed with a battery of 12 aeroallergens. Stools were assessed for the presence of helminths. Descriptive statistics were used, as well as univariate calculation of odds ratios.
ResultsSensitisation to aeroallergens was low (8%) and most sensitised children were asymptomatic. Most frequent sensitisations involved house dust mites, cockroach or fungi, and a high proportion of children (78.1%) were monosensitised. No relationship was detected between sensitisations and asthma, rhinitis or eczema. Place of residence, gender, age or helminthic infection did not affect the probability of having positive SPTs.
ConclusionsThe most frequent sensitisations in children from Bengo Province in Angola involve house dust mites, followed by cockroach and fungi. No relationship was found between atopic sensitisation and asthma or other allergic diseases.
The prevalence of asthma, rhinitis and eczema has been increasing, particularly in children1 and the International Study of Asthma and Allergies in Children (ISAAC) confirmed this trend when two timepoints were compared, at least for some age groups.2 A high proportion of these diseases is associated with atopy,3 with sensitisation profiles varying according to geoclimatic and socioeconomic factors, among others.4,5 Worldwide, there are some aeroallergens that are more frequently associated with allergic diseases in schoolchildren and adolescents, and these include dust mites, fungi, and cockroach, with geographical variations,5,6 although in areas such as the USA and Europe, sensitisation to various pollens is also relevant.5,7 Furthermore, some sensitisations may be actual risk factors for the development and severity of allergic diseases.6,8
In Africa, few studies of general sensitisation profiles have been performed, namely in children or adolescents. These include a study performed in Zimbabwe, in 650 allergic urban individuals, using a panel of 20 aeroallergens for skin prick tests (SPT), and which showed that the most prevalent sensitisations were to mites and grass pollens.9 However, the study involved individuals with a very broad age range, from one to 65 years and there was no discrimination of sensitisations according to age. Three other studies were performed in South Africa. One of these involved children, and showed that the most common sensitisations were to dust mites, Timothy grass and cat dander.10 However, this study only involved children with atopic dermatitis and results were based on specific IgE results and not on SPT results. The second study involved adolescents from urban areas, and showed that most common sensitisations were to mites and cockroach.11 A related study showed similar results and an urban-rural difference in sensitisations in children.12 However, these two studies only involved Xhosa children and adolescents. Another study was performed in Uganda, involving a cohort of children that were studied at three and nine years of age and it also showed a similar pattern of sensitisations.13 There are also a few studies that only focused on the prevalence of sensitisation to the most common aeroallergens such as dust mites and/or cockroach. These include a study performed in rural and urban 6–15 year-old schoolchildren in Ghana, which used SPT with house dust mites and cockroach, and showed a prevalence of sensitisation of 13.6% and 10.8% to these allergens, respectively,14 and another study carried out in 13−14 year old adolescents in Nigeria, which used SPT with mites, cockroach, mould, cat and mouse epithelia, and showed that mites and cockroach were the most prevalent ones, particularly in asthmatic adolescents.15 Other, very few, studies, were carried out in adults.16,17
It is clear that there are very few studies carried out at different latitudes, in Africa, in terms of sensitisation profiles and their relationship with allergic diseases. Furthermore, there are very few studies using a broad panel of aeroallergens for testing sensitisations, in children or adolescents. Finally, in Angola, there are no previously published studies in this context. Thus, the objectives of the present study were to assess the sensitisation profile of Angolan schoolchildren and to determine the relationship between such a pattern and sociodemographic factors as well as the expression of allergic diseases.
MethodsStudy area and populationThis study was performed in the Province of Bengo, in Caxito (urban) and Úcua (rural) areas, with the support of the Centro de Investigação de Saúde em Angola (CISA).
The climate is tropical, semi-dry, with annual relative temperatures between 22 and 32 °C.
The area under study is included in the CISA’s Health and Demographic Surveillance System (HDSS), which comprises 4700 km², corresponding to a population of around 60,000 inhabitants.18
Study designThis was a cross-sectional, observational study in 5-14-year-old children, performed between September and November 2017. Five schools (15%) were randomly selected out of a total of 33 schools in the geographical area of the study: three were selected from an urban area, and two from a rural area.
SampleAll schoolchildren within the adequate age range were invited to participate in the study via an information letter sent to their parents. Children with suspected infectious lung disease or other chronic infectious disease were excluded upon medical examination. All children whose parents did not consent to participate in the study were also excluded. The sample size was calculated with a 95% confidence interval and a margin of error not above 3%, considering a population of around 17,873 5-14-year-old children included in CISA’s HDSS, and on the basis of two previous ISAAC-formatted studies on the prevalence of asthma, allergic rhinitis and atopic eczema in 6–7-year-old children19 and 13−14 year-old adolescents20 from Luanda, Angola, as well as on a possible prevalence of atopy of around 25%.13 This yielded a recommended sample size of 1008 children.
Data collectionWritten questionnairesData were collected using the Portuguese version of the ISAAC questionnaires for children and adolescents,21 which contain questions about asthma, rhinitis and eczema symptoms. The ISAAC Phase III environmental exposure and risk factor questionnaire was also used.22 All questions were answered and explanations were given in a standardised fashion, in Portuguese, by a specifically trained team of researchers, with the collaboration of the parents or guardians, in the presence of the children.
DefinitionsIn accordance with the ISAAC phase III protocol, current asthma was defined as the presence of wheezing episodes in the previous 12 months, current rhinitis was recorded for sneezing, rhinorrhoea or nasal obstruction, not associated with flu, in the previous 12 months, and eczema was defined as the presence of itchy skin lesions which waxed and waned in the previous 12 months.21 Atopy was defined by positive SPT.23 Atopic monosensitisation was defined by positive SPT to a single aeroallergen, and polysensitisation by positive SPT to two or more non-related (or not obviously related) aeroallergens.32 Helminthic infection was defined by the presence of helminth adult worms, worm segments, eggs or larvae in stools.24
Allergen SPTSPT (LETI Laboratories, Barcelona, Spain) were performed by a team with training and experience in this type of test, and in accordance with the standardised procedure of the European Academy for Allergology and Clinical Immunology.25 A battery of 12 aeroallergens was used (Dermatophagoides pteronyssinus, Dermatophagoides farinae, Blomia tropicalis, grass mix, weed mix, Aspergillus species, Cladosporium species, Mucor mucedo, Alternaria alternata, cockroach mix, dog and cat epithelia). Histamine dihydrochloride (10 mg/ml) was used as a positive control, and allergen diluent control was used as a negative control. A small drop of each allergen extract was placed, in duplicate, on the volar aspect of the forearms, and subsequently pricked through using a metal lancet (Stallergènes, Antony, France). The longest diameter of each wheal was measured after 15 min. Wheals with a diameter at least 3 mm greater than that of the negative control were regarded as positive.
Analysis of parasitic infectionStool examinationsA sample of faeces from each child was obtained for assessment of the presence of helminths as adult worms, worm segments, eggs and larvae. Samples were processed by the ParasiTrap® concentration method (ParasiTrap Fixsepar Eco System, VWR international GmbH, Germany) and by the quantitative Kato-Katz® (Sterlitech kit KatoKatz Method, Kent, Washington, USA) method, for detecting Ancylostoma duodenale, Ascaris lumbricoides, Trichuris trichuria, Strongyloides stercoralis, Enterobius vermiculares, Hymenolepsis nana and Schistosoma mansoni. Slides were observed independently by two technicians between 30 min and two hours after being processed, in accordance with WHO recommendations.24
Ethical considerationsThis study was approved by the Ethics Committees of the Ministry of Health of Angola and the University of Beira Interior. It was also approved by the Directors of the selected schools. A written, informed consent form was also signed by parents / guardians of all participating children, which was sent to them two days prior to data collection. All children with symptoms of asthma, allergic rhinitis and eczema were referred to a pulmonology outpatient clinic, and simple aeroallergen eviction measures were given to parents of children with positive SPT. Anti-helminthic treatment (Albendazole and Praziquantel) was freely supplied to all children with clinical indication.
Statistical analysisData were processed and analysed using the IBM SPSS software, version 25. Categorical variables were described using frequencies and percentages, and quantitative variables were analysed by means, standard deviations, medians, and ranges. All possible associations between variables under study and potential risk factors were studied by logistic regression, which estimated odds ratios (OR). An adjusted version of OR was also studied, taking into account some of the variables, where applicable, and adjusted OR values were only shown when Wald test p value was lower than 0.2. Wald test was regarded as statistically significant when the p-value was lower than 0.05.
ResultsSociodemographic dataThe sample included 1023 schoolchildren aged between five and 14 years, from five randomly selected schools. This corresponded to a 99% reply rate to the invitation of participating in the study. There were slightly more boys than girls, most children were between 10 and 14 years old, and most were residing in urban areas (Table 1).
Sociodemographic data of study sample (n = 1023).
| Parameter | N (%) |
|---|---|
| Sex Girls Boys | 495 (48.4) 528 (51.6) |
| Age range (years) 5–9 10-14 | 434 (42.4)589 (57.6) |
| Residence Urban RuralMaternal schooling No schooling 1–4 years 5–9 years ≥ to 10 years Type of family income Irregular 1 parent regular Both parents regular | 619 (60.5)404 (39.5)190 (18.6)540 (52.8)227 (22.2)66 (6.4)499 (48.8)442 (43.2)82 (8) |
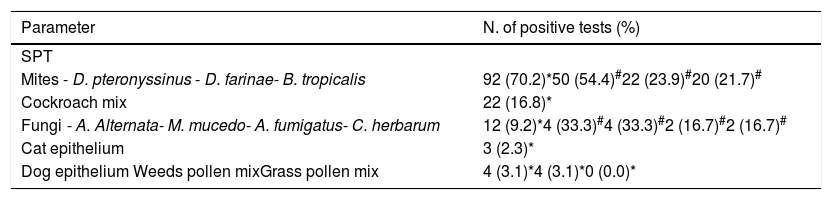
SPT to aeroallergens were positive in 82 (8.0 %; 95%CI 6.5;9.9) of the 1023 children, corresponding to 92 positive tests. The most frequently observed sensitisations were due to house dust mites (70.2%), particularly D. pteronyssinus, cockroach (16.8%) and fungi (9.2%) (Table 2).
Sensitisation to aeroallergens in 82 children with positive skin prick tests.
| Parameter | N. of positive tests (%) |
|---|---|
| SPT | |
| Mites - D. pteronyssinus - D. farinae- B. tropicalis | 92 (70.2)*50 (54.4)#22 (23.9)#20 (21.7)# |
| Cockroach mix | 22 (16.8)* |
| Fungi - A. Alternata- M. mucedo- A. fumigatus- C. herbarum | 12 (9.2)*4 (33.3)#4 (33.3)#2 (16.7)#2 (16.7)# |
| Cat epithelium | 3 (2.3)* |
| Dog epithelium Weeds pollen mixGrass pollen mix | 4 (3.1)*4 (3.1)*0 (0.0)* |
SPT- skin prick test: (*) - relative to total number of positive tests.
(#) – relative to total number of positive tests to mites or fungi, respectively.
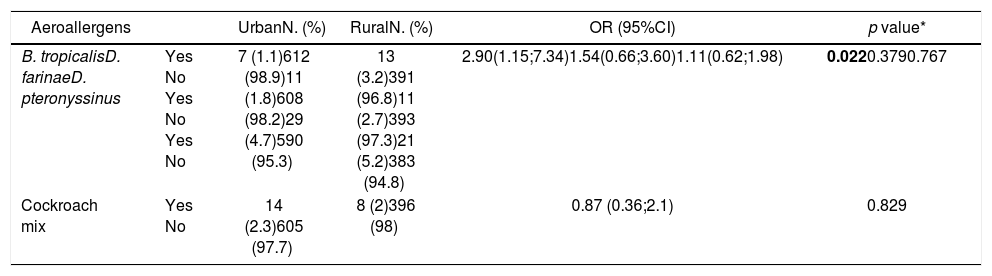
When we compared the prevalence of sensitisation to the most common aeroallergens between urban and rural children, no significant differences were observed, except for sensitisation to B. tropicalis, which was more frequent in rural children (Table 3).
Comparison of prevalence of sensitisation to the most common aeroallergens between urban and rural children.
| Aeroallergens | UrbanN. (%) | RuralN. (%) | OR (95%CI) | p value* | |
|---|---|---|---|---|---|
| B. tropicalisD. farinaeD. pteronyssinus | Yes No Yes No Yes No | 7 (1.1)612 (98.9)11 (1.8)608 (98.2)29 (4.7)590 (95.3) | 13 (3.2)391 (96.8)11 (2.7)393 (97.3)21 (5.2)383 (94.8) | 2.90(1.15;7.34)1.54(0.66;3.60)1.11(0.62;1.98) | 0.0220.3790.767 |
| Cockroach mix | Yes No | 14 (2.3)605 (97.7) | 8 (2)396 (98) | 0.87 (0.36;2.1) | 0.829 |
OR- odds ratio; 95%CI- confidence interval; * Wald test.
Regarding the sizes of the SPT wheals, for the most common aeroallergens, results showed no significant differences among dust mites or between mites and cockroach mix - D. pteronyssinus, D. farinae, B. tropicalis: mean + SD = 3.76 + 1.15 mm; median (range) = 3 (3–7)mm or between mites and cockroach mix - mean + SD = 3.09 + 0.29 mm; median (range) = 3 (3–4)mm.
In the 82 children with a positive SPT, 64 (78.1%) were monosensitised, and 18 (21.9%) were polysensitised. The most frequent polysensitisations involved mites and cockroach (n = 8; 44.4% of polysensitised children), followed by co-sensitisation to mites and fungi (n = 6; 33.3%). This pattern was not affected by the presence of allergic disease or its type. In fact, both in children with allergic disease and in asymptomatic children, the most frequently observed polysensitisations involved co-sensitisations to mites and cockroach (n = 2; 15.4% versus n = 6; 26.1%, respectively), without significant differences.
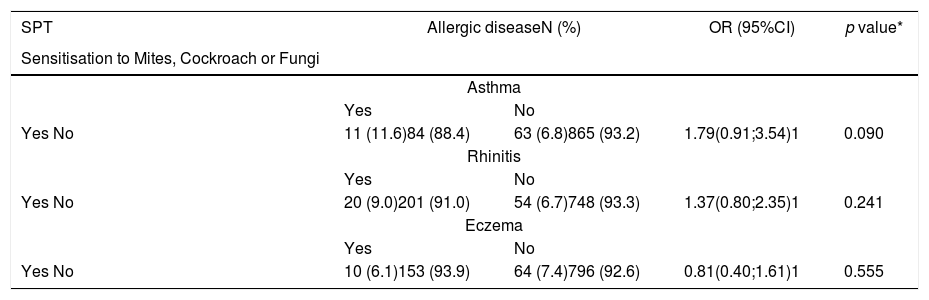
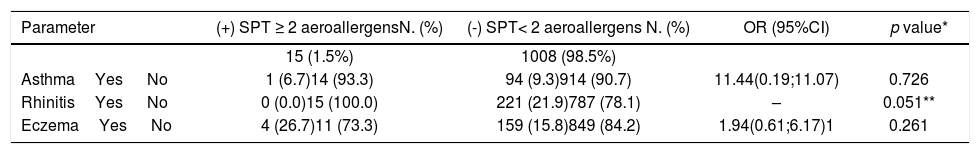
Relationship between allergic sensitisation and allergic diseaseRegarding the presence of allergic diseases in the 82 children with positive SPT, 11 (13.4%) had isolated asthma, 11 (13.4%) had isolated eczema, and 21 (25.6%) had isolated rhinitis. In addition, four (4.9%) children had both asthma and rhinitis, two (2.4%) had asthma and eczema. Interestingly, most sensitised children (n = 4; 54.9%) did not have any manifestations of allergic diseases. When the prevalence and pattern of sensitisation to the most common aeroallergens were compared between children with and without allergic disease, no significant differences were found (Table 4). However, a trend towards a significant increase in frequency of positive sensitisation tests was observed in children with asthma. Analysis of the association between the wheal size of the most common aeroallergens and specific allergic disease or being parasitised showed no significant results (data not shown). Finally, having positive SPT was not significantly predictive of having an allergic disease (asthma, rhinitis or eczema) (Table 5).
Relationship between the prevalence of sensitisation to the most common aeroallergens and prevalence of allergic diseases.
| SPT | Allergic diseaseN (%) | OR (95%CI) | p value* | |
|---|---|---|---|---|
| Sensitisation to Mites, Cockroach or Fungi | ||||
| Asthma | ||||
| Yes | No | |||
| Yes No | 11 (11.6)84 (88.4) | 63 (6.8)865 (93.2) | 1.79(0.91;3.54)1 | 0.090 |
| Rhinitis | ||||
| Yes | No | |||
| Yes No | 20 (9.0)201 (91.0) | 54 (6.7)748 (93.3) | 1.37(0.80;2.35)1 | 0.241 |
| Eczema | ||||
| Yes | No | |||
| Yes No | 10 (6.1)153 (93.9) | 64 (7.4)796 (92.6) | 0.81(0.40;1.61)1 | 0.555 |
SPT- skin prick test; OR- odds ratio; 95%CI- confidence interval; * Wald test.
Relationship between mono- and poly-sensitisations to aeroallergens and allergic diseases.
| Parameter | (+) SPT ≥ 2 aeroallergensN. (%) | (-) SPT< 2 aeroallergens N. (%) | OR (95%CI) | p value* |
|---|---|---|---|---|
| 15 (1.5%) | 1008 (98.5%) | |||
| AsthmaYes No | 1 (6.7)14 (93.3) | 94 (9.3)914 (90.7) | 11.44(0.19;11.07) | 0.726 |
| RhinitisYes No | 0 (0.0)15 (100.0) | 221 (21.9)787 (78.1) | – | 0.051** |
| EczemaYes No | 4 (26.7)11 (73.3) | 159 (15.8)849 (84.2) | 1.94(0.61;6.17)1 | 0.261 |
SPT- skin prick test; OR- odds ratio; 95%CI- confidence interval; * Wald test; **Fisher’s Exact Test.
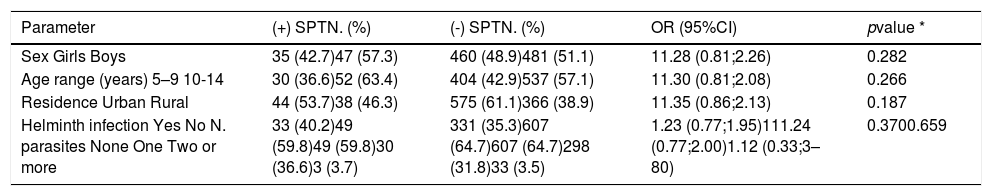
We next analysed some factors that may influence sensitisation to aeroallergens - place of residence (urban or rural), gender, age, and presence and intensity of helminthic infection (Table 6) or specific helminth types (data not shown). None of these factors were significantly associated with being sensitised to aeroallergens and this also applied to the relationship between other sociodemographic factors and atopy (data not shown).
Analysis of factors that may potentially affect sensitisation to aeroallergens.
| Parameter | (+) SPTN. (%) | (-) SPTN. (%) | OR (95%CI) | pvalue * |
|---|---|---|---|---|
| Sex Girls Boys | 35 (42.7)47 (57.3) | 460 (48.9)481 (51.1) | 11.28 (0.81;2.26) | 0.282 |
| Age range (years) 5–9 10-14 | 30 (36.6)52 (63.4) | 404 (42.9)537 (57.1) | 11.30 (0.81;2.08) | 0.266 |
| Residence Urban Rural | 44 (53.7)38 (46.3) | 575 (61.1)366 (38.9) | 11.35 (0.86;2.13) | 0.187 |
| Helminth infection Yes No N. parasites None One Two or more | 33 (40.2)49 (59.8)49 (59.8)30 (36.6)3 (3.7) | 331 (35.3)607 (64.7)607 (64.7)298 (31.8)33 (3.5) | 1.23 (0.77;1.95)111.24 (0.77;2.00)1.12 (0.33;3–80) | 0.3700.659 |
SPT- skin prick test; OR- odds ratio; 95%CI- confidence interval, * Wald test.
This is the first study of aeroallergen sensitisation in Angolan children and one of the few carried out in Africa. Using SPT we showed that sensitisation to aeroallergens was low and also that most sensitised children were asymptomatic. The most frequent sensitisations involved dust mites, cockroach or fungi, and a high proportion of the children (43.9%) were polysensitised. No relationship was detected between specific sensitisations and asthma, rhinitis or eczema.
In our study, SPT to aeroallergens were only positive in 82 of the 1023 children, suggesting a prevalence of atopy of only 8.0%. This is lower than in other African regions. In a cohort study involving 1170 nine-year-old children from Entebbe, Uganda, the prevalence of positive SPT was 25.2%13; in another study involving 600 university students living in the capital of Cameroon, Douala, SPT-based prevalence was 42.8%16; in another report in high school 15–24 year-old Xhosa adolescents, SPT-reported atopy was 32%,11 and, finally, in a study performed in Zimbabwe, involving 650 urban 1–63 year-old individuals (mean age = 24.7 years), around 52% were sensitised to Dermatophagoides pteronyssinus.9 Several factors may account for these discrepancies. First of all, genetic factors specific for each population, are well known to play a part. Secondly, climatic and geographical features impact upon exposure to outdoor allergens, and differential indoor conditions may also affect sensitisation to indoor allergens.26 Thirdly, although our population was mostly urban, most children frequently moved to rural regions during the weekends and holidays, to help their parents with rural chores. This was in contrast with the clearer urbanicity of volunteers in other studies. Finally, our results might also be explained by a possibly high infection of helminthic infections in the Bengo area. However, only around 30–40 % of children were infected and there were no differences in the prevalence of positive SPT between infected and non-infected children.
The most frequently observed SPT sensitisations were to house dust mites (70.2%), particularly D. pteronyssinus, and cockroach and fungi (9.2%). These results are similar to those from other studies in African children, adolescents and young adults,10,11,14,27,28 with some of them showing an urban-rural difference. In our study, however, we did not find any significant differences between living in urban or rural settings and SPT results, except for sensitisation to B. tropicalis which was more frequent in rural children, a finding similar to that found in South Africa, in a study involving 1-18-year-old children from a rural and an urban area.29 Apart from climate and geography, other reasons that may underlie this situation should be ascertained.
We did not detect children sensitised to grass pollens, as was also seen in other studies in Africa. The study in 9-year-old urban Ugandan children, showed that only 1.2% and 0.8% were sensitised to Bermuda grass or other pollens, respectively.13 In a Ugandan study on urban and periurban adult women, only three women reacted to Bermuda grass, and one to weed pollen mix.17 However, our results are in contrast with other reports. A Nigerian study in 13–14-year-old schoolchildren, showed SPT positivity to Southern grass mix in 7–17% of children,15 and another study in 1–63-year-old volunteers from Zimbabwe showed high percentages of sensitisation to pollens: 38% to Cynodon dactylon, 28% to maize pollen, and 26% to grass pollen mix.9 Various reasons may explain the discrepancy between these studies. Firstly, grass pollens in our SPT battery (mostly of the Pooideae subfamily) may not be adapted to the geographical area we studied. In fact, the Chloridoideae and Panicodideae subfamilies are more frequent in Southern Africa and cross-reactivity between these subfamilies and that of Pooideae subfamily is low.30 Secondly, different locations are associated with contrasting climatic settings, which influence vegetation content and pollen release.31 Such differences can be found between Angola and other areas of Africa. Finally, specific types of pollens also differ across regions.31 Further studies should be performed with a broader range of grass pollen sub-families.
Most (56.1%) of the sensitised children were monosensitised, and of the 43.9% of children who were polysensitised, the most frequent sensitisations occurred to different types of mites, followed by mites and cockroach or mites and fungi. Few African studies mention this aspect but in two that did, contrasting results were observed. In the Uganda study in 9-year-olds, monosensitisation was 89.1%16 whereas only 13.2% of university students were monosensitised, in the Cameroon study.16 Overall, polysensitisations are frequent in allergic individuals, and may increase the risk of allergic multimorbidity or severity of IgE-mediated symptoms.32,33 However, polysensitisation may depend upon multiple factors, including individual phenotypes of response, or other population-specific sociodemographic features, and its relevance should be based not only upon epidemiological data but also on clinical aspects.
Our study did not show any relationship between specific patterns of sensitisation, their magnitude and the presence of asthma, rhinitis or eczema. This contrasts with various studies which showed that children with allergic diseases are more frequently sensitised to aeroallergens. For instance, the study in high school Xhosa children in South Africa showed that the number of positive SPTs was positively associated with asthma and bronchial hyper-reactivity.11 In another study involving 13–14-year-old Nigerian schoolchildren, positive SPTs to aeroallergens were more frequent in asthmatic than in non-asthmatic children.15 In the Ugandan cohort study involving 9-year-old children, having positive SPTs significantly increased the risk of having wheeze, allergic rhinitis and eczema.13 These results are in agreement with large analyses of European data which showed that atopy increases the risk of allergic comorbidities.32 However, this association may not always be found, as shown by a study performed in 242 Tanzanian schoolchildren, in which no differences in SPT reactivity were observed between asthmatic and non-asthmatic children.34 In addition, a study performed in 7155 1-4-year-old rural and urban Ethiopian children showed no relationship between SPT-detected sensitisation to D. pteronyssinus or cockroach and the presence of wheeze.35
Our study did not show any significant association between factors such as place of residence (urban or rural), gender, age, helminthic infection and risk of being sensitised to aeroallergens. This is in contrast with various other studies in children, particularly in terms of the urban-rural axis. For instance, a study in 9-12-year-old Korean children from a rural village, a rural town and an urban city showed that the prevalence of allergic diseases and atopy was lower in children with rural, farming parents.36 Another study, performed in 50 rural and 50 urban children in India showed that rural children had lower prevalence of positive SPTs, self-reported asthma and rhinitis.37 Finally, a South African study in urban, peri-urban and rural Xhosa children showed that the prevalence of positive SPTs was significantly lower in rural than in urban or recently urbanised children.12 Various factors may underlie the difference between these studies and our report, namely the fact that urban children in our study also had frequent and regular contact with rural settings, namely farms, since infancy and this may have decreased urban-rural differences. Furthermore, rather than simply analysing rural-urban differences on their own, such differences should always be integrated into broader living profiles including factors such as affluence, indoor exposures to fumes, number of siblings or presence of animals in the house.38,39 Moreover, children in the study area (Bengo Province) resident in urban areas play outdoors for a good part of the day, in very similar conditions to those in rural areas. Furthermore, most of the children spend their school holidays in rural areas, helping family with subsistence farming.
Our study has some limitations. Firstly, the questionnaires were based on self-reporting and this may have been associated with memory bias. Secondly, part of those residing in rural areas had some limitations in understanding certain concepts in ISAAC questionnaire items, and this may have been associated with interpretation biases. Thirdly, our SPT battery may not have been capacitated to detect sensitisations to pollens in the region. This aspect should be further analysed with a broader range of pollen allergens. Another limitation is that, although we used validated ISAAC questionnaires, we did not fully study some personal and family factors (namely history of atopy) as well as various sociodemographic factors that may have interfered with sensitisation, namely number of siblings in the home, presence of animals at home or exposure to farm animals. Another limitation may have been due to the fact that we did not test specific aeroallergen-specific IgE levels, which might enrich our analysis. Finally, although our sample size was determined to have enough statistical power (1008 estimated; final 1023 children sampled), it may not have been sufficiently powered to study the influence of factors such as helminthic infections, on their own or in association with rural or urban living and other sociodemographic features, upon atopy. In spite of these limitations, our study is part of a set of very few studies on allergen sensitisations in African children, and the first one in Angola. In terms of the relationship between helminthic infections and the presence of atopy, our study did not show any significant association. Nevertheless, this relationship is not simple, should not be regarded in isolation and the results from African studies have been inconsistent.40
In conclusion, our results have shown that most frequent sensitisations in children from Bengo Province in Angola involve house dust mites, followed by cockroach and fungi. No relationship was found between atopic sensitisation and allergic diseases, helminthic infection or other sociodemographic factors. Future studies involving a larger sample of children from other Angolan regions, as well as a broader battery of allergens and further questions on environmental and sociodemographic aspects should be implemented to better clarify profiles of atopic sensitisation and factors that influence it in African children.
Conflicts of interestOL, FQ, JMRG and MB have no conflicts of interest and have not been funded.
MA has received support to attend international congresses from MSD and from AstraZeneca Angola.
LTB has received support to attend EAACI congresses from Victoria Laboratories and Menarini and has been paid lecture fees by Novartis, AstraZeneca, Merck Sharp & Dohme and Menarini.
JRP has received support to attend EAACI congress from Roxall.
The authors would like to acknowledge all CISA staff for their support during the field work, the local administration, the directory boards of all schools involved in the study, and all the children and their parents/guardians. This work was supported by the promoters of the Centro de Investigação em Saúde de Angola—Camões – Institute for Cooperation and Language, Calouste Gulbenkian Foundation, Bengo Provincial Government and the Ministry of Health of Angola—which played no role in either the design of the study or in interpreting the findings.
We would like to thank LETI Laboratories (Porto, Portugal) for having kindly supplied the aeroallergen batteries. No drug company had any input in the design or implementation of the study.